Abstract
High-speed Laser Cladding (HLC) technology was used to fabricate WC/316L composite coatings with varying WC contents on a 316 L alloy substrate. The microstructure of the coatings, along with the dissolution and precipitation behavior of WC and the wear mechanism, were investigated. The results show that during the cladding process, the edges of WC dissolve to form a W-rich zone. Upon cooling, W atoms nucleate and precipitate carbides at the WC/316L interface, resulting in strong interfacial bonding between WC and the 316 L substrate. The phases in the coating include austenite, WC, M2C, M7C3, and M3C2. As the WC content increases, the austenite content decreases. WC enhances the wear resistance of the coating by increasing hardness, dispersing the load on the matrix, and passivating wear debris. The wear rate of the 316 L + 30WC coating is only 27% of that of the 316 L alloy.
Similar content being viewed by others
Introduction
High-speed Laser Cladding (HLC) is an advanced surface modification technology that has rapidly developed in recent years. Unlike traditional laser cladding (LC), HLC shifts the laser-powder intersection above the substrate surface, allowing most of the laser energy to directly impact the powder. This causes the powder to enter a molten or semi-molten state before reaching the melt pool, reducing the time it spends in the pool and decreasing thermal input to the substrate. As a result, HLC significantly improves cladding efficiency and powder utilization1,2,3. Due to its high scanning speed and low melt pool temperature, HLC effectively suppresses the decomposition of ceramic particles, making it ideal for fabricating ceramic particle-reinforced metal matrix composite coatings. This technology has found widespread applications in surface strengthening in industries such as aerospace, automotive, and petroleum4,5,6.
316 L stainless steel is commonly used for its excellent corrosion resistance, formability, and biocompatibility. However, its relatively low hardness and wear resistance limit its performance in harsh conditions. WC ceramic particles, with high hardness, high melting point, and exceptional wear resistance, are ideal for reinforcing stainless steel-based composite materials. Hu et al.7 applied Ni/WC composite coatings to 304 steel using HLC, where the addition of WC particles refined the dendritic structure and enhanced the coating via fine grain and solid solution strengthening. The primary wear mechanisms identified were adhesive, abrasive, and oxidation wear. Chen et al.8 demonstrated that TiC/IN625 coatings produced by HLC exhibited lower surface roughness, smaller dendrite and grain sizes, and a higher proportion of low-angle grain boundaries compared to conventional laser cladding. Zhang et al.9 showed that HLC effectively prevents carbide precipitation and porosity formation, while promoting a uniform distribution of WC particles, which reduces stress localization and crack nucleation. Additionally, Ren et al.10 observed that in-situ VC (< 2 μm) was uniformly distributed in Fe-based coatings, benefiting from both HLC and in-situ synthesis, leading to a homogeneous hardness distribution and significant improvements in hardness and wear resistance-by factors of 4 and 10, respectively.
HLC enhances the performance of WC/316L coatings by inhibiting WC particle coarsening and promoting grain refinement in the 316 L matrix through rapid cooling11,12. By adjusting the laser parameters and WC content, the microstructure and properties of the coatings can be tailored for specific applications. However, while high cooling rates effectively suppress WC particle dissolution and coarsening, they also shorten the time for interfacial reactions, which may reduce the bonding strength between WC and the substrate. The relationship between WC content and the resulting microstructure and properties of the cladding is not yet fully understood. Therefore, this study aims to use HLC technology to fabricate WC/316L composite coatings, investigate the interfacial reactions and dissolution-precipitation behavior of WC particles, and clarify how WC content influences the coating’s microstructure and performance. This research will deepen the theoretical understanding of the high-speed laser cladding process and promote the application of HLC WC/316L composite coatings in wear protection for engineering components.
Materials and methods
The substrate material used in this experiment is 316 L alloy. The laser cladding powder is a composite, consisting of 316 L alloy powder mixed with WC particles in varying proportions, as detailed in Table 1. The 316 L alloy powder is produced via inert gas atomization, while the WC particles are commercially available cast WC. Both powders have particle sizes ranging from 15 to 53 μm, with 316 L exhibiting a spherical morphology and WC an irregular blocky shape, as shown in Fig. 1. Laser cladding was performed using a 10 kW Laserline diode laser in continuous mode, with process parameters of 6800 W laser power and a wavelength of 1064 nm, 10 m/min scanning speed, 85% overlap rate, and 5.5 r/min powder feed rate.
