Abstract
Perioperative neurocognitive disorder (PND) is a long-term perioperative complication that affects elderly surgical patients. As the aging population continues to grow, the prevention and treatment of PND have become important issues to address. Research has shown that ulinastatin significantly reduces the incidence of PND in elderly patients undergoing various types of surgery, including spinal and abdominal surgeries. However, the underlying mechanisms of this effect remain unclear. Transient receptor potential melastatin 4 (TRPM4) is a Ca2 + -activated, monovalent cation channel associated with many neurological diseases. In the present study, we aimed to investigate the relationship between ulinastatin and TRPM4 in PND and to determine its underlying mechanism. Forty eighteen-month-old male Sprague‒Dawley rats were randomly divided into four groups: the sham group, surgery group (internal fixation of tibial fracture), ulinastatin group (ulinastatin + surgery), and 9-phenanthrol group (TRPM4 channel inhibitor 9-phenanthrol + surgery). The neurocognitive function of the rats was detected using the Y-maze and new object recognition (NOR) tests. We used WB, qPCR, and IF to detect the expression levels of TRPM4 in different groups of rats. We also detected the degree of apoptosis in rat hippocampal cells. Surgery under inhaled sevoflurane anesthesia induced cognitive impairments and elevated the expression of TRPM4 in the hippocampus. Downregulation of TRPM4 expression by ulinastatin or 9-phenanthrol alleviated cognitive impairments after anesthesia and surgery. Furthermore, ulinastatin treatment decreased the level of apoptosis in the hippocampus. Ulinastatin downregulates TRPM4 expression and apoptosis to mitigate cognitive dysfunction in aged perioperative neurocognitive disorder rats, and ulinastatin is a promising neuroprotectant against PND.
Similar content being viewed by others
Introduction
Perioperative neurocognitive disorder (PND) refers to changes in cognitive function that can occur before and/or after surgery. This condition is characterized by symptoms including memory loss, depression, anxiety, and personality changes1. PND is most commonly observed in elderly patients, aged 65 and older, and can lead to increased hospitalization, higher costs, greater social burdens, and increased mortality rates. Although the exact mechanisms behind PND are not fully understood, several potential contributors have been identified, including neuroinflammation, oxidative stress, mitochondrial dysfunction, neurotransmitter dysfunction, and impaired synaptic function2,3,4,5,6.
As a widely accepted experimental model, the rat tibia fracture model was used in this study. At present, the underlying mechanisms of PND are related to hippocampal neuronal damage, inflammatory response, and oxidative stress7. The hippocampus is one of the main organs involved in cognitive dysfunction, and the apoptosis of hippocampal neurons, caused by the imbalance of BCL-2 and BAX, may be one explanation for the hippocampus’s relationship with cognitive dysfunction.
TRPM4 is a nonselective cation channel that is primarily permeable to Na+, activated by intracellular calcium, and modulated by ATP. Dysregulation of TRPM4 contributes to many pathological processes, including stroke, post-traumatic cerebral edema, spinal cord injury, and neurodegenerative diseases8. The mechanism may be related to the depolarization of neurons by TRPM4 currents, and the increase in TRPM4 currents may increase the degree of apoptosis in hippocampal neurons, compromise the integrity of the blood‒brain barrier, and lead to excessive sodium influx9. Studies have shown that blocking TRPM4 channels can correct the above pathological processes10. However, whether TRPM4 also plays a mechanistic role in postoperative cognitive dysfunction (POCD), remains unclear. Preoperative intraperitoneal injection of ulinastatin has a protective effect on postoperative cognitive impairment in rats9. Previous studies have indicated that ulinastatin offers protective benefits for perioperative neurocognitive function in patients undergoing various types of surgery, including fracture, abdominal, spinal, and cardiac procedures11,12,13.
On the basis of these findings, we aimed to verify the role of the TRPM4 pathway in a PND rat model, which could provide a new target for the clinical prevention of PND.
