Abstract
This study aims to investigate the effects of ulinastatin on therapeutic outcomes and inflammatory markers in children with septic shock. This study selected 117 children with septic shock admitted to our hospital from April 2023 to April 2024. Among them, 7 children had comorbidities, 3 dropped out during the research process, 5 did not continue to participate in the study due to other reasons, and 4 explicitly refused to participate. After screening and excluding the above - mentioned cases, A total of 98 children were allotted to experimental group (ulinastatin combined with routine antishock therapy, n = 49) and control group (routine antishock therapy, n = 49) using stratified random method. The therapeutic effects and levels of inflammatory markers were assessed before and after treatment using a double antibody sandwich ELISA. The outcome was significantly better in the experimental group compared to controls (P < 0.05). Prior to treatment, there were no significant differences in the levels of CD64, procalcitonin (PCT), C-reactive protein, neutrophil-to-lymphocyte ratio (NLR), and serum α-hydroxybutyrate dehydrogenase between the two groups (all P > 0.05). After 7 days of treatment, the experimental group exhibited significantly lower levels of CD64, PCT, C-reactive protein, NLR, and serum α-hydroxybutyrate dehydrogenase compared to the control group (all P < 0.001). Ulinastatin enhances the therapeutic efficacy in children with septic shock by reducing levels of associated inflammatory markers and promoting recovery.
Similar content being viewed by others
Introduction
Sepsis is defined as a dysregulated response of the body to an infection that can lead to life-threatening organ dysfunction. In severe cases, this condition may progress to septic shock, a critical state requiring immediate medical intervention1,2. According to statistics from 2018, the sepsis-related mortality rate in China is 6.7 per 100,000, which is significantly higher than that observed in developed countries3. Recent global data indicate that the weighted case fatality rate for sepsis and septic shock in children is markedly different between regions, with developing countries experiencing a rate of 31.7%, compared to 19.3% in developed nations4. These figures highlight the high mortality associated with septic shock and underscore the serious threat it poses to pediatric health5,6.
Surveys have shown that septic shock is a prevalent cause of mortality in neonatal intensive care units, with reported fatality rates ranging from 10 to 50% in pediatric emergency settings7. Children in septic shock often exhibit various degrees of organ dysfunction and metabolic disturbances due to factors such as infection and hypoxia. Furthermore, septic shock is characterized by a systemic inflammatory response syndrome, where inflammation plays a crucial role in its pathogenesis8.
Ulinastatin, a urinary trypsin inhibitor, has been shown to have beneficial effects in this context. It works by inhibiting the release of inflammatory mediators, stabilizing lysosomal membranes, and scavenging oxygen free radicals, thus mitigating the inflammatory response associated with septic shock9. Despite these potential benefits, there is limited clinical literature addressing the combined treatment of ulinastatin with other therapeutic agents in pediatric patients with septic shock.
In light of this, the present study aims to investigate the effects of ulinastatin on therapeutic outcomes and inflammatory markers in children with septic shock. By elucidating these effects, we hope to provide valuable clinical guidance for the treatment of pediatric patients suffering from this critical condition.
Patients and methods
Patients
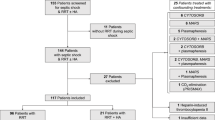
This study included 117 patients who met the inclusion criteria and visited our hospital from April 2023 to April 2024. Among them, there were 2 cases of primary adrenocortical insufficiency, 1 case of liver cancer, 2 cases of juvenile idiopathic arthritis, 1 case of systemic lupus erythematosus, 3 cases of type I diabetes, 4 cases of inflammatory bowel disease, 2 cases of hemorrhagic shock, 1 case of myocardial infarction shock, and 3 cases of abandonment of treatment. In the end, a total of 98 people participated in the final study (see FIG. 1). Cases were divided into control group (n = 49) and experimental group (n = 49) using random number table method. General data, before treatment and after treatment inflammatory factor level information, hospitalization information and adverse drug reaction information were collected. The above information was analyzed statistically.