To prepare the samples, the edge of the WC particles was precisely cut using focused ion beam (FIB) technology. Microstructural analysis of the cladding layer was conducted with a Hitachi SU 5000 scanning electron microscope (SEM) and a FEI Tecnai G2 F20 transmission electron microscope (TEM). Phase composition was identified using a BRUKER D8 ADVANCE X-ray diffraction (XRD) instrument. The hardness of the samples was measured with a 600MRD-S Rockwell hardness tester. Wear scar morphology and volume were analyzed with a ZYGO 3D white-light interferometer (WLI) profiler.
Results and discussion
Microstructure analysis
Figure 2 shows the microstructures of Fe-based cladding layers with varying WC contents fabricated by HLC, where WC particles appear white. No defects, such as cracks or pores, are observed in the cladding layers. The WC particles are evenly distributed across all layers, without sedimentation or aggregation, despite the significant density difference between WC and 316 L. This uniform distribution is attributed to the pre-melting of the powder above the melt pool during the cladding process and the shallow melt pool, which prevents WC particle settling13.
As WC content increases, both the concentration of WC particles in the cladding layer and their morphology change, as shown in Fig. 3. Compared to Fig. 1(b), the edges of the original WC particles are sharper, while those in the cladding layer are smoother. This is due to the reaction between WC and 316 L under high-energy laser irradiation, causing the dissolution of WC particle edges and forming a W-rich zone14. During cooling, W nucleates at the WC-316 L interface, precipitating as carbides, which enhances the bond between WC and the 316 L alloy.
To further explore the dissolution and precipitation of WC particles, FIB technology was employed to cut the edges of WC particles for sample preparation (Fig. 4). This technique enables precise sub-micron localization of the WC-316 L interface, overcoming sample tilt or delamination issues associated with traditional preparation methods. It also prevents interface tearing or plastic deformation due to shear stress, preserving the true morphological features and elemental diffusion behavior15. The prepared thin foil (58.58 nm thick) was examined by TEM, and, together with SEM and EDS analyses, provided further insights into the dissolution and precipitation processes.
Figure 5 presents the EDS mapping of the WC edge region in the cladding layer, showing that W diffuses into the 316 L matrix in a serrated pattern. At the edges of the WC particles, W mixes and precipitates with Cr and Fe, forming a structure that improves interface bonding and prevents WC particle detachment during friction16,17.
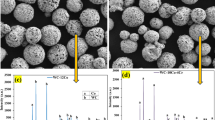
Figure 6 shows EDS analysis of the precipitated phases in the cladding layer. The precipitates are primarily composed of Fe, Cr, and W, with small amounts of Ni and Mo. Comparing Figs. 6(a) and 6(c), both contain Fe as the dominant element (over 50 at%), but the W content in Fig. 6(a) is 23.02 at%, whereas in Fig. 6(c) it is 7.95 at%. This difference is due to the absence of other precipitates surrounding the phase in Fig. 6(a), which allows W to diffuse and precipitate with Fe and Cr, resulting in higher W content. In Fig. 6(c), fine nanoscale precipitates and serrated WC particles lower the surrounding W concentration. The precipitate in Fig. 6(a) is approximately 100 nm, while that in Fig. 6(c) is about 200 nm. Despite similar W content, the larger volume of the precipitate in Fig. 6(c) leads to a lower W concentration.
Based on the selected area electron diffraction (SAED) pattern in Figs. 6(b), the identified diffraction spots corresponding to the (111), \(\:\text{(1}\stackrel{\text{-}}{\text{1}}\text{}\stackrel{\text{-}}{\text{1}}\text{)}\), and (200) planes yield a zone axis of [0 1 − 1], indicating a typical low-index crystallographic orientation. Combined with the measured interplanar angles, diffraction ring radii ratios, and the primary elemental composition (Fe, W, Cr, Ni, and C) obtained from EDS analysis, the phase is preliminarily identified as a metastable face-centered cubic (FCC) Fe-W-C-based solid solution formed under rapid solidification conditions18. Ni and Cr are likely incorporated as substitutional solutes, jointly stabilizing the FCC structure, with a possibility of forming γ-WC-like or metal-rich metastable phases. Such structures are commonly observed in non-equilibrium processes, such as laser cladding or gas atomization, and exhibit nanocrystalline features.