Results
Elevated TRPM4 expression in the hippocampus of PND model rats
To investigate the relationship between TRPM4 expression and PND formation in rats, we detected TRPM4 expression in the hippocampus of rats in the PND surgery group by using WB, qPCR, and IF. As shown in Fig. 1, TRPM4 expression in the hippocampus of PND-group rats was significantly elevated. These findings suggest that the occurrence of neurocognitive impairment in aged rats is associated with TRPM4 and that the process of PND development may be related to increased TRPM4 expression. The expression levels of TRPM4 in the groups treated with ulinastatin and 9-phenanthrol prior to surgery were lower than those in the surgery group. Ulinastatin may inhibit TRPM4 expression through a specific mechanism.
Elevated TRPM4 expression in the hippocampi of PND model rats. TRPM4 levels in the hippocampi of PND model rats were measured via western blotting (a, b). Differences in TRPM4 protein expression among the different groups were determined by immunofluorescence (c, d). Determination of TRPM4 RNA expression in the hippocampus by qPCR (e). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, sham versus surgery; #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001, surgery versus ulinastatin and 9-phenanthrol.
Inhibition of TRPM4 expression attenuates neurocognitive impairment in PND rats
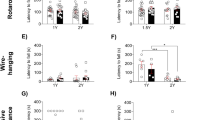
To verify whether TRPM4 inhibition ameliorates rat neurocognitive dysfunction, rat neurocognitive function was assessed by detecting the spontaneous alternation rate in the Y-maze test and the percentage of head probing time for new objects in the new object recognition test. As shown in Fig. 2, the spontaneous alternation rate was lower in the surgery group, as compared to the sham group, and higher in the 9-phenanthrol and ulinastatin groups than in the surgery group. The discrimination ratio of the NOR test was lower in the surgery group than in the sham group, and greater in the 9-phenanthrol and ulinastatin groups, as compared to the surgery group. These results suggest that inhibition of the TRPM4 pathway can protect the neurocognitive function of rats.
Inhibition of TRPM4 expression attenuates neurocognitive impairment in PND rats. Trajectory plots in the spontaneous alternating rate experiment in aged rats in the Y-maze (a). The spontaneous alternation rate was monitored in aged rats on the first and third postoperative days (b). The discrimination ratio of aged rats was monitored on the first and third postoperative days (c). Heatmap of paths explored in novel object recognition (NOR) in aged rats. The color transition from blue to red (0–10 s) represents an increasing amount of time spent exploring that area (d). Data are expressed as, the mean, standard error of the mean (SEM), *P < 0.05, **P < 0.01, ***P < 0.001, sham versus surgery; #P < 0.05, ##P < 0.01, surgery versus ulinastatin & 9-phenanthrol.
Inhibition of TRPM4 regulates the expression of apoptosis-related proteins in PND rat hippocampus
To further clarify the relationship between the inhibition of TRPM4 and apoptosis, we used WB and PCR to detect the expression of the apoptosis-related proteins BCL-2 and BAX, and the results are shown in Fig. 3. Compared with that in the sham group, the expression of BCL-2 in the PND group was decreased, and that in the ulinastatin and the 9-phenanthrol groups were elevated, as compared with that of the surgery group. The expression of BAX in the PND group was elevated as compared with that in the sham group, and decreased both in the ulinastatin group and the 9-phenanthrol group, as compared with that of the surgery group. The balance between the two was disrupted, and the degree of apoptosis in the rat hippocampus was increased. The trend of apoptosis-related protein expression also suggests that inhibition of TRPM4 expression could inhibit rat hippocampal apoptosis. TUNEL was used to detect apoptosis in the rat hippocampus, and the results, presented in Fig. 4, indicate that the rate of apoptosis in the surgery (PND) group increased. In contrast, the rates of apoptosis in both the 9-phenanthrol (TRPM4-inhibition) group and the ulinastatin prevention group were lower than those in the surgery group. These findings suggest that inhibiting TRPM4 reduces apoptosis in the rat hippocampus.