Sample size
The sample size was calculated as follows: n = 2[(u1-α + u1-β) s/σ]2, α = 0.05, β = 0.01, and n was 117. According to the regulations of the State Food and Drug Administration of China, 15% was taken as the shedding release rate, so the sample size of the group in this study was determined to be n = 117 × 1/ (1 ~ 0.15) = 98.12 ≈ 98. Therefore, 98 children with septic shock were selected as the study objects.
Inclusion and exclusion criteria
Inclusion criteria
(1) In line with the diagnostic criteria for pediatric septic shock in the 2012 International Guidelines for the Diagnosis and Treatment of Severe Sepsis and Septic Shock10; (2) Age: 6 to 12 years old; (3) The parents or guardians of the children voluntarily participated in the study, knew the research contents, able to cooperate with the treatment, and signed the informed consents.
Exclusion criteria
(1) Patients with severe major organ dysfunction, malignant tumor, primary adrenal cortical dysfunction, hematological diseases or autoimmune diseases; (2) Shock caused by non-infectious factors; (3) Patients who received glucocorticoids or other immunosuppressants within 3 months prior to admission; (4) Patients with a history of mental illness or mental impairment; (5) Patients who gave up treatment for various reasons, or failed to complete the 7-day treatment due to adverse drug reactions, death or other reasons; (6) Concomitant congenital disease or genetic disease.
This study carried out the spirit of blind method and adopted double blind method. In the process of implementing the blind method, the children, their families, doctors, nurses, examiners and data statisticians did not communicate with each other and completed their work independently. This study was approved by the ethics committee of the Huanggang Central Hospital of Yangtze University, and the informed consent forms were obtained from all patients.
Pathogen identification
All patients underwent pathogen identification upon admission. Blood cultures and samples from suspected infection sites (e.g., sputum, peritoneal drainage, urine, cerebrospinal fluid) were collected and analyzed using the VITEK 2 Compact system (bioMérieux) for microbial identification and antibiotic susceptibility testing. For suspected viral or fungal infections, PCR or (1,3)-β-D-glucan testing (G-test) was performed. The distribution of pathogens is summarized in Supplementary Table 1.
Treatment methods
After diagnosis of septic shock, the child was given 0.9% 250 ml sodium chloride solution 20 ml/kg intravenously for 20 min. After fluid resuscitation, if the blood pressure (venous pressure < 75mmHg, systolic pressure < 85mmHg) did not return to normal, intravenous dopamine was given 5 µg/kg·min. Pathogen identification and antibiotic selection were adjusted based on susceptibility results (Supplementary Table 1). Pathogen identification: 3 ml of venous blood was extracted from the children for pathogen identification, drug selection and use: ① Gram-positive bacteria: Vancomycin 15 mg/kg was given, the intravenous infusion time was not less than 60 min, and intravenous infusion was given every 8 h; (2) Gram-negative bacteria: ceftazidime was given 150 mg/kg, each intravenous infusion time was 30 min, every 8 h; ③ Anaerobic bacteria: metronidazole 30 mg/kg, each intravenous infusion time of 30 min, every 8 h intravenous infusion. Acid inhibition therapy: omeprazole 0.8 mg/kg, once a day, intravenous injection, injection time of not less than 30 min; Famotidine 0.5-1 mg /kg/ day, divided into 2 intravenous injections, injection time of not less than 2 min. Atomizing treatment: salbutamol solution, dose is 2.5-5 mg/time, add 2-3 ml normal saline, atomized inhalation, once every 6 h; Budesonide 0.5-1 mg/time, diluted with about 2 ml of normal saline, atomized inhalation, once every 6 h. At the same time, according to the patient’s organ function, the corresponding organ function maintenance treatment.
The experimental group: on the basis of the above treatment, ulinastatin was given 20,000 U·kg-1 and diluted in 50mL 5% glucose injection before intravenous infusion for about 1 h, twice a day. 2 groups were treated for 5 days.