Figure 7 shows XRD analysis of HLC WC/316L composite coatings with different WC content. The main phases in all layers include austenite, WC, M2C, M7C3, and M3C2, where M represents metal elements like Fe, Cr, and W19. As WC content increases, the intensity of WC diffraction peaks rises, while that of austenite decreases. Fe, Cr, W, and Mo form mixed carbides, including M2C, M7C3, and M3C2, with M2C primarily containing W and Mo, and M7C3 and M3C2 consisting mainly of Fe and Cr.
Hardness and wear resistance
Figure 8 presents the hardness of 316 L alloy and WC/316L composite coatings. As WC content increases, the Rockwell hardness of the cladding layer significantly improves. With 10 wt% WC, hardness reaches 28.5 HRC, a 39% increase compared to 316 L. At 30 wt% WC, it rises to 45.2 HRC, a 120% improvement. This hardness increase is due to several factors20,21,22: (1) the contribution of WC particles, known for their high hardness and wear resistance, (2) the particle reinforcement effect, which alters stress distribution and hinders deformation, and (3) solid solution strengthening and phase interactions between WC and the stainless steel matrix.
Figure 9 shows wear rates for 316 L alloy and WC/316L composite coatings. As WC content increases, the wear rate decreases, indicating improved wear resistance. The wear rate of 316 L alloy is 7.18 × 10⁻² mm³N⁻¹m⁻¹, which decreases to 3.51 × 10⁻² mm³N⁻¹m⁻¹ with 10 wt% WC, and further drops to 1.92 × 10⁻² mm³N⁻¹m⁻¹ at 30 wt% WC.
Figure 10 illustrates the volume loss wear rate of 316 L alloy and WC/316L composite coatings. The wear track of the 316 L alloy is rough, with deep, wide grooves and flaky spalling due to its lower hardness. The primary wear mechanisms are adhesive and abrasive23.
In contrast, the composite coating wear tracks are smoother, with smaller grooves and less plastic deformation, indicating that abrasive wear predominates, with slight adhesive wear7,24,25,26. The enhanced wear resistance of the WC/316L composite coating is attributed to three factors: (1) The addition of WC enhances the coating hardness, which, according to Archard’s wear law, significantly lowers the wear rate; (2) WC particles serve as rigid supports, distributing external loads over a larger area, thus reducing stress on the matrix, limiting plastic deformation and material removal, and preventing deep embedding of abrasive particles. This leads to a finer distribution of wear debris and reduces secondary wear caused by larger particles; (3) Upon impact with abrasive particles, microfracturing occurs at the WC interface, increasing the curvature of the cutting edge and reducing cutting efficiency in subsequent wear cycles.
Conclusion
-
(1)
During HLC, the edges of WC particles dissolve, forming a W-rich zone. Upon cooling, W nucleates and precipitates carbides at the WC/316L interface, resulting in a strong bond between WC and the 316 L matrix.
-
(2)
The phases in the coating include austenite, WC, M2C, M7C3, and M3C2. As WC content increases, the amount of austenite decreases.
-
(3)
The wear resistance of the WC/316L composite coating is attributed to three primary mechanisms: increased hardness, load distribution within the matrix that reduces wear debris size, and passivation of wear debris, which minimizes cutting.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Zhou, L. et al. Research status and prospect of extreme high-speed laser cladding technology. Opt. Laser Technol. 168, 109800 (2024).
Yuan, W. et al. A comparative study on microstructure and properties of traditional laser cladding and high-speed laser cladding of Ni45 alloy coatings. Surf. Coat. Technol. 405, 126582 (2021).
Wang, Z., Zhang, J., Zhang, F. & Qi, C. Study on the microstructure and properties of a laser cladding Fe-Ni-Al coating based on the invar effect. Sci. Rep. 14, 11685 (2024).
Koruba, P. et al. Ultra-High speed laser cladding (UHSLC) technology for stellite 6 functional coatings deposition in aviation industry. Weld. Technol. Rev. 89, 6 (2017).
Liang, Y. et al. A review on coatings deposited by extreme high-speed laser cladding: processes, materials, and properties. Opt. Laser Technol. 164, 109472 (2023).
Pacheco, J. T. et al. Status of high-speed laser cladding process: an up-to-date review. Prog Addit. Manuf. 9, 1857–1868 (2024).
