Abstract
Stingless bee propolis has gained global attention as a novel source of bioactive compounds with potential applications in food and medicine to promote human health. However, research on Australian stingless bee propolis remains limited as much of propolis is discarded by Australian beekeepers due to a lack of understanding its value. This study aimed to assess and compare the quality and chemical diversity of propolis from two Australian stingless bee species Tetragonula carbonaria and Tetragonula hockingsi across Queensland and New South Wales. Chemical assays revealed that T. hockingsi propolis had significantly higher total phenolic and flavonoid contents than T. carbonaria. Australian stingless bee propolis also showed high phenolic but moderate flavonoid content compared to international propolis sources. The phenolic content displayed geographical dependence, decreasing from northern to southern regions. The analyses of uHPLC-QTOF MS and 1H NMR profiling identified nine distinct propolis types, showcasing diverse chemical composition with the presence of flavan-3-ol, dihydroflavonol, flavanone, tannin, lignan, phenolic acid, cinnamic acid glycoside, chalcone, and alkyl-phenylketone compounds. These findings provide a foundation for future research into the value and therapeutic potential of Australian stingless bee propolis products, contributing to the development of the Australian propolis industry.
Similar content being viewed by others
Introduction
Bees are the most important group of insect pollinators and approximately 20,000 species have been described1. Stingless bees, belonging to the tribe Meliponini within the family Apidae, are closely related to honeybees, orchid bees, and bumblebees, and represent the largest monophyletic group of eusocial bees with over 60 genera and 600 species2. Stingless bees are widely distributed in the warm, humid environments of tropical and subtropical regions across the Americas, Australia, Asia, and Africa3. The keeping of stingless bees (Meliponiculture) plays a significant role in environmental conservation by pollinating native plant species, subsequently helping reduce environmental degradation and deforestation4. Due to their atrophied stingers, stingless bees often rely on propolis, a resinous material made from various plant resins and beeswax, to physically and chemically deter intruders5. Additionally, they use propolis as the primary material for nest construction and to help maintain hive homeostasis, including temperature and humidity6. As a result, stingless bees normally produce propolis in greater quantity compared to honeybee species7.
The use of propolis as a therapeutic agent for humans has been developed independently by various ancient cultures over millennia with Ancient Egyptians, Greeks, Persians, and Romans reportedly using propolis to treat ulcers and wounds2. Similarly, indigenous populations in India developed folk medicine that included stingless bee propolis for the treatment of ailments8. Modern research has confirmed the therapeutic benefits of stingless bee propolis, highlighting its antioxidant, antibacterial, antifungal, antiviral, anti-inflammatory, and anticancer properties9. As stingless bees collect resins and materials from various flora surrounding their hives, the chemical composition of their propolis varies depending on the local environment10. In general, its biological activities are largely attributed to the presence of small molecules belonging to polyphenol and terpenoid chemical classes11. However, research on stingless bee propolis is still in its infancy compared to that of honeybee propolis.
Most studies on stingless bee propolis have initially focused on American bee species. Since 2010, chemical composition and biological activity of propolis from 24 distinct bee species across eight genera, primarily in Brazil, have been investigated. These species include Frieseomelitta longipes12, Melipona beecheii13, Melipona fasciculata14, Melipona interrupta15, Melipona marginata16, Melipona mondury17, Melipona orbignyi18, Melipona quadrifasciata19,20, Melipona quadrifasciata anthidioides21,22, Melipona scutellaris23, Melipona seminigra 24, Melipona subnitida25, Nanotrigona testacularis24,26, Plebeia droryana27, Plebeia remota28, Scaptotrigona bipunctata29, Scaptotrigona depilis22, Scaptotrigona jujuyensis30, Scaptotrigona polysticta31, Scaptotrigona postica32, Tetragona clavipes33, Tetragonisca angustula20, Tetragonisca fiebrigi34, Trigona spinipes35. More recently, research on Asian stingless bee propolis has significantly increased, with studies conducted in Brunei, India, Indonesia, Malaysia, the Philippines, Thailand, and Vietnam on propolis from 21 bee species belonging to nine genera including Geniotrigona thoracica36,37, Heterotrigona itama36,37,38, Lepidotrigona terminate39, Lepidotrigona ventralis39, Lisotrigona cacciae40, Lisotrigona furva41, Tetragonula biroi42, Tetragonula fuscibasis43, Tetragonula fuscobalteata43, Tetragonula iridipennis44, Tetragonula laeviceps45,46, Tetragonula pagdeni39, Tetragonula sapiens47, Tetrigona apicalis48, Tetrigona binghami37, Tetrigona melanoleuca46, Timia apicalis43, Trigona incisa49, Trigona minor50, Trigona thoracica51, and Wallacetrigona incisa 52. Approximately 87% of studies on the bioactivity of stingless bee propolis are in vitro, with only a limited number (13%) focusing on in vivo research2.
In Australia there are 11 species of stingless bees which are part of the Tetragonula and Austroplebia genera53. These species mainly inhabit urban and forested areas of the east coast including Queensland and some northern regions of New South Wales, as well as northern areas of Western Australia and the Northern Territory53. Previous studies on Australian propolis produced by T. carbonaria bees demonstrated its therapeutic potential, including antibacterial54, antioxidant55, anti-inflammatory55, and vasomodulation56 activities. Stingless bee propolis from Australia is expected to contain uncommon bioactive compounds, since 84% of the flora is unique to the country57. The identification of tetragocarbone B (1)58 and tomentosenol A (2)59 (Fig. 1) in T. carbonaria propolis highlights the uniqueness of Australian stingless bee propolis. These compounds are trimethylated monomeric phloroglucinol derivatives that have not been found in propolis from other parts of the world. Tetragocarbone B has shown to possess an anti-inflammatory property60 while tomentosenol A exhibited an anti-fibrotic activity59.
Two species, Tetragonula carbonaria and Tetragonula hockingsi, play crucial roles in pollinating Australian native flora and horticultural crops61. These bees have recently gained interest as alternative pollinators in Queensland and part of New South Wales, particularly for crops such as macadamia, mango, lychee, avocado, and strawberry, following a Varroa mite outbreak in 202262. Their predominant propagation for pollination services results in the massive production of propolis. However, much of the propolis produced by these bees is often discarded by Australian beekeepers due to a lack of understanding its value. This study aimed to assess the quality and chemical diversity of Australian stingless bee propolis produced by T. carbonaria and T. hockingsi, comparing both species and collection regions across two eastern states of Australia, Queensland and New South Wales (Fig. 2). Propolis extraction yield as well as total phenolic and flavonoid contents, which are key chemical quality control parameters of propolis63,64, were assessed. The chemical diversity of stingless bee propolis was analysed through their1H nuclear magnetic resonance (NMR) spectra, high-performance liquid chromatography with diode array detection (HPLC–DAD), and ultra high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (uHPLC-QTOF MS) profiling. The findings of this study will establish a foundation for further investigations into the chemical composition and therapeutic properties of Australian T. carbonaria and T. hockingsi propolis, contributing to the knowledge of Australian propolis and the sustainability of both domestic and global propolis industries.