Inhibition of TRPM4 regulates the expression of apoptosis-related proteins in PND rats. Determination of BAX levels in the rat hippocampus by western blotting (a, c). The BCL-2 levels in rat hippocampus were determined via western blotting (b, d). Caspase levels in the rat hippocampus were determined via western blotting (e, f). Statistical analysis was performed on data from five independent slices by using one-way ANOVA and post hoc Bonferroni correction: *P < 0.05, **P < 0.01, ***P < 0.001; sham versus surgery, #P < 0.05, ##P < 0.01; surgery versus ulinastatin and 9-phenanthrol.
Apoptosis rate in the hippocampus using the TUNEL assay. Immunofluorescence staining for assessment of neuronal apoptosis in the hippocampal CA1 area at 24 h after PND in the sham, surgery, ulinastatin, and 9-phenanthrol groups (a). A TUNEL-positive signal was observed in the nucleus of the cell and appeared circular, small, or granular. Green-stained cells are apoptotic cells, and the blue-stained region represents the cell nuclei; magnification, 200 × . The results of the quantitative analysis of the neuronal apoptosis rate are shown in (b). The green fluorescence-stained region represents apoptotic cells, and the blue fluorescence represents cell nuclei. The data are presented as the means ± SEM. *P < 0.05 versus the sham group; #P < 0.05 versus the surgery group; **P < 0.01 versus the sham group; ##P < 0.01 versus the surgery group.
Inhibition of TRPM4 improves mitochondrial structural changes in PND rats
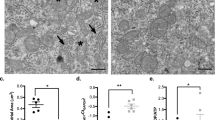
To further explore the possible mechanisms of apoptosis in the surgery-group rat hippocampus, we initially explored the mitochondrial morphology of each group, and the results (Fig. 5) reveal that the mitochondria in the hippocampal cells of rats in the PND group, and the mitochondrial morphology of the TRPM4-inhibition group were altered, suggesting that the inhibition of TRPM4 expression has a certain protective effect on PND-group rat mitochondria.
Inhibition of TRPM4 improves mitochondrial structural changes in PND rats. Representative ultrastructural changes in mitochondria of the sham, PND, ulinastatin and 9-phenanthrol groups (a). Morphological analysis of mitochondria shows the average area of mitochondria in each group (b). The organization of the mitochondrial cristae was analyzed, and the figure shows the percentage of mitochondria with intact cristae (c). Statistical analysis was performed on data from three independent slices by using one-way ANOVA and post hoc Bonferroni correction: *P < 0.05, **P < 0.01, ***P < 0.001, sham versus surgery; #P < 0.05, ##P < 0.01, surgery versus ulinastatin & 9-phenanthrol.
Discussion
Our results indicate that inhibition of TRPM4 expression significantly reduces hippocampal neuron apoptosis and improves neurocognitive function in PND rats. We observed that presurgical TRPM4 inhibition reduced neuronal apoptosis in the hippocampus and improved learning and memory function in this rat model, which is consistent with the findings of Charlene P. Poore14.
TRPM4, a Ca2+-sensitive member of the TRPM protein family, functions as a selective monovalent cation channel that is impermeable to divalent cations15. This membrane protein has a broad tissue distribution and plays a significant role in various pathological processes through its regulation of membrane potential dynamics16. Physiologically, TRPM4 is predominately associated to cardiac physiology and vascular tone regulation in the circulatory system, kidney physiology in the urinary system, nervous system and neuronal activity, various type of neurohumoral regulation, and immunity17. It is pathologically associated with arrhythmia, myocardial ischemia–reperfusion injury, stroke, cerebral ischemia–reperfusion injury, subarachnoid hemorrhage, and cancer of some organs18. Several prior studies have indicated the potential of TRPM4 inhibition in the treatment of patients with various central nervous system disorders. TRPM4 may also become a therapeutic entry point for other system-related diseases in the future. A specific inhibitor of the TRPM4 channel, 9-phenanthol can regulate a variety of physiological processes through TRPM4 current inhibition, thereby exerting beneficial effects in a variety of pathological conditions19. The compound 9-phenanthrol inhibits TRPM4 expression and reduces the expression of apoptosis-related molecules (e.g., BAX) and inflammatory cytokines (e.g., IL-6)22.