Observation indicators
Patient basic information including patient age, gender, PCIS score11, APACHE II score12, SOFA score13, VOS score, pSOFA score, underlying diseases, and antibiotic usage.
Treatment outcomes
Primary outcome indicators
Evaluate the patient’s treatment outcomes X days after treatment according to the 2012 International Guidelines for the Diagnosis and Treatment of Severe Sepsis and Septic Shock10:
-
• Markedly Effective: Shock symptoms disappear, and heart rate and white blood cell count return to normal.
-
• Effective: Shock symptoms are alleviated, with at least one of the following indicators abnormal: heart rate or white blood cell count.
-
• Ineffective: No improvement or worsening of the condition.
Secondary outcome indicators
Measure CD64(Normal value: range from1–10%), PCT(Normal value: <0.05ng/mL), CRP(Normal value: <10 mg/mL), neutrophil-to-lymphocyte ratio (NLR) (Normal value: range from1–3%), Erythrocyte Sedimentation Rate (ESR) (Normal value: range from 0% to 16 mm/h)and serum α-hydroxybutyrate dehydrogenase levels (Normal value: range from180 to 430 U/L) 7 days after treatment, duration of mechanical ventilation, length of hospital stay, length of stay in ICU, adverse drug reactions, nausea, diarrhea, headache.
Measurement and calculation methods
Blood Test Indicators: Collect 4 mL of elbow venous blood in the morning after fasting. Centrifuge at 3500 revolutions per minute for 10 min to extract serum.
Determination of CD64 index: CD64 index was determined by flow cytometry. A FACSCantoll flow cytometer was used with monoclonal antibody CD64-PE provided by BD Company. 5µL of CD64-PE antibody and 50 µL of anticoagulant whole blood were added to a special flow tube, mixed thoroughly, and incubated in the dark for 20 min. Subsequently, 500 µL hemolysin was added, mixed well, and left in the dark for 10 min. By centrifugal (500 g, 5 min), went to the clear liquid, with PBS to join 500 mu L PBS suspension cells after washing. The neutrophil population and lymphocyte population were labeled. The fluorescence intensity of CD64 expressed on neutrophils and lymphocytes was measured, and the neutrophil CD64 index was calculated as the ratio of fluorescence intensity of neutrophils to lymphocytes. PCT: immunofluorescence quantitative analysis method was used to detect PCT, and the operation was carried out in strict accordance with the instructions of the detection kit (provided by Shanghai Huiying Biological Technology Co., LTD.). CRP: Enzyme-linked immunosorbent assay (ELISA) was used to detect CRP in strict accordance with the instructions of the detection kit (provided by Shanghai Kanglong Biological Technology Co., LTD.). NLR determination: Fasting venous blood of all patients was collected before operation, and EDTA-Na2 anticoagulation was performed. SYSMEX XN550 automatic hematology analyzer and supporting reagents were used to measure blood routine, and NLR was calculated. α-hydroxybutyric acid dehydrogenase: detection kits provided by Beijing Jiuqiang biological technology co., LTD., by using enzyme-linked immunosorbent determination.
Statistical analysis
All data were statistically analyzed using SPSS 26.0 (IBM, USA), and excel was used to establish the database. All the data were tested for normal distribution. Non-normal distribution measurement data were expressed as median (quartile)[M (P25, P75)]. The measurement data conforming to normal distribution were expressed as \(\bar {x}+s\), and LSD method was used to compare the data between and within groups. The categorical data were expressed as percentage (%), and Chi-square chi-2 test was used for comparison. P <0.05 was considered statistically significant.
Results
Comparison of general data between the two groups
There were no significant differences between the two groups in terms of gender, age, PCIS score, APACHE II score, SOFA score, VOS score, pSOFA score, or related primary diseases (P > 0.05). The detailed results are presented in Table 1.
Comparison of therapeutic effect between the two groups
The total effective rate in the experimental group was significantly higher than that in the control group (P < 0.05), as shown in Table 2.