Map of Australia with stingless bee propolis collection regions. The map was created using Microsoft Excel and BioRender (https://www.biorender.com/).
Results and discussion
Propolis quality assessment
The pure resin yield from 170 propolis samples exhibited considerable variability, with a mean percentage yield of 51% (Table S1 in Supplementary Information), ranging from a minimum of 15% to a maximum of 86% (Figure S1A in Supplementary Information). Comparison of extraction yield between bee species revealed no significant differences between the propolis produced by T. carbonaria and T. hockingsi (Fig. 3A). However, the stingless bee propolis demonstrated double the extraction yield compared to that produced by honeybees (Apis mellifera) in our previous study65.
Quality parameters of Australian stingless bee propolis. (A) Species comparison of extraction yield, (B) Species comparison of total phenolic content, (C) Species comparison of total flavonoid content, (D) Species comparison of antioxidant activity, (E) Comparison of total phenolic content based on collection region, (F) Comparison of antioxidant activity based on collection region. Asterisks indicate levels of statistical significance (* P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001 **** P ≤ 0.0001). Figure created using Biorender.com.
The total phenolic content (TPC) of Australian stingless bee propolis extracts in this study ranged from 34.6 to 314.5 mg gallic acid equivalent (GAE)/g dry extract (Figure S1B in Supplementary Information). The results indicated that T. hockingsi propolis had a greater TPC than that of T. carbonaria propolis, with averaged values of 195.4 ± 2.2 mg GAE/g dry extract compared to 158.4 ± 1.8 mg GAE/g dry extract (Fig. 3B). Unpaired two-tailed t-tests indicated a statistically significant difference (*** P ≤ 0.001) in TPC between propolis from T. carbonaria and T. hockingsi. Both types of propolis exhibited TPC levels approximately two to three times higher than that of A. mellifera propolis with an averaged TPC of 68 mg GAE/g dry extract (Table S1 in Supplementary Information).
In terms of total flavonoid content (TFC), Australian stingless bee propolis ranged from 2.1 to 27.1 mg quercetin equivalent (QE)/g dry extract (Figure S1C in Supplementary Information). A comparison of the TFC between the two Australian stingless bee species (Fig. 3C) revealed that T. hockingsi samples had a significantly higher TFC (14.6 ± 0.2 mg QE/g dry extract) compared to T. carbonaria samples (11.5 ± 0.2 mg QE/g dry extract). Overall, the TFC of propolis produced by these two stingless bee species is approximately two times lower than that of A. mellifera propolis (Table S1 in Supplementary Information).
Australian propolis generally contains higher levels of phenolic and flavonoid contents than propolis from Indonesia, Thailand, and the Philippines (Table 1). The comparison of these parameters between Australian propolis and those from Argentina, Brazil and Malaysia is challenging due to differences in reference units (mg GAE per gram of extract versus per gram of raw material). However, it can be seen that most propolis produced by stingless bees exhibits TPC values that are over ten times greater than TFC. Notably, the ratio of TPC to TFC is 58 for Tetragonula biroi propolis in the Philippines and 39 for Melipona quadrifasciata propolis in Brazil. In term of Australian stingless bee propolis, this ratio is relatively comparable between two species, at approximately 13–14. Overall, these data suggest that stingless bees likely prefer foraging for plant resins which are rich in non-flavonoid compounds.
Testing propolis extracts at a concentration of 100 µg/mL revealed that T. hockingsi propolis exhibited higher antioxidant activity, with a free radical scavenging property ranging from 69 to 100% (Figure S1D in Supplementary Information). In contrast, T. carbonaria samples showed a broader range of scavenging activity, from 39 to 100% (Figure S1D in Supplementary Information). Samples having over 50% scavenging activity at 100 µg/mL were further tested to determine their IC50 values (with lower IC50 value indicating better antioxidant activity). Unpaired two-tailed t-tests indicated a statistically significant difference (* P ≤ 0.05) in IC50 between propolis from T. carbonaria and T. hockingsi. The top 10% of both T. hockingsi and T. carbonaria propolis demonstrated IC50 values for free radical scavenging ranging from 4.9 to 7.8 µg/mL which are relatively comparable to the known antioxidant compound, vitamin C (ascorbic acid) having its IC50 value of 5.0 µg/mL (Table S2 in Supplementary Information). On average, T. hockingsi propolis demonstrated more potent antioxidant activity as evidence by its lower IC50 values than T. carbonaria propolis (14.1 µg/mL versus 18.8 µg/mL) (Fig. 3D). Ecologically, T. hockingsi bees are primarily found in the more tropical regions of northern Queensland, while T. carbonaria bees distribute in subtropical areas67. The results demonstrate that geographical distribution of these two species highly influences their total phenolic content and antioxidant activity.
Data analysis between TPC and collection region showed two comparisons of significance (** P ≤ 0.001) between Central QLD, Brisbane City and Sydney (Fig. 3E). Lower antioxidant IC50 values (Fig. 3F) were found to correspond to further northern collections and positively correlate with the increase in TPC (Fig. 3E). These results indicate that stingless bee propolis collected in northern east-coast Australia has significantly higher antioxidant activity than samples collected further south. Similar trends were observed when analysing datasets for each bee species (Figure S2 in Supplementary Information). These findings suggest that propolis from warmer regions contains higher phenolic compounds and antioxidant activity compared to those from cooler areas. This phenomenon may be attributed to the fact that plants under thermal stress tend to accumulate more phenolic compounds68,69. Therefore, propolis produced by T. hockingsi bees exhibits higher TPC and superior antioxidant property compared to that of T. carbonaria bees.
Propolis chemical diversity assessment
Chemical composition of pure propolis has been known to consist of mainly polyphenolic and terpenoid compound classes70, which can be confirmed by 1H NMR profiles. Polyphenolic compounds exhibit aromatic protons in the range of 6.5 to 7.8 ppm, while terpenoids show saturated and olefinic methine and methylene protons in two separated regions from 1.0 to 2.5 ppm (saturated signals) and from 4.5 to 6.0 ppm (olefinic signals)65. The 1H NMR analysis (Fig. 4) revealed that approximately 80% of the tested propolis samples were high in polyphenols, and 18% were rich in terpenoids. Additionally, about 2% of the samples contained a significantly high level of sugars which were indicated by 1H signals between 3 and 5 ppm65, suggesting potential contamination with honey when propolis was harvested.
The principal component analysis (PCA) for species comparison provides a graphical representation of the NMR peaks between the T. carbonaria and T. hockingsi propolis groups. The PCA for species comparison (Fig. 5A) revealed some differences between T. carbonaria and T. hockingsi propolis, with a maximum difference between species of over 30% composition of PC1 and a maximum difference between species of over 10% composition of PC2 for samples within the Hotelling’s T2 95% confidence ellipses. Additionally, the sparse partial least-squares discriminant analysis (sPLS-DA) for species comparison (Fig. 5B) further supported these findings by demonstrating a better separation of Hotelling’s T2 ellipses between T. carbonaria and T. hockingsi samples. This indicates a distinct difference in chemical composition of these two propolis groups.
