Abstract
CKD progression is associated with declining vitamin D levels, which correlate with various comorbidities. Total serum 25-hydroxyvitamin D (Total-25(OH)D) may not accurately reflect vitamin D deficiency in these patients. This study aimed to evaluate the vitamin D spectrum in CKD and kidney transplant recipients (KTRs). We recruited 38 stage 3 & 4 CKD patients and 38 patients with ESRD, who were followed for six-months post-KT. Total-25(OH)D and VDBP were measured using ELISA. Free and bioavailable-25(OH)D concentrations were calculated using Bikle formula. ESRD patients had lower total-25(OH)D, free-25(OH)D, and bioavailable-25(OH)D, compared to stage 3 & 4 CKD patients. VDBP levels showed no significant difference. Six months post-KT, a continued decline in free and bioavailable-25(OH)D was observed, whilst VDBP was increased. No significant change was found in total-25(OH)D levels. On regression analysis, bioavailable-25(OH)D and creatinine could independently predict CKD stage (based on eGFR). Total-25(OH)D, free-25(OH)D, and bioavailable-25(OH)D showed significant decline, with increasing severity of CKD. Notably, even after KT, they did not show significant improvement. Elevated VDBP post-KT probably contributed to continued decline in free and bioavailable-25(OH)D. Further research with extended follow-up is warranted to assess whether these alterations have a significant impact on long-term patient outcomes.
Similar content being viewed by others
Introduction
A progressive decline in functional nephrons is found in chronic kidney disease (CKD). With the progression of CKD to more advanced stages and eventually to end-stage renal disease (ESRD), the kidney’s ability to produce 1α-hydroxylase diminishes, which results in lower synthesis of 1,25-dihydroxyvitamin D (1,25(OH)2D) which is the active form of 25-hydroxyvitamin D (25(OH)D). These changes in vitamin D metabolism is an important contributor for low calcium levels and secondary hyperparathyroidism that result in CKD-mineral bone disorder (CKD-MBD)1.
Studies show that lower vitamin D concentrations are linked with several comorbidities in these patients. These include reduced bone mineral density, quicker progression of CKD, faster buildup of calcium in blood vessels, worsening of kidney transplant function, and an increased risk of cardiovascular disease and death2,3,4,5,6. According to the free hormone theory, hormones free from binding proteins exhibit biological activity, whereas hormones attached to proteins are comparatively inert. A lesser portion (10–15%) is weakly coupled to albumin, whereas the (85–90%) bulk of 25(OH)D and 1,25(OH)2D which is circulating in the blood is securely bound to vitamin D binding protein (VDBP). The quantity of vitamin D, which is not bound to protein in circulation, is less than 1%7.
Therefore, changes in the levels of VDBP or how strongly it binds to 25(OH)D can affect the concentration of free-25(OH)D available. The most accurate indicator of vitamin D deficiency may not be total-25 (OH) D concentrations. For instance, patients with high VDBP levels can seem to have enough 25(OH)D, but they might be lacking in a vitamin D that is bioavailable. On the other hand, individuals with low VDBP levels will have a decreased total-25(OH)D, but they may also have enough amounts of vitamin which is bioavailable8. Reports suggest that the bioavailable or free-25(OH)D has a stronger correlation with markers that are related to mineral metabolism than levels of total-25(OH)D8. The objective of our study was to evaluate total-25(OH)D, calculated free-25(OH)D, and bioavailable-25(OH)D levels and their relationship with VDBP in patients with CKD. We also studied how these parameters vary in different stages of CKD and in kidney transplant recipients (KTRs).
Results
Thirty-eight pre-dialysis CKD patients (stages 3 and 4) and 38 CKD patients in stage 5 undergoing dialysis and scheduled for renal transplantation were recruited in the study. Table 1 provides the research participants’ baseline demographic information. The median age of the pre-dialysis CKD patients was significantly larger than the patients with ESRD. Body mass index was not significantly different between the two groups. Compared to the CKD patients with stage 3 and 4 group, we observed that patients with ESRD had a significant increase in systolic blood pressure. We did not find any significant difference in the duration of the CKD between the groups.