Comparison of inflammation indexes between the two groups
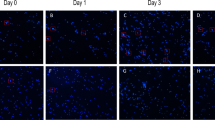
Before treatment, there were no significant differences in the levels of CD64, PCT, C-reactive protein, NLR, ESR or serum α-hydroxybutyrate dehydrogenase between the two groups (all P > 0.05). However, after 7 days of treatment, the levels of CD64, PCT, C-reactive protein, NLR, ESR and serum α-hydroxybutyrate dehydrogenase in the experimental group were significantly lower than those in the control group, with statistical significance (P < 0.001 for all), as shown in Table 3; Fig. 2.
Comparison of prognosis and adverse drug reactions between the two groups
The duration of mechanical ventilation, length of hospital stay, and ICU stay were significantly shorter in the experimental group compared to the control group (P < 0.001). Additionally, the incidence of adverse drug reactions was lower in the experimental group, with statistical significance (P < 0.001), as shown in Table 4.
Discussion
Sepsis in children is a systemic inflammatory response syndrome triggered by various infections and trauma, which can further progress to severe sepsis and septic shock. This condition leads to tissue hypoperfusion and organ dysfunction, severely threatening the health and life of children14. Previous studies15 have highlighted that when patients develop septic shock, inflammatory mediators in the body are released in a cascaded manner. These mediators include both pro-inflammatory and anti-inflammatory substances, which not only exacerbate and expand the body’s inflammatory response but also disrupt immune system balance. Therefore, it is crucial to actively intervene and block the chain reaction of inflammation. However, current clinical strategies for anti-inflammatory and anti-infection therapy primarily rely on drug treatments, which remain unstable and are associated with numerous complications. Consequently, exploring more effective treatment methods is of significant importance.
Ulinastatin is composed of 143 amino acids, with a relative molecular mass of approximately 67,000. It contains two Kunitz - type inhibitory active regions, namely cyclic structures formed by six cysteine residues through three disulfide bonds. This structure endows ulinastatin with a variety of biological activities and serves as the basis for its anti - inflammatory effects. Ulinastatin is a broad - spectrum protease inhibitor. It can improve the microcirculation state in shock, increase blood oxygen saturation, reduce ischemia - reperfusion injury, promote oxygen metabolism, and thus achieve the goals of protecting the body and alleviating symptoms. Additionally, ulinastatin can modulate immune function, inhibit inflammation, eliminate free radicals, and protect the functions of vital organs such as the liver, kidneys, and lungs. Relevant studies have indicated that ulinastatin, when added to conventional treatment for septic shock, improves capillary permeability and enhances the effect of blood resuscitation, significantly promoting better clinical outcomes. Ulinastatin also reduces the production of pro-inflammatory factors, downregulates the body’s immune response, inhibits the progression of inflammation, and prevents the excessive inflammatory response, thus protecting the body from inflammatory damage9.
The results of this study demonstrated that the total effective rate in the experimental group was significantly higher than that of the control group (P < 0.05), suggesting that ulinastatin effectively improves the clinical symptoms of children with septic shock, with clear therapeutic benefits. This finding is consistent with previous studies. CD64, a high-affinity Fc gamma type I receptor (FcyRI) for immunoglobulin IgG, is primarily expressed on the surface of antigen-presenting cells such as monocytes, macrophages, and dendritic cells under normal conditions, while its expression on neutrophils is generally low16. In recent years, CD64 has emerged as a promising biomarker. Turan et al.17 also proposed that CD64 could be used as an effective diagnostic marker for septic shock. When septic shock occurs, pathogen-associated molecular patterns, such as bacterial endotoxins, stimulate the immune system, prompting monocytes, macrophages, and other cells to release inflammatory mediators, such as IL-1β, IL-6, and TNF-α. These mediators then induce neuroendocrine cells to produce large amounts of procalcitonin (PCT), which, in turn, stimulates monocytes and macrophages to release even more inflammatory mediators, thereby exacerbating the systemic inflammatory response syndrome. This process results in vascular endothelial cell damage, increased vascular permeability, and a reduction in effective circulating blood volume. Simultaneously, PCT significantly disrupts the balance between coagulation and fibrinolysis, upregulating tissue factor expression, activating the extrinsic coagulation pathway, inhibiting fibrinolysis, and promoting a hypercoagulable state. This leads to microthrombi formation, which blocks microvessels and worsens tissue hypoxia and organ dysfunction. Additionally, PCT severely affects vascular tone by interfering with the synthesis or release of vasoactive substances like nitric oxide, causing abnormal contraction of vascular smooth muscle and resulting in unstable blood pressure. Persistent hypotension ultimately leads to hypoperfusion of tissues and organs. The dynamic change in PCT levels is crucial for guiding the use of antibacterial drugs. If PCT decreases slowly or even increases during treatment, adjustments in antibacterial therapy may be necessary, and drug-resistant bacterial infections should be considered. Furthermore, persistently high PCT levels are closely associated with high mortality rates17.