Inhibition of TRPM4 has a protective effect on chronic cerebral hypoperfusion20. TRPM4 also contributes to the death of mossy cells after status epilepticus21. Inhibition of TRPM4 reduces hippocampal neuronal apoptosis and enhances learning and memory functions14. Given the role of TRPM4 inhibition in the treatment of various neurological diseases, we believe that inhibition of the TRPM4 pathway may have a beneficial effect on PND. Therefore, we investigated whether inhibition of TRPM4 improves cognitive dysfunction in a PND rat model induced by tibial fracture reduction and internal fixation, and further explored the possible mechanisms involved. Our current study revealed that the model leads to cognitive dysfunction in aged rats, resulting in increased apoptosis of hippocampal neurons. Inhibition of the TRPM4 pathway may significantly improve the cognitive function of rats by inhibiting the apoptosis of hippocampal neurons. Our results suggest that inhibition of the TRPM4 pathway has a significant neuroprotective effect in PND rats.
A recent study demonstrated that in a chronic hypoxia rat model, TRPM4 inhibition attenuates M4P antibody-induced hippocampal neuronal apoptosis, ameliorates long-term potentiation (LTP) impairment, and enhances learning/memory functions14. The inhibitor of TRPM4, 9-phenanthrol, significantly reduces brain water content, blood‒brain barrier disruption, neuronal apoptosis, and neurobehavioral deficits in a cerebral edema model22. Trpm4 deficiency in a status epilepticus mouse model significantly attenuated neuronal loss, cell necrosis, and apoptosis, as well as astrocytosis and microgliosis in the hippocampus and piriform cortex23. We found that TRPM4 expression was increased in aged PND rats by WB, PCR, IF, and other experimental methods. Y-maze and novel object recognition experiments were also used to explore the protective effects on cognitive function in aged PND rats.
Ulinastatin is a serine protease inhibitor that is present in human blood and urine. It has a strong inhibitory effect on a variety of serine proteases, such as trypsin and thrombin. Ulinastatin can inhibit inflammation, restrain apoptosis, improve immune function, and protect tissues and organs. It has been used to treat pancreatitis and sepsis, among other conditions. In addition, recent studies have shown that ulinastatin can significantly reduce the occurrence of POCD in elderly patients after general anesthesia, inhibit the inflammatory response, and reduce cell and tissue damage. Ulinastatin administration prior to general anesthesia can prevent neuronal damage, and can reduce cognitive decline after general anesthesia. The protective effect of ulinastatin was associated with the inhibition of microglial activation. Its target in the activation of microglia is the M1 phenotype24. Our research revealed that preoperative administration of ulinastatin can inhibit TRPM4 expression and protect cognitive function in elderly PND unit rats.
Clinically, PND primarily occurs in elderly patients, and age is an independent risk factor. In addition, some studies have shown that inhaled general anesthesia is a risk factor for PND. To better simulate clinical situations, inhaled general anesthesia and an orthopedic model in senile PND unit rats, was used in the present study. We found that general anesthesia and the model caused cognitive impairment in elderly rats and that inhibiting TRPM4 significantly improved the cognitive behavior of PND rats.
Our study indicates that TRPM4 is a significant target for addressing perioperative neurocognitive dysfunction. Ulinastatin may help protect neurocognitive function by inhibiting TRPM4 and reducing apoptosis in the hippocampus of aged rats. Therefore, ulinastatin shows promise as a drug for preventing perioperative neurocognitive impairment.