2D score PCA (A) and sPLS-DA (B) plots of Australian stingless bee propolis based on bee species; 2D score PCA (C) and sPLS-DA (D) plots of Australian stingless bee propolis based on collection region. Samples in the Hotelling’s T2 ellipses show a 95% confidence level. Samples outside any Hotelling’s T2 ellipses are labelled with collection region. Created using BioRender.com with chemometric analysis from MetaboAnalyst 6.0 (https://www.metaboanalyst.ca/).
The PCA for geographical location (Fig. 5C) showed that most samples overlapped, except for those collected from Sydney which had a higher PC2 (minimum of 10%) than samples from all other locations within their appropriate Hotelling’s T2 95% confidence ellipses. The sPLS-DA (Fig. 5D) indicated that samples from Brisbane showed consistent low variation in chemical composition and shared some similarities with other regions. The sPLS-DA results suggested that there was not much distinct propolis present in Sunshine Coast and Wide Bay Burnett compared to Brisbane. In contrast, Central QLD, Far North QLD, and Sydney displayed a large diversity in their compositions. Propolis samples from Sydney showed the highest variation with a range of 20% on component 2 with 95% confidence provided by Hotelling’s T2 ellipse. The sPLS-DA also indicated some similarities between Central and Far North QLD propolis as demonstrated by overlapping Hotelling’s T2 ellipse. The differences in chemical composition of propolis in Central and Far North QLD compared to Sydney are likely attributable to variations in flora, with Central and Far North QLD being more tropical while Sydney is more subtropical.
Propolis classification and compound identification
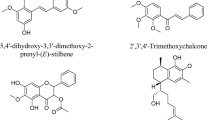
Through the analysis of HPLC–DAD profiles, propolis samples in this study were classified into nine groups (Table 2). A representative chromatogram for each group underwent comprehensive UV and MS/MS analyses to identify their major components (Table 3). Of the 26 compounds detected, 17 compounds were successfully identified, and their tentative chemical structures are shown in Fig. 6. Twelve of them belong to the flavonoid family while the remaining five are classified as non-flavonoid phenolic compounds. The identified flavonoid compounds are classified into four classes: flavan-3-ol (one compound), dihydroflavonol (three compounds), flavanone (six compounds) and chalcone (two compounds). The identified non-flavonoid phenolic compounds belong to five classes: tannin, lignan, phenolic acid, cinnamic acid glycoside, and alkyl-phenylketone (one compound for each class). The identification of diverse non-flavonoid phenolic compound classes as major chemical components in stingless bee propolis further supports the high level of total phenolic content.
Among the 17 compounds identified, six compounds including padmatin (E), pinoresinol (F), 1-O-cinnamoyl-6-O-p-coumaroyl-β-D-glucopyranose (I), 3-methyl-1-(2,4,6-trihydroxyphenyl)-1-butanone (K), 4-hydroxy-2',4',6'-trimethoxychalcone (O), and 2’-hydroxy-3',4',6'-trimethoxydihydrochalcone (Q) have not been previously reported in stingless bee propolis. Catechin (A), ellagic acid (B), katuranin (C), cinnamic acid (G), naringenin (H), and pinocembrin (I) have been identified in the propolis of various Melipona species, including M. quadrifasciata19,71, M. quadrifasciata anthidioides72, M. fasciculata73,74, M. scutellaris73 and M. subnitida75. Folerogenin (J) and sakuranetin (L) have been reported as a component of Trigona spinipes propolis35. Sakuranetin (L) has also been found in Tetragonula carbonaria propolis together with eriodictyol (D), cryptostrobin (N) and demethoxymatteucinol (P)54,76. Eight compounds (A-H) have exhibited strong antioxidant activity77,78,79,80,81,82,83,84, which may contribute to the potent antioxidant effect of propolis extracts observed in this study. In addition, these compounds, along with other flavonoids J, L, M and N have also demonstrated anti-inflammatory property77,78,79,80,81,82,83,84,85,86,87,88. Compounds F, G, and H have been known for their neuroprotective effects82,83,84. The remaining identified compounds I, K, O, P and Q have been sparsely studied in the literature regarding their potential bioactivities. Understanding the biological activities of these major compounds in Australian propolis could guide future developments in using this natural resource to advance human health.
No distinctly observable difference in chemical profiles of propolis produced by T. carbonaria and T. hockingsi bees was identified as evidenced by the profiles of samples collected in Central Queensland, Wide Bay Burnett, Sunshine Coast and Brisbane regions (propolis types 3–5). The chemical profiling clearly highlighted the unique composition of Far North Queensland propolis (types 1 and 2), which displayed distinct profiles compared to those from Sydney (type 9). Unique chemical profiles were also found in Central Queensland (type 3) and some regions in Brisbane (type 6). Some samples shared common compounds across a wide geographical distribution. Notably, a flavan-3-ol catechin (A) was detected as a major component in propolis from Central QLD to Sydney (propolis types 3, 5, and 7–9) while a flavanone naringenin (H) was universally present as a major or minor constituent in propolis from Far North Queensland to Sydney (propolis types 1–6, and 8–9). Only sakuranetin (L) and demethoxymatteucinol (P) was found to be present in all nine propolis types with the UV absorbance peaks being visible across all nine types. Additionally, cryptostrobin (N) was found to be a major constituent in propolis from Central QLD to Sydney. These results suggests that bees are likely able to find similar resins across multiple regions for their propolis production and compounds A, H, L and P may play a key role for bees’ defensive system.
The co-presence of compounds in both propolis types 4 and 5 indicates that these types share a common botanical source, potentially found from Wide Bay Burnett to Brisbane. Interestingly, propolis type 9 appears to be a mixture of types 7 and 8 which were collected from the southern regions of Brisbane as they share similar compounds. This indicates that these propolis type were likely produced from similar plant species, distinct from those in the northern regions. Corymbia torelliana is likely one of the resin sources used by the bees as compounds M, N, and P have previously been identified in the resin of this plant species54. These chemical profiles serve as a valuable resource for identifying the origins of these propolis samples in the future.
Conclusion
In conclusion, this is the first comprehensive study to overview the quality and chemical diversity of Australian propolis produced by stingless bees T. carbonaria and T. hockingsi. The results indicate that Australian stingless bee propolis possesses potent antioxidant activity due to its high level of polyphenol compounds consisting primarily of non-flavonoid phenolics. The antioxidant capacity of propolis suggests that it could be used as an ingredient in the preparation of functional foods and dietary supplements.