C-reactive protein (CRP) is an acute-phase protein synthesized and secreted by the liver, and its levels rise in response to inflammation17. The neutrophil-to-lymphocyte ratio (NLR) can rapidly assess the systemic immune-inflammatory response in patients. The increase in NLR is mainly due to a rise in neutrophil count and a reduction in lymphocyte count, which correlates with the extent of the systemic inflammatory response in patients18. Some studies19 suggest that during infection, a large number of inflammatory mediators are released, exacerbating myocardial damage, and as the disease progresses, this damage worsens. Serum α-hydroxybutyrate dehydrogenase (α-HBDH) is a key enzyme in the oxidation of ketone bodies and is highly sensitive to myocardial injury. A rise in α-HBDH levels typically indicates hypoxia in myocardial and brain tissue. Liberski et al.17 emphasized that NLR, CRP, and PCT could effectively predict the severity of septic shock. Duan et al.20 concluded that effective treatment could significantly reduce levels of CD64, PCT, CRP, and NLR in children with septic shock.
In this study, the results showed that after 7 days of treatment, the levels of CD64, PCT, CRP, NLR, and serum α-HBDH in the experimental group were significantly lower than those in the control group (P < 0.001). This suggests that ulinastatin effectively reduces the levels of these biomarkers and decreases inflammatory factors in children with septic shock. However, this study has several limitations. Firstly, the sample size is relatively small. Secondly, the age and gender of the children were not stratified. Thirdly, the reasons and mechanisms underlying the significant effects of the combined drug therapy were not thoroughly explored. In future studies, we will expand the sample size and conduct multi-center research to further confirm the effect of combination therapy. Additionally, animal studies will be performed to explore the mechanisms of the combined actions of the three drugs.
Conclusion
Ulinastatin can effectively improve the clinical symptoms of children with septic shock, demonstrating significant efficacy. It effectively regulates the immune balance, allowing children to return to normal life as soon as possible. This effect may be achieved by rapidly correcting abnormal levels of CD64, procalcitonin, C-reactive protein, NLR, and serum α-hydroxybutyrate dehydrogenase, thereby reducing the inflammatory response.
Data availability
Data is provided within the manuscript.
References
Garcia, P. C. R., Tonial, C. T. & Piva, J. P. Septic shock in pediatrics: the state-of-the-art. J. Pediatr. 96(suppl1), 87–98 (2020).
Lee, E. P. et al. Hemodynamic monitoring and management of pediatric septic shock. Biomed. J. 45(1), 63–73 (2022).
Wang, N. et al. The role of eSOFA score in 28-day, 90-day and 1-year prognosis assessment of patients with sepsis. Chin. Emerg. Med. 43(09), 711–715 (2023).
Patel, J. J., Shukla, A. & Heyland, D. K. Enteral nutrition in septic shock: A pathophysiologic conundrum. JPEN J. Parenter. Enter. Nutr. 45(S2), 74–78 (2021).