Materials and methods
Animals
Eighteen-month-old male SD rats weighing 450–500 g were obtained from the Jinan Pengyue Animal Company. The rats were housed in an air-conditioned room maintained at 19–23 °C, with a relative humidity of 40–50%, and a 12-h light/dark cycle. The SD rats were labeled, numbered, and divided into four groups using a random number table. Finally, the rats involved in the experiment were euthanized by cervical dislocation after anesthesia. The study protocol was approved by the Ethics Committee of Weifang Medical University, and all of the animal experiments adhered to the ARRIVE guidelines.
Animal model
The PND model was built by performing internal fixation of tibial fractures in rats. 9-phenanthrol (0.5 mg/kg) was intraperitoneally injected 30 min before surgery. Ulinastatin (100,000 U/kg) was also intraperitoneally injected 30 min before surgery. Briefly, aged rats were anesthetized with 1.5–2% sevoflurane and 100% oxygen using a rat anesthesia mask. A 1.0–1.5 cm longitudinal incision was made from the left hind limb knee joint downward4. The fibula and the muscles around the tibia were isolated, and osteotomy was performed with a tear at the junction from the knee to the middle third of the tibia. A 1 mm stainless steel rod was subsequently inserted into the canal to the distal third of the tibia. After the fracture was produced, the skin was sutured with nylon, and 2% lidocaine was applied to the wound to relieve pain25.
Y-maze
Spontaneous alternation behavior was assessed through a single session of the Y- maze. The Y-maze used in the present study was made from black Plexiglas. It has three arms with the following dimensions: 25 cm high, 50 cm long, and 10 cm wide, at angles of 120 degrees, which were marked as A, B, and C. Twenty-four hours post-surgery, each animal was placed at the end of an arm and allowed to explore the maze for 8 min14. Successive entry in each arm was defined as spontaneous alternation behavior. A blinded observer registered the visited arm sequence corresponding to each animal. The spontaneous alternation percentage was calculated as, (number of alternations/total entries − 2) × 100. Between trials, the Y-maze was washed with a 75% ethanol solution. Additionally, the total number of arm entries was considered as an index of locomotor activity, and the rats that performed fewer than 6 arm entries in 8 min were omitted from the analysis26.
Novel object recognition (NOR) test
The NOR test was performed in a black Plexiglas box (length: 72 cm, width: 72 cm, height: 50 cm). In the first trial, a rat was placed in the box (with two identical objects placed) to explore for 5 min and then returned to its home cage. After 24 24-h intervals (testing sessions), the rat went through the second trial, in which one of the two (familiar) objects was replaced by a new one. Interaction was defined as looking, licking, sniffing, or touching an object while sniffing. The discrimination ratio for each rat was calculated as, ETN/(ETF + ETN), where ETN is equal to the exploration time(s) spent by the rat interacting with the novel object and ETF equals the exploration time(s) spent by the rat interacting with the familiar object27,28.
WB
Total proteins were isolated from rat hippocampal tissues using RIPA lysis buffer (Beyotime P0013B, Shanghai, China). The protein concentration was determined using an Enhanced BCA Protein Assay Kit (Solarbio PC0020, Beijing, China), and 25–40 μg of protein was resolved via 10–12% sodium dodecyl sulfate‒polyacrylamide gel electrophoresis (SDS‒PAGE, Solarbio P1200-2, Beijing, China) and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore). The membranes were subsequently blocked with 5% dried skim milk in TBST for 2 h, and then incubated overnight at 4 °C with primary antibodies for detection of TRPM4 (1:1000, Sigma SAB2102548), BAX, BCL-2, caspase3, cleaved-caspase3, and β-actin (all 1:500, Abclonal A0207, A11025, A2156, and AC026, respectively, Wanleibio WL02117, China). The membranes were then incubated with HRP-conjugated secondary detection antibodies (Proteintech SA00001-2, Wuhan, China). Immunoreactive proteins were subsequently detected with ECL Plus Reagent (Beyotime P0018AS, Shanghai, China). The value of each band was normalized to that of β-actin. The quantification of the immunoblots was performed using ImageJ software29.