Chemical analysis by 1H NMR and uHPLC-QTOF MS profiles facilitated the classification of nine propolis types and shed a light into the chemical diversity of propolis collected from the northern to southern regions of Eastern coast Australia. Although not all compounds were determined from the uHPLC-QTOF MS data mining, the identification of multiple compound classes suggested that Australian stingless bee propolis has a diverse chemical composition. Further investigation into the undetermined compounds in this study may lead to the discovery of novel bioactive compounds that could serve as candidates for pharmaceutical development. The findings of this study will enhance awareness of Australian stingless bee propolis in both domestic and global markets and contribute to the growth of propolis industry in Australia.
Methods
Solvents and reagents
All solvents including ethanol (EtOH), methanol (MeOH), acetonitrile (MeCN), water and formic acid used for extraction and chromatography are LC–MS grade and were purchased from Merck. Dimethylsulfoxide-d6 (DMSO-d6) for NMR analysis was purchased from Merck. Gallic acid, Folin-Ciocalteu reagent, quercetin, potassium acetate (CH3COOK), aluminium nitrate (Al(NO3)3), used for colorimetric assays were purchased from Merck. 2,2-diphenyl-1-picrylhydrazyl (DPPH) used for antioxidant assay was purchased from Merck.
Propolis collection and extraction
In this study, 170 raw propolis samples including T. carbonaria propolis (122 samples) and T. hockingsi propolis (48 samples) were collected across the eastern coast of Australia from Queensland (QLD, 159 samples) and New South Wales (NSW, 11 samples) by local beekeepers from 2022 and 2023 (Fig. 2). Sample details were provided in Table S3 (Supplementary Information). The raw propolis was frozen and ground into a powder before 0.5 g of the powdered propolis was transferred into a 15 mL falcon tube. After which 5 mL of 70% ethanol was added to each tube and briefly vortexed to mix. The samples were left in tubes and incubated in a 65 °C water bath for 30 min in darkness and were vortexed every 10 min. After the 30-min incubation, the samples were agitated through sonication for 2 min, vortexed for 2 min and then placed back into the ultrasonic water bath for 3 min, for a total of 5 min sonication time. The solution was then centrifuged at 4,000 rpm for 15 min at 4 °C. Once centrifuged, the supernatant was transferred into a 15 mL test tube. The supernatant was then dried overnight through centrifugal evaporation in a GeneVac using the low boiling point method. The extract was then dried further in a vacuum desiccator for 30 min. Once sufficiently dried, the extract was finally weighed to obtain the extraction yield and kept in darkness at -20 °C for NMR and uHPLC-QTOF MS sample preparation.
Determination of total phenolic content
Total phenolic content was determined using the Folin-Ciocalteu colorimetric method as previously described65. In brief, 80 µL of the Folin–Ciocalteu solution was added to 410 µL of H2O, followed by the addition of 10 µL of propolis extracts (10 mg/mL) or 10 μL of gallic acid standard solution in MeOH and then 500 µL of 10% (m/v) aqueous sodium carbonate. MeOH was used as a blank sample (negative control). The samples in Eppendorf tubes were incubated in darkness at room temperature for 60 min before being plated to a 96-well plate (200 µL/well). Finally, the absorbance at 760 nm was measured on a Perkin Elmer Enspire microplate reader. All measurements were performed in triplicates, the mean values were interpolated in a gallic acid calibration curve and the total phenolic content was expressed as mg Gallic Acid Equivalents (GAE) per gram of dry extract.
Determination of total flavonoid content
To determine total flavonoid content, the aluminium chloride colorimetric method was used as previously described65 with some modifications. The aluminium chloride colorimetric method was completed by adding 20 µL of 10% Al(NO3)3 in H2O and 20 µL of 1 M CH3COOK in water to 950 µL of MeOH. 10 µL of 10 mg/mL propolis extract in MeOH or quercetin in MeOH was then added. The samples in Eppendorf tubes were then incubated at room temperature for 45 min in darkness before being plated in duplicate onto a 96 well plate (200 µL per well). The absorbance was then measured through spectrophotometry at 415 nm on a Perkin Elmer Enspire microplate reader. Quercetin was used as a chemical equivalent for quantification in this assay.
Evaluation of antioxidant activity using DPPH free radical scavenging assay
To determine the antioxidant activity, the DPPH free radical scavenging assay was used with propolis extracts and different concentrations as previously described65. Briefly, the DPPH solution was prepared on the day of measuring at a concentration of 100 µM in MeOH. The propolis extracts (200 μL) at different concentrations were added to 600 μL of DPPH solution in Eppendorf tubes. The mixtures were kept in the dark at room temperature for 20 min before being plated to a 96-well plate (200 µL/well) and measured at 518 nm using a Perkin Elmer Enspire microplate reader. All evaluations were performed in triplicates. Gallic acid and MeOH were used as positive and negative controls. The % inhibition of the DPPH radical for each sample was normalised and calculated using the following formula:
where AS is the absorbance of the sample, AP is the absorbance of the positive control and AB is the absorbance of the blank sample (negative control).
An IC50 curve for each extract was generated using GraphPad Prism 10.4.1 (https://www.graphpad.com/) with a 95% confidence interval. The IC50 values were determined as the concentration required to inhibit 50% of DPPH free radicals.
1H NMR analysis and processing
To prepare samples for NMR, dry propolis extract was dissolved in 500 µL of Dimethylsulfoxide-d6 (DMSO-d6) to produce a 50 mg/mL propolis sample which was then transferred to a 10 mm NMR tube. The NMR data acquisition was conducted on a Bruker Ascend 400 MHz NMR instrument equipped with a 5 mm room temperature probe housed at The University of the Sunshine Coast. The acquisition method consisted of obtaining 1H NMR spectra at 300 K. The data obtained from 1H NMR was then analysed using Bruker Topspin 4.4.0 software. The spectrum was recorded using a standard pulse sequence with a 90° pulse length of 9.61 µs, 64 scans, a spectral width of 16 ppm, a relaxation delay of 5 s, and an acquisition time of 3.75 s. The spectra were referenced to the DMSO residual solvent signal at 2.50 ppm. Metabolomic analysis for chemical profile development was conducted using MetaboAnalyst 6.0 (https://www.metaboanalyst.ca/).