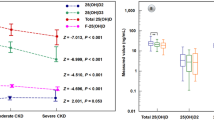
While comparing baseline biochemical parameters between patients with stage 3 and 4 CKD and patients with ESRD, we found a significant increase in creatinine and phosphorous in patients with ESRD. Calcium levels were significantly decreased in the patients with stage 3 and 4 CKD (Supplementary Table 1). Total-25(OH)D, free-25(OH)D, and bioavailable-25(OH)D levels were significantly decreased in the patients with ESRD compared to stage 3 and 4 CKD patients. VDBP levels were not significantly different between patients with ESRD and stage 3 and 4 CKD patients (Fig. 1).
We followed up the patients with ESRD at baseline for six months after their renal transplantation. When we compared the baseline biochemical parameters between the patients with ESRD and follow-up after six months of transplantation, we found a significant decrease in creatinine, phosphorous, alkaline phosphatase, and total protein. Calcium level and estimated glomerular filtration rate (eGFR) were higher in the KTRs compared to patients with ESRD on dialysis treatment (Supplementary Table 2). On follow-up, we found a significant reduction in free-25(OH)D and bioavailable-25(OH)D in the KTRs, compared to their levels before transplantation. VDBP levels were significantly higher in the KTRs than in patients with ESRD. We found no significant change in the total-25(OH)D levels (Fig. 2).
When we checked for the correlation between the study parameters in patients with stage 3 and 4 CKD and ESRD, we found a significant positive correlation between free-25(OH)D, bioavailable-25(OH)D, and total-25(OH)D. We also observed that eGFR has a significant positive correlation with free-25(OH)D, bioavailable-25(OH)D, and total-25(OH)D, indicating the reduction in these parameters with advancing stages of CKD. VDBP has a significant negative correlation with free-25(OH)D and bioavailable-25(OH)D (Supplementary Table 3).
Using the CKD stage based on eGFR as the dependent variable (in CKD cases prior to transplantation) and bioavailable-25(OH)D, creatinine, alkaline phosphatase, and BMI as independent predictor variables, a multiple linear regression model was constructed (Table 2) and we observed that bioavailable-25(OH)D (β = 0.142, p = 0.043) and creatinine (β = 0.241, p = 0.000) were independent predictors of CKD stage (based on eGFR).
Discussion
As kidney function declines, the body’s ability to maintain bone mineral homeostasis progressively diminishes. Vitamin D is necessary for maintaining bone health, and its insufficiency has been linked to a quicker progression of CKD9. Vitamin D acts by blocking the nuclear factor kappa B (NF-κB) and renin-angiotensin pathways, which lowers the risk of CKD and slows down its progression9. The NF-κB route stimulates inflammation and fibrogenesis, while the renin-angiotensin pathway causes vasoconstriction, fibrogenesis, kidney damage, and renal failure10,11.
In our study, we observed a significantly lower total-25(OH)D level in the patients with ESRD scheduled for kidney transplantation compared to the patients with stage 3 and 4 CKD. This result was concordant with the previous literatures, which reported that vitamin D levels were associated with the level of kidney function, especially in an advanced stage of CKD12,13,14. This may be due to the loss of VDBP and 25(OH)D in the urine of severe proteinuria patients15. However, total-25(OH)D levels were not correlated to serum VDBP in our study, which is concordant with Preka et al., and Kalousova et al.16,17. We also observed that total-25(OH)D was associated with lower concentrations of free and bioavailable-25(OH)D, implying that variables other than the urine loss of VDBP are probably involved in altered vitamin D metabolism. The observed reduction in 25(OH)D levels in ESRD patients compared to those with earlier CKD stages is a consequence of several interconnected physiological derangements. One of the primary contributors is the markedly reduced activity of renal 1α-hydroxylase, the enzyme that facilitates the conversion of 25(OH)D to 1,25(OH)₂D. In ESRD, the drastic loss of functional kidney tissue leads to a marked decrease in the availability and activity of this enzyme. While this primarily affects calcitriol production, it has downstream effects that can contribute to lower 25(OH)D levels over time18. Additionally, the substantial rise in fibroblast growth factor 23 (FGF-23) concentrations, which becomes more pronounced as CKD progresses and peaks in ESRD, plays a crucial role. Elevated FGF-23 inhibits 1α-hydroxylase activity, further reducing calcitriol production, and simultaneously induces 24-hydroxylase, an enzyme that facilitates the degradation of both 25(OH)D and 1,25(OH)₂D. As a result, increased FGF-23 levels in ESRD promotes the breakdown of vitamin D metabolites, thereby contributing to the reduced circulating levels of 25(OH)D19.