De Backer, D., Ricottilli, F. & Ospina-Tascón, G. A. Septicshock: Amicro circulation disease. Curr. Opin. Anaesthesiol. 34(2), 85–91 (2021).
Foster, D. M. & Kellum, J. A. Endotoxic septic shock: diagnosis and treatment. Int. J. Mol. Sci. 24(22), 16185 (2023).
Spaggiari, V. et al. Neonatal septic shock, a focus on first line interventions. Acta Biomed. 93(3), e2022141 (2022).
Gunjan, K. et al. Echocardiographic characteristics in neonates with septic shock. Eur. J. Pediatr. 183(4), 1849–1855 (2024).
Hang, C. C. et al. Effects of Ulinastatin on renal perfusion evaluated by doppler ultrasonography in a Porcine model of septic shock. Exp. Ther. Med. 22(5), 1324 (2021).
Gao, G., Feng, Z. & Chang, Z. G. 2012 International guidelines for the diagnosis and treatment of severe Sepsis and septic shock. Chin. Crit. Care Emerg. Med. 25, 501–505 (2013).
Zhou, Y., Ye, H. & Lu, W. Serum substance P concentration in children with traumatic brain injury: A first report. World Neurosurg. 147, e200–e205 (2021).
Tang, Y. et al. Clinical relevance of neutrophil/lymphocyte ratio combined with APACHEII for prognosis of severe heatstroke. Heliyon 9(10), e20346 (2023).
Wang, X. et al. Application prospect of the SOFA score and related modification research progress in Sepsis. J. Clin. Med. 12(10), 3493 (2023).
Weiss, S. L. et al. Surviving Sepsis campaign international guidelines for the management of septic shock and Sepsis-Associated organ dysfunction in children. Pediatr. Crit. Care Med. 21(2), e52–e106 (2020).
Godfred-Cato, S. AbramsJY,BalachandranN,etal.Distinguishing multisystem inflammatory syndromein children from COVID-19,Kawasaki disease and toxic shock syndrome. PediatrInfect Dis. J. 41(4), 315–323 (2022).
Seree-Aphinan, C. et al. Distinguishing sepsis from infection by neutrophil dysfunction: A promising role of CXCR2 surface level. Front. Immunol. 23(11), 608–616 (2020).
Turan, Y. B. The role of proadrenomedullin, Interleukin 6 and CD64 in the diagnosis and prognosis of septic shock. BMC Anesthesiol. 17;23(1), 278 (2023).
Liang, P. & Yu, F. Predictive value of procalcitonin and neutrophil-to-lymphocyte ratio variations for bloodstream infection with septic shock. Med. Sci. Monit. 5(28), 935–966 (2022).
Zhang, S. S. et al. Changes of serum creatine kinase isoenzyme, α-hydroxybutyrate dehydrogenase and prealbumin levels before and after treatment of neonatal septic shock and its clinical significance. J. Practical Clin. Med. 23(09), 72–76 (2019).
Duan, H. N. et al. Effects of continuous blood purification on CD64, procalcitonin, C-reactive protein and NLR in children with sepsis. Chin. J. Integr. Nephrop. 22(7), 597–599 (2021).
Author information
Authors and Affiliations
Contributions
GS, CM, ZW, CZ, and CL had primary responsibility for the study, protocol development, patient enrollment and outcome assessment and for writing the manuscript. RH, JF, YL and DP performed patient enrollment, taking ECG, final data analyses and contributed to the writing of the manuscript. GAS, ZL, DL and HJ contributed to protocol development, data analyses, outcome assessment and manuscript writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Huanggang Central Hospital of Yangtze University, and the study was performed in accordance with the Helsinki II declaration. Informed consent was obtained from all the study subjects before enrollment.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, S., Cheng, S., Zhang, Q. et al. Effects of ulinastatin on therapeutic outcomes and inflammatory markers in pediatric septic shock patients. Sci Rep 15, 16624 (2025). https://doi.org/10.1038/s41598-025-00629-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-00629-8