Chemometric analysis
1H NMR acquisition data from 0 to 8 ppm was exported to MetaboAnalyst 6.0 software (https://www.metaboanalyst.ca/) 89 in a comma-separated value (CSV) format. PCA90 and sPLS-DA91 were conducted to demonstrate the variations in sample composition depending on their geographical location and stingless bee species.
uHPLC-QTOF MS analysis
To prepare samples for uHPLC-QTOF MS, dry propolis extract was dissolved in methanol to produce a sample concentration of 10 mg/mL and stored in darkness at -20 °C in 2 mL Agilent LC–MS vials before uHPLC-QTOF MS analysis. The analysis was conducted on an Agilent 1290-uHPLC-6546-QTOF MS equipped with a DAD detector and was injected (2 µL) onto a Zorbax Eclipse plus C18 column (2.1 × 50 mm, 1.8 µm) with a guard column. The LC-QTOF method was completed in 1D and was completed in reverse phase utilising a gradient consisting of H2O (solvent A, 0.1% formic acid) and MeCN (solvent B, 0.1% formic acid) with a sample flow rate of 0.4 mL per minute. uHPLC analysis utilised a DAD with 8 wavelengths being analysed, consisting of 200 nm, 260 nm, 280 nm, 300 nm, 320 nm, 360 nm, 420 nm, and 460 nm. The LC gradient consisted of a 15-min sample run time at a constant sample flow rate of 0.4 mL/min. The gradient program started at 2% solvent B for 0.5 min, increased to 100% solvent B for 9 min, kept at this level for the next 3 min, then reduced to 2% solvent B for 1 min, and finally re-equilibrated at 2% solvent B for 1.5 min.
The QTOF MS analysis was completed in positive and negative ion modes and was ionised with a Dual Agilent Jet Stream (Dual AJS) electrospray ionisation (ESI) ion source set to follow an ionisation energy formula described by \(\left( {5 \times \frac{m/z}{{100}}} \right) + 10\), with the MS scan to be completed in tandem MS (MS2, MS/MS) mode. The TOF–MS fragmentor and skimmer were set at 120 V and 45 V, respectively. Acquisition was completed in positive and negative polarity modes with the analysed mass range being from 100 to 1,050 Da. The carrier gas source used in MS was nitrogen at 250 °C, with a flow rate of 12 L per minute. Analysis of QTOF-MS spectra was conducted using SIRIUS Version 6.0.7 (https://bio.informatik.uni-jena.de/software/sirius/).
Compound identification
The prediction of compounds in propolis samples was performed using DAD uHPLC-QTOF MS, controlled by Agilent MassHunter Workstation Software Version B.08.00 (https://www.agilent.com/en/promotions/masshunter-mass-spec) and SIRIUS Version 6.0.7 (https://bio.informatik.uni-jena.de/software/sirius/). Through DAD chromatograms, at least five major peaks were relatively identified for each propolis type by comparing their signals at eight different wavelengths: 200, 260, 280, 300, 320, 360, 420 and 460 nm. After identifying these major UV peaks, initial classification of compound family (flavonoid or non-flavonoid phenolics) was determined by considering the number of carbon atoms, unsaturated degrees from each compound’s molecular formula and their UV absorbance signals94,95. Compound identification was conducted using MS/MS data in both positive and negative modes utilising the molecular formula identifier (MFI), fingerprint predictor (CSI:FingerID), and compound class predictor (CANOPUS) within the SIRIUS software with MS/MS mass accuracy threshold ≤ 10 ppm. The positive and negative MS2 data for each major compound were searched using the online database searching (CSI:FingerID) feature within SIRIUS across 21 libraries including Biocyc, Blood exposome, CHEBI, COCONUT, FooDB, GNPS, HMDB, HSDB, KNApSAcK, LipidMaps, Maconda, MeSH, MiMeDB, NORMAN, Plantcyc, PubChem (bio and metabolites, drug, food, safety and toxic)92, SuperNatural, TeroMOL, YMDB, and LOTUS93. Compounds with a confidence value < 80% were deemed not determined (n.d). MS1 and MS2 data of compounds A-Q were detailed in Figures S3-S5 (Supplementary Information).
Statistical analysis
Statistical analysis was completed using GraphPad Prism Version 10.4.1 (https://www.graphpad.com/). Unpaired two-tailed t-tests were conducted to compare the means of the yield, total phenolic content, and antioxidant activity between two species groups. One-way ANOVA with post-hoc Tukey’s test was used to compare the means of each collection region. Statistical significance was expressed using asterisks. All error bars present are in the form of standard error mean (SEM).
Data availability
All data generated or analysed during this study are included in this paper and its Supplementary Information file.
References
Zattara, E. E. & Aizen, M. A. Worldwide occurrence records suggest a global decline in bee species richness. One Earth 4, 114–123. https://doi.org/10.1016/j.oneear.2020.12.005 (2021).
Zulhendri, F. et al. Propolis of stingless bees for the development of novel functional food and nutraceutical ingredients: a systematic scoping review of the experimental evidence. J. Funct. Foods 88, 104902. https://doi.org/10.1016/j.jff.2021.104902 (2022).
Rasmussen, C. & Cameron, S. A. Global stingless bee phylogeny supports ancient divergence, vicariance, and long distance dispersal. Biol. J. Linn. Soc. 99, 206–232. https://doi.org/10.1111/j.1095-8312.2009.01341.x (2010).
De Carvalho, R. M. A., Martins, C. F. & Mourão, J. D. S. Meliponiculture in Quilombola communities of Ipiranga and Gurugi, Paraíba state, Brazil: an ethnoecological approach. J. Ethnobiol. Ethnomed. 10, 3. https://doi.org/10.1186/1746-4269-10-3 (2014).
Lehmberg, L., Dworschak, K. & Blüthgen, N. Defensive behavior and chemical deterrence against ants in the stingless bee genus Trigona (Apidae, Meliponini). J. Apic. Res. 47, 17–21. https://doi.org/10.1080/00218839.2008.11101418 (2008).
Shanahan, M. & Spivak, M. Resin use by stingless bees: a review. Insects 12, 719 (2021).
Salleh, S. N. A. S., Johari, W. L. W. & Hanapiah, N. A. M. A comprehensive review on chemical compounds, biological actions and potential health benefits of stingless bee propolis. Sains Malays. 51, 733–745 (2022).
Choudhari, M. K., Punekar, S. A., Ranade, R. V. & Paknikar, K. M. Antimicrobial activity of stingless bee (Trigona sp.) propolis used in the folk medicine of Western Maharashtra, India. J. Ethnopharmacol. 141, 363–367. https://doi.org/10.1016/j.jep.2012.02.047 (2012).
Popova, M., Trusheva, B. & Bankova, V. Propolis of stingless bees: a phytochemist’s guide through the jungle of tropical biodiversity. Phytomedicine 86, 153098. https://doi.org/10.1016/j.phymed.2019.153098 (2021).
Kämper, W., Kaluza, B. F., Wallace, H., Schmitt, T. & Leonhardt, S. D. Habitats shape the cuticular chemical profiles of stingless bees. Chemoecology 29, 125–133. https://doi.org/10.1007/s00049-019-00282-4 (2019).
Tran, T. D. et al. Lessons from exploring chemical space and chemical diversity of propolis components. Int. J. Mol. Sci. 21, 14 (2020).
De Souza, E. C. A. et al. Chemical compositions and antioxidant and antimicrobial activities of propolis produced by frieseomelitta longipes and apis mellifera BEES. Quim. Nova 41, 485–491. https://doi.org/10.21577/0100-4042.20170208 (2018).