We observed no significant change in total 25(OH)D levels six months after kidney transplantation, consistent with findings from previous studies in KTRs20,21,22. Although the transplanted kidney adequately replaces the need for dialysis, it may not immediately restore all metabolic functions to optimal levels. Subtle impairments in vitamin D homeostasis may persist during the early post-transplant period. Previous study have reported that it may take up to 18 months for vitamin D status to improve following renal transplantation23. Our results align with earlier reports suggesting that immunosuppressive therapy may interfere with vitamin D metabolism, thereby limiting improvements in vitamin D levels despite the recovery of renal function24.
Vitamin D metabolism influences CKD risk, and the evidence for this relationship continues to increase. However, the underlying molecular mechanism is yet unknown. One emerging hypothesis centers on the regulatory effect of vitamin D within the renin-angiotensin-aldosterone system (RAAS), an important pathway responsible for maintaining blood pressure, vascular function, and kidney health25. Vitamin D serves as a negative endocrine regulator of RAAS25. This dysregulation is believed to contribute not only to hypertension but also to inflammation, fibrosis, and progressive renal damage.
Clinical studies have provided additional evidence for this link, revealing a negative relationship between serum vitamin D levels and both RAAS activation and blood pressure26. These findings suggest that disturbances in vitamin D homeostasis may promote CKD progression, at least in part, through RAAS-mediated mechanisms.
We found no significant difference in the VDBP level of the dialysis patients scheduled for kidney transplantation compared to the CKD stage 3 and 4 patients. Similar result was found by Prytula et al. and Preka et al. where serum VDBP levels were not significantly different in the pre-dialysis group and who were on peritoneal dialysis17,27. We found a significant increase in the VDBP level after six months of kidney transplantation. This result was concordant with Preka et al., who also found a similar result17. Even with low levels of 25(OH)D, the post-transplant group has high VDBP in our study. VDBP has a significant negative correlation with free-25(OH) D and bioavailable-25(OH)D. As the VDBP level increases, it binds with the total-25(OH)D leading to a reduction in free and bioavailable-25(OH)D. Generally, the complex of VDBP and total-25(OH)D is filtered through the glomerulus, and then it is reabsorbed by tubular epithelial cells at the brush border via receptor-mediated processes involving megalin and cubilin28. Low serum VDBP in our study may be because kidneys during renal failure have a reduced ability to transport VDBP from the glomerular filtrate into the renal tubules. Significant proteinuria can result in the loss of both VDBP and total-25(OH)D in the urine, as the megalin/cubulin transport system becomes overloaded. An increase in VDBP post-transplant can be attributed to the restoration of renal function post-transplant, which improves the reabsorption capacity of proximal tubular cells16,29,30.
Our study found a significant difference between the free-25(OH)D and bioavailable-25(OH)D levels between patients scheduled for kidney transplantation and pre-dialysis CKD cases. We also observed that eGFR has a significant positive correlation with free-25(OH)D and bioavailable-25(OH)D, indicating the reduction in these parameters with advancing stages of CKD. According to Denburg et al.‘s findings consistent with ours, children with CKD stages 2 to 5 had lower total and free-25(OH)D levels as their disease progressed. However, their study showed no notable differences in serum VDBP levels31. Both free-25(OH) D and bioavailable-25(OH) D levels were also found to be reduced after six months of kidney transplantation in our study. These results were concordant with Preka et al.17, who also found less free-25(OH)D and bioavailable-25(OH)D levels even after kidney transplantation. Although total-25(OH)D concentrations were not significantly altered, the elevated levels of VDBP in the post-transplant group likely contributed to the continued decline in free and bioavailable-25(OH)D levels.