qPCR
Total RNA was extracted from hippocampal tissues using TRIzol. The RNA was then converted to cDNA using a ReverTra Ace qPCR RT kit (Vazyme R323). Gene expression was analyzed by RT‒qPCR using SYBR Green PCR Master Mix (Vazyme Q711), and β-actin was used as an internal control. The TRPM4 forward primer sequence was CTTCGAGCACTTCCGTGTCT, and the TRPM4 reverse primer sequence was CACAGACTCCAAGTCAGCA. All tests were performed in triplicate, and gene expression was normalized against the expression of β-actin. Relative gene expression was then calculated as fold changes using the 2−∆∆CT method30.
TUNEL
Brain tissue samples were quickly removed, fixed with 4% paraformaldehyde, embedded in paraffin at 4 °C, left overnight, and sectioned into 4 μm thick slices. Apoptotic cells were visualized by in situ detection of DNA fragmentation (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)) according to manufacturer’s instructions (Elabscience Biotechnology Co., Ltd., Wuhan, China). Five slices were selected randomly from each group and analyzed with a Leica DM2500 microscope. The apoptosis index (AI) was calculated via ImageJ software using the formula, number of apoptotic cells/total number of cells × 100%. Slices of brain tissues were further analyzed with Hoechst 33,258 (Beyotime Biotechnology) and visualized under a fluorescence microscope to determine the degree of nuclear fragmentation in apoptotic cells31.
TEM
Four percent paraformaldehyde-fixed prefrontal cortex (PFC) and hippocampal samples (3 mm × 3 mm) from the control, surgery, ulinastatin, and 9-phenanthrol groups were collected and fixed in 2% glutaraldehyde in 0.1 mol/L sodium phosphate buffer (pH 7.4) overnight at 4 °C. After dehydration with a graded ethanol series, the sample was embedded in Epon812 and sectioned using a Leica EM UC6 ultramicrotome (Leica Co., Vienna, Austria). The samples were viewed and photographed via transmission electron microscopy (TEM) (JEM-2000EX, JEDL, Japan)32.
IF
Half of the brain tissue of three randomly selected rats from each group was fixed overnight in 4% paraformaldehyde (Solarbio P1110, China). The tissue blocks were dehydrated with sucrose, embedded in oct, and cut into 10-micron coronal sections at − 20 °C. After being blocked with 5% BSA (Solarbio SW3015, China) for 30 min, the slices were incubated with a TRPM4 primary antibody at 4 °C overnight. After being washed with PBS the following day, the sections were incubated with fluorescein at 37 °C for 40 min, and then stained with DAPI (Solarbio C0065, Beijing, China)23. The images were obtained by fluorescence microscopy, and the results were analyzed by ImageJ software33.
The animals were intracardially perfused with normal saline, followed by 4% paraformaldehyde, under anesthesia. Following fixation with 4% paraformaldehyde and dehydration with sucrose, the brain was cut into 10 μm thick coronal slices at − 20 °C. ImageJ software (National Institutes of Health, Bethesda, Maryland, USA) was used to count the number of TH-positive neurons in six parallel sections containing the SN of each animal.
Statistical analysis
GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA) was used to analyze the data, the Brown-Forsythe test was used to verify a normal distribution, and the data are expressed as the means ± SEM. Repeated measures analysis of variance (ANOVA) was used for comparisons between groups, and p < 0.05 was considered significant34.
Data availability
Raw data are deposited in the Supplementary Files. The datasets generated and/or analyzed during the current study are not publicly available due to data protection but are available from the corresponding author on reasonable request.
References
Evered, L. et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-20181. J. Alzheimer’s Dis. JAD 66(1), 1–10 (2018).
Wu, W. S. et al. Clemastine ameliorates perioperative neurocognitive disorder in aged mice caused by anesthesia and surgery. Front. Pharmacol. 12, 738590 (2021).
Muscat, S. M. et al. Selective TLR4 antagonism prevents and reverses morphine-induced persistent postoperative cognitive dysfunction, dysregulation of synaptic elements, and impaired BDNF signaling in aged male rats. J. Neurosci. 43(1), 155–172 (2023).