Yam-Puc, A. et al. Pentacyclic triterpenes and other constituents in propolis extract from Melipona beecheii collected in Yucatan, México. Rev. Bras. Farmacogn. 29, 358–363. https://doi.org/10.1016/j.bjp.2019.01.006 (2019).
Batista, M. C. A. et al. Chemical composition and antioxidant activity of geopropolis produced by Melipona fasciculata (Meliponinae) in flooded fields and cerrado areas of Maranhão State, northeastern Brazil. Acta Amazon. 46, 315–322 (2016).
Silva, E. C. C. D., Perêa, M. M., Nunomura, R. D. C. S., Nunomura, S. M. & Zilse, G. A. C. Constituintes fenólicos e atividade antioxidante da geoprópolis de duas espécies de abelhas sem ferrão amazônicas. Quim. Nova 36, 628–633 (2013).
Cardozo, D. V. et al. Variabilidade química de geoprópolis produzida pelas abelhas sem ferrão Jataí, Mandaçaia e Mandurí. Rev. Virtual Quim. 7, 2456–2474 (2015).
Santos, T. et al. Melipona mondury produces a geopropolis with antioxidant, antibacterial and antiproliferative activities. An. Acad. Bras. Cienc. 89, 2247–2259. https://doi.org/10.1590/0001-3765201720160725 (2017).
Santos, H. F. D. et al. Chemical profile and antioxidant, anti-inflammatory, antimutagenic and antimicrobial activities of geopropolis from the stingless bee Melipona orbignyi. Int. J. Mol. Sci. 18, 953 (2017).
Hochheim, S. et al. Determination of phenolic profile by HPLC–ESI-MS/MS, antioxidant activity, in vitro cytotoxicity and anti-herpetic activity of propolis from the Brazilian native bee Melipona quadrifasciata. Rev. Bras. Farmacogn. 29, 339–350. https://doi.org/10.1016/j.bjp.2018.12.010 (2019).
Torres, A. R. et al. Chemical characterization, antioxidant and antimicrobial activity of propolis obtained from Melipona quadrifasciata and Tetragonisca angustula stingless bees. Braz. J. Med. Biol. Res. 51, e7118 (2018).
Dos Santos, C. M. et al. Chemical composition and pharmacological effects of geopropolis produced by Melipona quadrifasciata anthidioides. Oxid. Med. Cell. Longev. 2017, 8320804 (2017).
Bonamigo, T. et al. Antioxidant, cytotoxic, and toxic activities of propolis from two native bees in Brazil: Scaptotrigona depilis and Melipona quadrifasciata anthidioides. Oxid. Med. Cell. Longev. 2017, 1038153 (2017).
Da Cunha, M. G. et al. Antiproliferative constituents of geopropolis from the bee Melipona scutellaris. Planta Med. 82, 190–194 (2016).
Lavinas, F. C. et al. Brazilian stingless bee propolis and geopropolis: promising sources of biologically active compounds. Rev. Bras. Farmacogn. 29, 389–399. https://doi.org/10.1016/j.bjp.2018.11.007 (2019).
De Sousa, D. M. N. Prospecção fitoquímica, toxicidade in vitro e avaliação das atividades anti-radicalar e antibacteriana da geoprópolis da abelha jandaíra. Acta Vet. Bras. 9, 134–140 (2015).
Fernandes, A. Jr., Leomil, L., Fernandes, A. & Sforcin, J. The antibacterial activity of propolis produced by Apis mellifera L. and Brazilian stingless bees. J. Venom. Anim. Toxins 7, 173–182 (2001).
Bonamigo, T. et al. Antioxidant and cytotoxic activity of propolis of Plebeia droryana and Apis mellifera (Hymenoptera, Apidae) from the Brazilian Cerrado biome. PLoS ONE 12, e0183983 (2017).
Surek, M. et al. Propolis authentication of stingless bees by mid-infrared spectroscopy and chemometric analysis. LWT 161, 113370. https://doi.org/10.1016/j.lwt.2022.113370 (2022).
Cisilotto, J. et al. Cytotoxicity mechanisms in melanoma cells and UPLC-QTOF/MS2 chemical characterization of two Brazilian stingless bee propolis: uncommon presence of piperidinic alkaloids. J. Pharm. Biomed. Anal. 149, 502–511 (2018).
Brodkiewicz, Y. et al. Studies of the biological and therapeutic effects of Argentine stingless bee propolis. J. Drug Deliv. Ther. 8, 382–392. https://doi.org/10.22270/jddt.v8i5.1889 (2018).
Lorini, A., Wobeto, C., Bonaldo, S. M., Botelho, S. & Sinhorin, A. P. Chemical composition and antifungal activity of propolis on Aspergillus flavus. Biosci. J. 34, 1298–1307 (2018).
Coelho, G. R. et al. Antiviral action of hydromethanolic extract of geopropolis from Scaptotrigona postica against antiherpes simplex virus (HSV-1). Evid. Based Compl. Alternat. Med. 2015, 296086 (2015).
Bergamini, A. P. C. et al. Chemical profile, antioxidant, antifungal, and cytotoxic activities of propolis from the stingless bee Tetragona clavipes. Braz. J. Microbiol. 56, 251–262. https://doi.org/10.1007/s42770-024-01591-9 (2025).
Campos, J. F. et al. Antimicrobial, antioxidant, anti-inflammatory, and cytotoxic activities of propolis from the stingless bee Tetragonisca fiebrigi (Jatai). Evid. Based Compl. Alternat. Med. 2015, 296186 (2015).
Freitas, M. O., Ponte, F. A., Lima, M. A. S. & Silveira, E. R. Flavonoids and triterpenes from the nest of the stingless bee Trigona spinipes. J. Braz. Chem. Soc. 19, 532–535 (2008).
Ibrahim, N., Zakaria, A. J., Ismail, Z. & Mohd, K. S. Antibacterial and phenolic content of propolis produced by two Malaysian stingless bees, Heterotrigona itama and Geniotrigona thoracica. Int. J. Pharmacogn. Phytochem. Res. 8, 156–161 (2016).
Abdullah, N. A. et al. Phytochemicals, mineral contents, antioxidants, and antimicrobial activities of propolis produced by Brunei stingless bees Geniotrigona thoracica, Heterotrigona itama, and Tetrigona binghami. Saudi J. Biol. Sci. 27, 2902–2911. https://doi.org/10.1016/j.sjbs.2020.09.014 (2020).
Abdullah, N. A. et al. Physicochemical analyses, antioxidant, antibacterial, and toxicity of propolis particles produced by stingless bee Heterotrigona itama found in Brunei Darussalam. Heliyon 5, e02476 (2019).
Vongsak, B., Kongkiatpaiboon, S., Jaisamut, S., Machana, S. & Pattarapanich, C. In vitro alpha glucosidase inhibition and free-radical scavenging activity of propolis from Thai stingless bees in mangosteen orchard. Rev. Bras. Farmacogn. 25, 445–450 (2015).