A multiple linear regression model was developed to explore the relationship between the CKD stage measured by eGFR and the independent predictor variables: bioavailable-25(OH)D, creatinine, alkaline phosphatase, and BMI. These variables were chosen for their direct relevance to CKD pathophysiology and progression. Bioavailable-25(OH)D reflects the active form of vitamin D, which is essential for immune function and mineral metabolism, both critical in CKD contexts. Creatinine serves as a key indicator of renal function, directly reflecting the decline in glomerular filtration rate. Alkaline phosphatase is included to capture the comorbidities associated with CKD (disturbances in bone turnover and mineral metabolism and calcification). Lastly, BMI provides insights into adiposity-related metabolic risks that can accelerate kidney damage. Together, these variables offer a comprehensive view of the multifactorial processes underlying CKD severity. We found that creatinine and bioavailable-25(OH)D can independently predict CKD stage (based on eGFR: indicator of decreasing renal function). Together, these predictors provide valuable insights into how biochemical markers are linked to the pathogenesis and progression of chronic kidney disease.
Our findings indicate that bioavailable-25(OH)D could provide additional insights into vitamin D status and disease progression in CKD, beyond what is provided by total-25(OH)D alone. However, our study was limited by a small sample size. Additionally, although the six-month follow-up post-transplant provides valuable insights, it may not adequately reflect long-term changes in vitamin D metabolism or associated clinical outcomes. Hence, further research is needed with a larger cohort and extended follow-up duration, as these alternative markers in clinical assessment may help improve the understanding and management of vitamin D deficiency in CKD and post-transplant patients.
Conclusion
Our research indicates that levels of total-25(OH)D, free-25(OH)D, and bioavailable-25(OH)D decrease as the severity of CKD progresses. Notably, even after kidney transplantation, these levels did not show significant improvement. Although total-25(OH)D concentrations were not significantly altered, the elevated levels of VDBP in the post-transplant group probably contributed to the continued decline in free and bioavailable-25(OH)D levels. Further research with extended follow-up is necessary to assess whether these changes have a significant impact on long-term patient outcomes.
Methods
Study participants
This prospective observational study was conducted at a tertiary care centre in Puducherry. Based on the average number of ESRD patients who undergo renal transplantation annually at our centre in Puducherry; 38 patients were recruited for each group in the study. We recruited 38 consecutive pre-dialysis South Indian CKD patients in stages 3 and 4 (aged 18 to 70 years) who were attending the renal clinic during the study period. Exclusion criteria included pregnant or lactating women, patients with immobilization for more than three months, inherited diseases of bone, inherited causes of CKD, malignancy or neoplasia and active infections. Similarly, 38 consecutive ESRD patients undergoing maintenance dialysis and scheduled for renal transplantation were recruited from the nephrology outpatient department and were followed for six months post-transplantation. The Institutional Ethics Committee for Observational Studies approved the study. This study adhered to the Indian Council of Medical Research (ICMR) 2017 Ethical Guidelines for Biomedical Research involving Human Participants. Every study participant gave written informed consent, before their enrolment in the study.
Biochemical analysis
Graft function was assessed by the nephrologist through routine clinical evaluation. Biochemical parameters such as fasting blood sugar, creatinine, lipid profile, calcium, phosphorous, alkaline phosphatase, uric acid, total protein, and albumin were evaluated before transplantation and 6 months post-transplantation to evaluate renal function and overall metabolic status in cases. eGFR was estimated using the CKD-Epidemiology Collaboration (CKD-EPI) formula to assess the severity of CKD. Five millilitres of blood were drawn from each participant and transferred to an EDTA tube. The plasma was separated using centrifugation and kept in storage at -80 °C until further processing. Using commercially available ELISA kits, plasma concentrations of total-25(OH)D (Calbiotech, USA; Catalog no: VD220B, the standard curve range was between 2.5 ng/ml to 150 ng/ml, with a sensitivity of 2.5 ng/ml) and VDBP (Elabscience, USA; Catalog no: E-EL-H6167, the standard curve range was between 0.16 ng/ml to 10 ng/ml, with a sensitivity of 0.09 ng/ml) of all the research participants were measured in accordance with the manufacturer’s instructions. Standards and samples were run in duplicates, and the average of the values were taken to improve the repeatability of measurements. The absorbance was read using a microplate reader at 450 nm. The intra-assay and inter-assay coefficients of variation for the assays were within acceptable limits (< 10% for intra-assay and < 15% for inter-assay). To ensure validity and reproducibility, quality control samples provided in the kits were included during each run. Calibration curves were generated using the provided standards, and sample concentrations were calculated using curve-fitting software.