Netto, M. B. et al. Oxidative stress and mitochondrial dysfunction contributes to postoperative cognitive dysfunction in elderly rats. Brain Behav. Immun. 73, 661–669 (2018).
Liu, Y. et al. Prehabilitative resistance exercise reduces neuroinflammation and improves mitochondrial health in aged mice with perioperative neurocognitive disorders. J. Neuroinflamm. 19(1), 150 (2022).
Zhang, X. et al. Activated brain mast cells contribute to postoperative cognitive dysfunction by evoking microglia activation and neuronal apoptosis. J. Neuroinflamm. 13(1), 127 (2016).
Wei, P. H. et al. Human umbilical cord-derived mesenchymal stem cells ameliorate perioperative neurocognitive disorder by inhibiting inflammatory responses and activating BDNF/TrkB/CREB signaling pathway in aged mice. Stem Cell Res. Ther. 14(1), 263 (2023).
Riquelme, D. et al. TRPM4 expression during postnatal developmental of mouse CA1 pyramidal neurons. Front. Neuroanat. 15, 643287 (2021).
Wei, S. H. et al. Comparison of anti-oncotic effect of TRPM4 blocking antibody in neuron, astrocyte and vascular endothelial cell under hypoxia. Front. Cell Dev. Biol. 8, 562–584 (2020).
Bovet-Carmona, M. et al. Disentangling the role of TRPM4 in hippocampus-dependent plasticity and learning: An electrophysiological, behavioral and FMRI approach. Brain Struct. Funct. 223(8), 3557–3576 (2018).
Zhang, M. et al. Ulinastatin may significantly improve postoperative cognitive function of elderly patients undergoing spinal surgery by reducing the translocation of lipopolysaccharide and systemic inflammation. Front. Pharmacol. 9(1007), 9 (2018).
Zhou, M. et al. Effect of ulinastatin combined with dexmedetomidine on postoperative cognitive dysfunction in patients who underwent cardiac surgery. Front. Neurol. 10(1293), 19 (2019).
Duan, M. et al. Effect of ulinastatin on early postoperative cognitive dysfunction in elderly patients undergoing surgery: A systemic review and meta-analysis. Front. Neurosci. 15(618589), 21 (2021).
Poore, C. P. et al. TRPM4 blocking antibody reduces neuronal excitotoxicity by specifically inhibiting glutamate-induced calcium influx under chronic hypoxia. Neurobiol. Dis. 191, 106408 (2024).
Kovács, Z. M. et al. Pharmacological modulation and (patho)physiological roles of TRPM4 channel-part 1: Modulation of TRPM4. Pharmaceuticals (Basel, Switzerland) 15(1), 81 (2022).
Chubanov, V. et al. TRPM channels in health and disease. Nat. Rev. Nephrol. 20(3), 175–187 (2024).
Dienes, C. et al. Pharmacological modulation and (patho)physiological roles of TRPM4 channel-part 2: TRPM4 in health and disease. Pharmaceuticals (Basel, Switzerland) 15(1), 40 (2021).
Vandewiele, F. et al. TRPM4 inhibition by meclofenamate suppresses Ca2+-dependent triggered arrhythmias. Eur. Heart J. 43(40), 4195–4207 (2022).
Chen, B. et al. TRPM4-specific blocking antibody attenuates reperfusion injury in a rat model of stroke. Pflugers Arch. 471(11–12), 1455–1466 (2019).
Hazalin, N. A. M. N. et al. TRPM4 inhibition improves spatial memory impairment and hippocampal long-term potentiation deficit in chronic cerebral hypoperfused rats. Behav. Brain Res. 393, 112781 (2020).
Mundrucz, L. et al. TRPM4 regulates hilar mossy cell loss in temporal lobe epilepsy. BMC Biol. 21(1), 96 (2023).