Georgieva, K. et al. Phytochemical analysis of Vietnamese propolis produced by the stingless bee Lisotrigona cacciae. PLoS ONE 14, e0216074 (2019).
Oanh, V. T. K. et al. New dihydrochromene and xanthone derivatives from Lisotrigona furva propolis. Fitoterapia 149, 104821 (2021).
Cumbao, J. L. T. et al. Total phenolics, total flavonoids, antioxidant activity and antibacterial property of propolis produced by the stingless bee, Tetragonula biroi (Friese), from Laguna and Quezon, Philippines. Philippine Entomolog. 30, 63–74 (2016).
Kustiawan, P. M., Puthong, S., Arung, E. T. & Chanchao, C. In vitro cytotoxicity of Indonesian stingless bee products against human cancer cell lines. Asian Pac. J. Trop. Biomed. 4, 549–556 (2014).
Kothai, S. & Jayanthi, B. Evaluation of antioxidant and antimicrobial activity of stingless bee propolis (Tetragonula iridipennis) of Tamilnadu, India. Int. J. Pharm. Pharm. Sci. 6, 81–85 (2014).
Umthong, S., Phuwapraisirisan, P., Puthong, S. & Chanchao, C. In vitro antiproliferative activity of partially purified Trigona laeviceps propolis from Thailand on human cancer cell lines. BMC Complement. Altern. Med. 11, 1–8 (2011).
Sanpa, S. et al. Antibacterial compounds from propolis of Tetragonula laeviceps and Tetrigona melanoleuca (Hymenoptera: Apidae) from Thailand. PLoS ONE 10, e0126886 (2015).
Pujirahayu, N., Suzuki, T. & Katayama, T. Cycloartane-type triterpenes and botanical origin of propolis of stingless Indonesian bee Tetragonula sapiens. Plants 8, 57 (2019).
Mohamed, W. A. S. et al. GC-MS evaluation, antioxidant content, and cytotoxic activity of propolis extract from peninsular Malaysian stingless bees, Tetrigona apicalis. Evid. Based Complement. Alternat. Med. 2020, 8895262. https://doi.org/10.1155/2020/8895262 (2020).
Kustiawan, P. et al. Exploration of apoptotic effect in cancer cells treated with stingless bee Trigona incisa propolis native to East Kalimantan, Indonesia. Planta Med. 81, 18 (2015).
Nguyen, H. X., Nguyen, M. T., Nguyen, N. T. & Awale, S. Chemical constituents of propolis from Vietnamese Trigona minor and their antiausterity activity against the PANC-1 human pancreatic cancer cell line. J. Nat. Prod. 80, 2345–2352 (2017).
Shehu, A. et al. Antifungal properties of Malaysian Tualang honey and stingless bee propolis against Candida albicans and Cryptococcus neoformans. J. Appl. Pharm. Sci. 6, 44–50 (2016).
Arung, E. T. et al. Prenylated flavonoids as antioxidant and melanin inhibitors from stingless bee (Wallacetrigona incisa) propolis. Nat. Prod. Commun. 15, 3. https://doi.org/10.1177/1934578X20911272 (2020).
AussieBees. Australian stingless bees. https://www.aussiebee.com.au/australian-stingless-bees.html#question1 (2024).
Massaro, C. F. et al. Anti-staphylococcal activity of C-methyl flavanones from propolis of Australian stingless bees (Tetragonula carbonaria) and fruit resins of Corymbia torelliana (Myrtaceae). Fitoterapia 95, 247–257. https://doi.org/10.1016/j.fitote.2014.03.024 (2014).
Hamilton, K. D., Brooks, P. R., Ogbourne, S. M. & Russell, F. D. Natural products isolated from Tetragonula carbonaria cerumen modulate free radical-scavenging and 5-lipoxygenase activities in vitro. BMC Complement. Altern. Med. 17, 232. https://doi.org/10.1186/s12906-017-1748-6 (2017).
Massaro, F. C. et al. Effect of Australian propolis from stingless bees (Tetragonula carbonaria) on pre-contracted human and porcine isolated arteries. PLoS ONE 8, e81297. https://doi.org/10.1371/journal.pone.0081297 (2013).
Locher, C., Semple, S. J. & Simpson, B. S. Traditional Australian aboriginal medicinal plants: an untapped resource for novel therapeutic compounds?. Future Med. Chem. 5, 733–736. https://doi.org/10.4155/fmc.13.44 (2013).
Nishimura, E. et al. Structure determination of monomeric phloroglucinol derivatives with a cinnamoyl group isolated from propolis of the Stingless Bee, Tetragonula carbonaria. Asian J. Org. Chem. 5, 855–859. https://doi.org/10.1002/ajoc.201600106 (2016).
Hamilton, K. D. et al. Anti-fibrotic potential of tomentosenol a, a constituent of cerumen from the Australian native stingless bee, Tetragonula carbonaria. Antioxidants 11, 56 (2022).
Venigalla, M. et al. Identification of tetragocarbone C and sideroxylin as the most potent anti-inflammatory components of Syncarpia glomulifera. Fitoterapia 150, 104843. https://doi.org/10.1016/j.fitote.2021.104843 (2021).
Reynolds, O. L. & Robinson, M. Australian Native Bee Strategic RD&E Plan (2022–2027). AgriFutures Australia 3, 1–40 (2022).
Cook, J. Stingless bees as effective managed pollinators for Australian horticulture. Hort Innov. 2022, 1–62 (2022).
Escriche, I. & Juan-Borrás, M. Standardizing the analysis of phenolic profile in propolis. Int. Food Res. 106, 834–841. https://doi.org/10.1016/j.foodres.2018.01.055 (2018).
Woisky, R. G. & Salatino, A. Analysis of propolis: some parameters and procedures for chemical quality control. J. Apic. Res. 37, 99–105. https://doi.org/10.1080/00218839.1998.11100961 (1998).
Tran, C. T. N. et al. Quality assessment and chemical diversity of Australian propolis from Apis mellifera bees. Sci. Rep. 12, 13574. https://doi.org/10.1038/s41598-022-17955-w (2022).
Fikri, A. M., Sulaeman, A., Marliyati, S. A. & Fahrudin, M. Antioxidant activity and total phenolic content of stingless bee propolis from Indonesia. J. Apic. Sci. 63, 139–147 (2019).
Tetragonula carbonaria versus Tetragonula hockingsi—a comparison (2024, accessed 31 Oct 2024). https://tetranativebees.com/native-bee-blog/f/tetragonula-carbonaria-vs-tetragonula-hockingsi-a-comparison.
Rivero, R. M. et al. Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 160, 315–321. https://doi.org/10.1016/S0168-9452(00)00395-2 (2001).
Mansinhos, I. et al. Impact of temperature on phenolic and osmolyte contents in in vitro cultures and micropropagated plants of two mediterranean plant species Lavandula viridis and Thymus lotocephalus. Plants 11, 3516 (2022).