Plasma albumin, serum VDBP concentrations, and total-25(OH)D were used to calculate the free-25(OH) D concentration, based on the equation by Bikle et al.7. Plasma albumin and free- 25(OH) D concentrations were used to calculate the bioavailable-25(OH)D concentration7.
\({\text{Free-25}}\left( {{\text{OH}}} \right){\text{D }}={\text{ }}\frac{{{\text{Total-25}}\left( {{\text{OH}}} \right){\text{D}}}}{{{\text{1}}+{\text{ }}\left( {{{\text{K}}_{{\text{alb}}}}{\text{X Albumin}}} \right){\text{ }}+{\text{ }}\left( {{{\text{K}}_{{\text{VDBP}}}}{\text{X VDBP}}} \right)}}\)
\({\text{Bioavailable-25 }}\left( {{\text{OH}}} \right){\text{ D}}\,=\,{\text{Free-25 }}\left( {{\text{OH}}} \right){\text{D X }}({{\text{K}}_{{\text{alb}}}}{\text{X Albumin}}\,+\,{\text{1}})\)
Statistical analysis
We used IBM SPSS Statistics version 20 for our analysis. Frequencies and percentages were used to describe the categorical data. Continuous data were represented as the mean with standard deviations or the median with interquartile ranges. Parameters between pre-dialysis CKD patients and pre-transplant dialysis cases were examined using the independent Student’s t-test or the Mann-Whitney “U” test. The Wilcoxon test was used to compare the paired samples. The correlation between the studied parameters was examined using Spearman rank correlation. Multiple linear regression analysis was conducted to determine the independent factors which can predict CKD stage (based on eGFR). P < 0.05 was considered as statistically significant, and all the analyses were done at a 5% level of significance.
Data availability
Raw data for the dataset are not publicly available to preserve individuals’ privacy. However, it shall be made available by the corresponding author upon reasonable request.
References
Bhan, I. et al. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident Hemodialysis patients. Kidney Int. 82, 84–89 (2012).
Mucsi, I. et al. Serum 25(OH)-vitamin D levels and bone metabolism in patients on maintenance Hemodialysis. Clin. Nephrol. 64, 288–294 (2005).
Milinković, N. L. et al. Relation between 25(OH)-vitamin D deficiency and markers of bone formation and resorption in haemodialysis patients. Clin. Lab. 55, 333–339 (2009).
de Boer, I. H. et al. Serum 25-hydroxyvitamin D and change in estimated glomerular filtration rate. Clin. J. Am. Soc. Nephrol. 6, 2141–2149 (2011).
de Boer, I. H. et al. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J. Am. Soc. Nephrol. 20, 1805–1812 (2009).
Obi, Y. et al. Vitamin D deficiency predicts decline in kidney allograft function: a prospective cohort study. J. Clin. Endocrinol. Metab. 99, 527–535 (2014).
Bikle, D. D. et al. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J. Clin. Endocrinol. Metab. 63, 954–959 (1986).
Powe, C. E. et al. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J. Bone Miner. Res. 26, 1609–1616 (2011).
Ravani, P. et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 75, 88–95 (2009).
Li, Y. C. et al. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J. Steroid Biochem. Mol. Biol. 89–90, 387–392 (2004).
Sun, J. et al. Increased NF-kappaB activity in fibroblasts lacking the vitamin D receptor. Am. J. Physiol. Endocrinol. Metab. 291, E315–322 (2006).
Satirapoj, B., Limwannata, P., Chaiprasert, A., Supasyndh, O. & Choovichian, P. Vitamin D insufficiency and deficiency with stages of chronic kidney disease in an Asian population. BMC Nephrol. 14, 206 (2013).
Restrepo Valencia, C. A. & Aguirre Arango, J. V. Vitamin D 25(OH)D) in patients with chronic kidney disease stages 2–5. Colomb Med. (Cali). 47, 160–166 (2016).
Abdul Razzaque, M. R. et al. 25-Hydroxyvitamin-D deficiency in chronic kidney disease stages III, IV, and V in South Asian population: a retrospective cohort. SAGE Open. Med. 11, 20503121221148613 (2023).