Ma, P. et al. The TRPM4 channel inhibitor 9-phenanthrol alleviates cerebral edema after traumatic brain injury in rats. Front. Pharmacol. 14, 1098228 (2023).
Chen, X. et al. Knockout of transient receptor potential melastatin 4 channel mitigates cerebral edema and neuronal injury after status epilepticus in mice. J. Neuropathol. Exp. Neurol. 79(12), 1354–1364 (2020).
Cho, E. H. et al. The preventive effect of urinary trypsin inhibitor on postoperative cognitive dysfunction, on the aspect of behavior, evaluated by Y-maze test, via modulation of microglial activity. Int. J. Mol. Sci. 25(5), 2708 (2024).
Zhou, Y. L. et al. Tregs dysfunction aggravates postoperative cognitive impairment in aged mice. J. Neuroinflamm. 20(1), 75 (2023).
Xu, Z. L. et al. Effects of ginsenosides on memory impairment in propofol-anesthetized rats. Bioengineered 13(1), 617–623 (2022).
Postu, P. A. et al. Memory-enhancing effects of origanum majorana essential oil in an Alzheimer’s amyloid beta1–42 rat model: A molecular and behavioral study. Antioxidants (Basel, Switzerland) 9(10), 919 (2020).
Gómez-Galán, M. et al. Dysfunctional astrocytic regulation of glutamate transmission in a rat model of depression. Mol. Psychiatry 18(5), 582–594 (2013).
Osborne, A. L. et al. Improved social interaction, recognition and working memory with cannabidiol treatment in a prenatal infection (poly I:C) rat model. Neuropsychopharmacology 42(7), 1447–1457 (2017).
Park, J. et al. The anti-inflammatory effects of angiogenin in an endotoxin induced uveitis in rats. Int. J. Mol. Sci. 21(2), 413 (2020).
Attafi, I. M. et al. Lead nitrate induces inflammation and apoptosis in rat lungs through the activation of NF-κB and AhR signaling pathways. Environ. Sci. Pollut. Res. Int. 29(43), 64959–64970 (2022).
Dumbuya, J. S. et al. Effects of hydrogen-rich saline in neuroinflammation and mitochondrial dysfunction in rat model of sepsis-associated encephalopathy. J. Transl. Med. 20(1), 546 (2022).
Sun, Z. et al. ZiBu PiYin recipe prevents diabetes-associated cognitive decline in rats: Possible involvement of ameliorating mitochondrial dysfunction, insulin resistance pathway and histopathological changes. BMC Complement. Altern. Med. 16(200), 8 (2016).
Münster-Wandowski, A. et al. Distinct localization of SNAP47 protein in GABAergic and glutamatergic neurons in the mouse and the rat hippocampus. Front. Neuroanat. 11(56), 13 (2017).
Acknowledgements
Not applicable.
Funding
This study was supported by Mechanism of BRD4 regulating mitochondrial calcium homeostasis to protect glomerular endothelial cells from acute kidney injury, Shandong Provincial Fund, ZR2022MH134, 202301-202512.
Author information
Authors and Affiliations
Contributions
XXH and LJY wrote the manuscript text and completed the figures; LKL and HHR prepared the funding, reviewed and checked the article, ZCY completed the methodology and part of the figures, and ZMM completed part of the data analysis and proofreading.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was carried out in compliance with the ARRIVE guidelines. All methods are reported in accordance with ARRIVE guidelines (https://arriveguidelines.org) for the reporting of animal experiments. All the animal experiments were carried out according to the National Institutes of Health Guidelines for Care and Use of Laboratory Animals and were approved by the Animal EthicsCommittee of theShandong Second Medical University (Approval No.2023SDL359).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xia, X., Li, J., Zhang, M. et al. Ulinastatin modulates TRPM4 expression and apoptosis to mitigate cognitive dysfunction in aged perioperative neurocognitive disorder rats. Sci Rep 15, 17774 (2025). https://doi.org/10.1038/s41598-025-01394-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-01394-4