Tran, T. D. et al. Lessons from exploring chemical space and chemical diversity of propolis components. Int. J. Mol. Sci. 21, 4988 (2020).
Ferreira, B. L. et al. Southern-Brazilian geopropolis: a potential source of polyphenolic compounds and assessment of mineral composition. Food Res. Int. 126, 108683. https://doi.org/10.1016/j.foodres.2019.108683 (2019).
Rubinho, M. P. et al. A comprehensive characterization of polyphenols by LC-ESI–QTOF-MS from Melipona quadrifasciata anthidioides geopropolis and their antibacterial, antioxidant and antiproliferative effects. Nat. Prod. Res. 34, 3139–3144. https://doi.org/10.1080/14786419.2019.1607851 (2020).
Araújo, K. S. D. S. et al. Physicochemical properties and antioxidant capacity of propolis of stingless bees (Meliponinae) and Apis from two regions of Tocantins, Brazil. Acta Amazon. 46, 61–68 (2016).
Dutra, R. P. et al. Phenolic acids, hydrolyzable tannins, and antioxidant activity of geopropolis from the stingless bee Melipona fasciculata Smith. J. Agric. Food Chem. 62, 2549–2557. https://doi.org/10.1021/jf404875v (2014).
De Sousa-Fontoura, D. M. N. et al. Wound healing activity and chemical composition of geopropolis from Melipona subnitida. Rev. Bras. Farmacogn. 30, 367–373. https://doi.org/10.1007/s43450-020-00030-8 (2020).
Ebner, D. W., Woods, D. C. & Tran, T. D. Bioactive Polyphenolic Compounds from Propolis of Tetragonula carbonaria in the Gibberagee Region, New South Wales, Australia. Foods 14, 965 (2025).
Devi, S. A Centum of Valuable Plant Bioactives (eds. Muhammad, M. & Farooq, A.) 525–544 (Academic Press, 2021).
Saldanha, E. et al. in Polyphenols: Mechanisms of Action in Human Health and Disease (Second Edition) (eds. Ronald, R. W. et al.) 3–9 (Academic Press, 2018).
Patel, K. & Patel, D. K. Biological potential of aromadendrin against human disorders: recent development in pharmacological activities and analytical aspects. Pharmacol. Res. Mod. Chin. Med. 11, 100424. https://doi.org/10.1016/j.prmcm.2024.100424 (2024).
Islam, A., Islam, M. S., Rahman, M. K., Uddin, M. N. & Akanda, M. R. The pharmacological and biological roles of eriodictyol. Arch. Pharm. Res. 43, 582–592. https://doi.org/10.1007/s12272-020-01243-0 (2020).
Oršolić, N. Allergic inflammation: effect of propolis and its flavonoids. Molecules 27, 19 (2022).
Jean-Louis, D., Amos, S. & Simpson, D. In Pharmacognosy (Second Edition) (eds. Simone, B. M. & Yuri, N. C.) 295–309 (Academic Press, 2024).
Ruwizhi, N. & Aderibigbe, B. A. Cinnamic acid derivatives and their biological efficacy. Int. J. Mol. Sci. 21, 5712. https://doi.org/10.3390/ijms21165712 (2020).
Uçar, K. & Göktaş, Z. Biological activities of naringenin: a narrative review based on in vitro and in vivo studies. Nutr. Res. 119, 43–55. https://doi.org/10.1016/j.nutres.2023.08.006 (2023).
Ali, D. E. et al. In vivo anti-gastric ulcer activity of 7-O-methyl aromadendrin and sakuranetin via mitigating inflammatory and oxidative stress trails. J. Ethnopharmacol. 335, 118617. https://doi.org/10.1016/j.jep.2024.118617 (2024).
Stompor, M. A review on sources and pharmacological aspects of sakuranetin. Nutrients 12, 513. https://doi.org/10.3390/nu12020513 (2020).
Rasul, A. et al. Pinocembrin: a novel natural compound with versatile pharmacological and biological activities. Biomed. Res. Int. 2013, 379850. https://doi.org/10.1155/2013/379850 (2013).
Vechi, G. et al. Cryptostrobin and catechin isolated from Eugenia mattosii D. Legrand leaves induce endothelium-dependent and independent relaxation in spontaneously hypertensive rat aorta. Pharmacol. Rep. 71, 950–957. https://doi.org/10.1016/j.pharep.2019.05.006 (2019).
Pang, Z. et al. MetaboAnalyst 6.0: towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 52, 398. https://doi.org/10.1093/nar/gkae253 (2024).
Herbert-Pucheta, J. E. et al. Nuclear magnetic resonance metabolomics with double pulsed-field-gradient echo and automatized solvent suppression spectroscopy for multivariate data matrix applied in novel wine and juice discriminant analysis. Molecules 26, 56 (2021).
Lê Cao, K.-A., Boitard, S. & Besse, P. Sparse PLS discriminant analysis: biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinform. 12, 253. https://doi.org/10.1186/1471-2105-12-253 (2011).
Kim, S. et al. PubChem 2023 update. Nucleic Acids Res. 51, D1373–D1380. https://doi.org/10.1093/nar/gkac956 (2022).
Rutz, A. et al. The LOTUS initiative for open knowledge management in natural products research. Elife 11, e70780. https://doi.org/10.7554/eLife.70780 (2022).
Wang, G., Tang, W. & Bidigare, R. R. In Natural Products: Drug Discovery and Therapeutic Medicine (eds. Lixin, Z. & Arnold, L. D.) 197–227 (Humana Press, 2005).
Bae, E. et al. Study of double bond equivalents and the numbers of carbon and oxygen atom distribution of dissolved organic matter with negative-mode FT-ICR MS. Anal. Chem. 83, 4193–4199. https://doi.org/10.1021/ac200464q (2011).
Acknowledgements
The authors thank the Australian Native Bee Association, Sugarbag Bees and Australian beekeepers who generously donated their propolis to this study. T.D.T. acknowledges the Advance Queensland Industry Research Fellowship (AQIRF033-2023RD6), the Department of Industry, Science, Energy and Resources (ICG001958), the UniSC Launch Partnership, Native Beeings and the Van Rooyen Group for their financial support. We also acknowledge the Australian Research Council for funding the NMR instrument (ARC LE140100119) and the UniSC GROW for support towards an Agilent uHPLC-QTOF MS system.
Author information
Authors and Affiliations
Contributions
T.A.H., H.M.W. and T.D.T. contributed to project conceptualisation. T.A.H and T.D.T. contributed to sample collection. D.C.W. and M.A.O. contributed to experiments and data interpretation. T.D.T. supervised and administered the project. D.C.W. and T.D.T. wrote the original draft and all authors contributed to the review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Woods, D.C., Olsson, M.A., Heard, T.A. et al. Quality assessment and chemical diversity of Australian propolis from Tetragonula carbonaria and Tetragonula hockingsi stingless bees. Sci Rep 15, 17928 (2025). https://doi.org/10.1038/s41598-025-03011-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-03011-w