Melamed, M. L. & Thadhani, R. I. Vitamin D therapy in chronic kidney disease and end stage renal disease. Clin. J. Am. Soc. Nephrol. 7, 358–365 (2012).
Kalousova, M. et al. Vitamin D binding protein is not involved in vitamin D deficiency in patients with chronic kidney disease. BioMed. Res. Int. 492365 (2015).
Preka, E. et al. Free 25-hydroxyvitamin-D concentrations are lower in children with renal transplant compared with chronic kidney disease. Pediatr. Nephrol. 35, 1069–1079 (2020).
Zappulo, F. et al. Vitamin D and the kidney: two players, one console. Int. J. Mol. Sci. 23, 9135 (2022).
Seiler, S., Heine, G. H. & Fliser, D. Clinical relevance of FGF-23 in chronic kidney disease. Kidney Int. Suppl. 76, S34–42 (2009).
Mehrotra, S., Sharma, R. K. & Patel, M. R. Vitamin D, 1,25-Dihydroxyvitamin D, FGF23, and graft function after renal transplantation. Indian J. Nephrol. 29, 242–247 (2019).
Stavroulopoulos, A., Cassidy, M. J. D., Porter, C. J., Hosking, D. J. & Roe, S. D. Vitamin D status in renal transplant recipients. Am. J. Transplant. 7, 2546–2552 (2007).
Korntip P., Nakul D., Joanna S., Erik L. & Suphamai B. Changes in vitamin D levels after kidney transplantation. Transplantation 102, S655–S656 (2018).
Filipov, J. J. & Dimitrov, E. P. Vitamin D after kidney transplantation: metabolism and clinical importance. EMJ Nephrol. 5, 75–82 (2017).
Filipov, J. J., Zlatkov, B. K., Dimitrov, E. P. & Svinarov, D. Relationship between vitamin D status and immunosuppressive therapy in kidney transplant recipients. Biotechnol. Biotechnol. Equip. 29, 331–335 (2015).
Li, Y. C. Vitamin D regulation of the renin-angiotensin system. J. Cell. Biochem. 88, 327–331 (2003).
Jacoby, D. S. & Rader, D. J. Renin-angiotensin system and atherothrombotic disease: from genes to treatment. Arch. Intern. Med. 163, 1155–1164 (2003).
Prytuła, A. et al. Urinary and dialysate losses of vitamin D-binding protein in children on chronic peritoneal dialysis. Pediatr. Nephrol. 27, 643–649 (2012).
Nykjaer, A. et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 96, 507–515 (1999).
Bikle, D. D. & Schwartz, J. Vitamin D Binding Protein, total and free vitamin D levels in different physiological and pathophysiological conditions. Front. Endocrinol. 10, 317 (2019).
Mirković, K. et al. Urinary vitamin D binding protein: a potential novel marker of renal interstitial inflammation and fibrosis. PLoS One. 8, e55887 (2013).
Denburg, M. R. et al. Vitamin D bioavailability and catabolism in pediatric chronic kidney disease. Pediatr. Nephrol. 28, 1843–1853 (2013).
Acknowledgements
Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India, provided funding for this study through an Intramural Research Grant [JIP/Res/Intramural/Phs2/2020-21] in the name of the corresponding author (MR).
Author information
Authors and Affiliations
Contributions
The study was designed and planned by MR and SP. The laboratory work was done by GP and MR. Statistical analysis was performed by GP and MR. Patient enrolment, clinical data collection, organisation and analysis was done by GP, MR, SP, JS, and SS. The manuscript was written by MR and GP. The manuscript was critically reviewed by all authors and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declaration
This study was approved by the Institutional Ethics Committee for Observational Studies (Project number: JIP/IEC/2019/ 0546) and conducted in concordance with the Indian Council of Medical Research (ICMR) Ethical Guidelines for Biomedical Research involving Human Participants, 2017. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Priyadarshini, G., Parameswaran, S., Sahoo, J. et al. The vitamin D spectrum: insights into 25(OH)D and VDBP in chronic kidney disease and post-transplant. Sci Rep 15, 18395 (2025). https://doi.org/10.1038/s41598-025-03035-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-03035-2