Hu, Z. et al. Effect of WC content on microstructure and properties of high-speed laser cladding Ni-based coating. Opt. Laser Technol. 155, 108449 (2022).
Chen, L. et al. Effect of surface morphology and microstructure on the hot corrosion behavior of TiC/IN625 coatings prepared by extreme high-speed laser cladding. Corros. Sci. 201, 110271 (2022).
Zhang, Y. et al. Microstructure and wear resistance of Ni-based WC coating by ultra-high speed laser cladding. Acta Metall. Sin. 56, 1530–1540 (2020).
Ren, Y. et al. In situ synthesized VC reinforced Fe-based coating by using extreme high-speed laser cladding. Mater. Lett. 315, 131962 (2022).
He, B. Effect of WC content on microstructure and properties of WC/316L laser coatings. Ferroelectrics 608, 1–12 (2023).
Ding, H. et al. Laser cladding WC/Fe composite coating on nose rail of turnout: microstructure characteristics and wear behaviors. Wear 205929 (2025).
Muvvala, G., Karmakar, D. P. & Nath, A. K. Monitoring and assessment of tungsten carbide wettability in laser cladded metal matrix composite coating using an IR pyrometer. J. Alloy Compd. 714, 514–521 (2017).
Yang, Y. et al. Nano and micron WC particles reinforced Hastelloy C22 coatings manufactured by directed energy deposition. J. Alloy Compd. 970, 172540 (2024).
Sugiyama, M. & Sigesato, G. A review of focused ion beam technology and its applications in transmission electron microscopy. Microsc 53, 527–536 (2004).
Zhang, G. S., Xing, J. D. & Gao, Y. M. Impact wear resistance of WC/Hadfield steel composite and its interfacial characteristics. Wear 260, 728–734 (2006).
Li, Y. et al. Theoretical calculations on the adhesion, stability, electronic structure, and bonding of Fe/WC interface. Appl. Surf. Sci. 257, 5671–5678 (2011).
Antoni-Zdziobek, A., Shen, J. Y. & Durand-Charre, M. About one stable and three metastable eutectic microconstituents in the Fe-W-C system. Int. J. Refract. Met. Hard Mater. 26, 372–382 (2007).
Alvarez, P. et al. Analysis of the process parameter influence in laser cladding of 316L stainless steel. J. Manuf. Mater. Process. 2, 55 (2018).
Shen, X. et al. Microstructure and properties of WC/Ni-based laser-clad coatings with different WC content values. Materials 15, 6309 (2022).
Wei, A. et al. Effect of WC on microstructure and wear resistance of Fe-based coating fabricated by laser cladding. Coatings 12, 1209 (2022).
Bian, Y. et al. Effect of powder feeding rate on mass fraction of WC particles, microstructure evolution and micro-hardness of WC-Ni composite coating by laser cladding. J. Alloys Compd. 1013, 178432 (2025).
Zhuang, D. D. et al. Effect and action mechanism of ultrasonic assistance on microstructure and mechanical performance of laser cladding 316L stainless steel coating. Surf. Coat. Technol. 433, 128122 (2022).
Qiulin, W. et al. Extreme High speed laser cladding 316L coating. J. Phys. Conf. Ser. 012083 (2021). (1965).
Xiao, Q. et al. Wear mechanisms and micro-evaluation on WC particles investigation of WC-Fe composite coatings fabricated by laser cladding. Surf. Coat. Technol. 420, 127341 (2021).
Lu, J. Z. et al. Wear properties and microstructural analyses of Fe-based coatings with various WC contents on H13 die steel by laser cladding. Surf. Coat. Technol. 369, 228–237 (2019).
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 52301001) and Key Research and Development Program of Jiangxi Province(Grant No. 20232BBE50018).
Author information
Authors and Affiliations
Contributions
Ziqiang Pi wrote the main manuscript text, Kaiping Du prepared figures 1, Xing Chen prepared Figs. 2 and 3, Zhaoran Zheng prepared figures 8, Chen Wang prepared figures 10. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ziqiang, P., Kaiping, D., Xing, C. et al. Investigation of the dissolution-precipitation behavior and properties of high-speed laser cladding WC/316L composite coatings. Sci Rep 15, 17564 (2025). https://doi.org/10.1038/s41598-025-02413-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02413-0