Abstract
Autoantibodies directed against carbamylated proteins (anti-CarP) have been recently identified as predictors for RA development. The aim of this study was to determine the positivity of anti-CarP antibodies in a real-life cohort of RA patients and their association with radiological damage, disability and disease activity. In this open-label, observational, cross-sectional study 69 RA patients and 16 healthy controls (HC) were recruited. The study was approved by institutional Ethical Committee. Circulating levels of anti-CarP antibodies were determined by commercial ELISA anti-CarP quantitative sandwich immunoassay. Articular X-rays were evaluated to define the presence of bone erosions. Disease activity (DAS28-CRP) and disability (HAQ-DI) were assessed. Pearson χ2 test, Wilcoxon test or Kruskall–Wallis test was used. Spearman rank-order correlation coefficient was applied for continuous variables. Multivariable logistic regression model was performed. Anti-CarP positivity was found in 35% of RA patients and in no HC. One quarter of seronegative RA patients were anti-CarP positive. A positive correlation between levels of anti-CarP antibodies with DAS28-CRP (p = 0.0003; Spearman r = 0.4829) and HAQ-DI (p = 0.0003; Spearman r = 0.4253) was found. 87% of anti-CarP positive patients had erosions. At multivariable logistic regression analysis, RA disease activity (OR 1.31, 95% CI [1.14, 1.63]) and circulating levels of anti-CarP antibodies (OR 1.66, 95% CI [1.28, 2.37]) were independent predictors of bone erosions. Our study confirms that anti-CarP antibodies are associated with a more aggressive RA course and are independent predictors of bone erosions.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune articular disease with systemic manifestations1. The positivity for rheumatoid factor (RF) and anti-citrullinated peptide antibodies (ACPA) in RA patients is fundamental for diagnostic purposes, and their circulating levels have been found to have a prognostic value2. While the role of RF and ACPA positivity has been established for a long time, more recently, a new class of autoantibodies—anti-carbamylated protein antibodies (anti-CarP)—has been suggested as a promising disease biomarkers3.
Carbamylation is a post-translational process which consists in the conversion of lysine to homocitrulline, in the presence of cyanate4. Renal failure is known to be associated with increased carbamylation of proteins due to increase in circulating urea levels5. Moreover, cigarette smoking, which is a relevant risk factor for RA development, not only favours citrullination but also increases carbamylation by raising the cyanate concentration6. Of note, it was shown that carbamylation can take place in the synovium, and can be enhanced in presence of chronic inflammation as the activation of myeloperoxidase, released from neutrophils, shifts the balance from thiocyanate to cyanate7. Given this background, and the close relationship between smoking, chronic inflammation and RA development, the role of carbamylation was investigated also in RA and it was found to be active in inflamed joints4,5,6,7. This had led to the development of commercially available essays for the detection of circulating anti-CarP antibodies.
Pecani et al. in their study that included a large cohort of Italian patients, confirmed the data already existing on the diagnostic accuracy of anti-CarP in RA and demonstrated that anti-CarP antibodies have relatively low sensitivity and slightly higher specificity (47% and 92%, respectively) compared to ACPA and RF8. These antibodies have been observed in patients with arthralgias and their presence was found to be a predictive factor for the development of RA independently of the positivity of ACPA, with which they partially cross-react9,10,11. Moreover, recent studies have also highlighted how anti-CarP can be predictive for the development of more severe phenotypes of the disease both in the general RA population and in ACPA negative RA patients3,12,13.
The aim of our study was to determine the frequency of positive anti-CarP antibody in a real-life cohort of RA patients on synthetic and biologic disease-modifying anti rheumatic drugs (DMARDs) and to evaluate the association between anti-CarP antibodies and radiological damage as well as disease activity and disability.
Materials and methods
The study was designed as open-label, observational which included consecutive 69 RA patients (classified according to the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) 2010 criteri14) on treatment with synthetic and biological DMARd, attending the Clinic of Rheumatology, Military Medical Academy, Belgrade (Serbia). The study was approved by the institutional Ethical Committee and all patients signed the Informed Consent. The research was performed in accordance with relevant guidelines and regulations.
The exclusion criteria were: presence of other systemic connective tissue diseases, fibromyalgia, compromised renal function. The control group consisted of 16 healthy individuals.
A structured questionnaire was used to collect the socio-demographic characteristics of the patients (age, gender and smoking habits).
Disease features, previous and concomitant treatments were recorded, while the disease activity score (DAS28-CRP)15 was determined for the assessment of RA activity, and the assessment of patients’ functional ability was performed using the Health Assessment Questionnaire Disability Index (HAQ-DI)16.
To assess the presence of bone erosions, x-rays of hands and feet performed within 3 months of the baseline visit were evaluated. Following laboratory parameters performed within 1 week of the baseline visit were evaluated in all RA patients: complete blood count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), serum creatinine level.
Circulating levels of anti-CarP antibodies (IgG) were determined by a commercial enzyme-like immunosorbent assay (ELISA) anti-CarP quantitative immunoassay (Novatein Biosciences, MA, USA). Anti-CarP antibody levels were expressed as ng/ml concentration values. As in most already published works, and according to the manufacturer’s recommendations8,17, values above the 95th percentile HC were considered positive -cut-off value 6 ng/ml. The presence and circulating levels of ACPA were determined by anti-CCP2 ELISA. The dosage of anti-CarP, ACPA and RF was performed as well within 1 week of the baseline visit in all RA patients. All HC were tested as well for both ACPA and RF.
Statistical analysis
The methods of descriptive and analytical statistics were used in statistical data processing. Youden’s index (maximum potential efficiency of biomarker when equal weight is given to both sensitivities and specificities derived from Receiver Operating Curve) is determined for the calculated cut-off value for the positivity of anti-CarP antibodies. Pearson χ2 test, Wilcoxon test or Kruskall-Wallis test was used, as appropriate. The Kruskal–Wallis test, a non-parametric method for comparing more than two independent groups, was used to compare anti-CarP levels across multiple independent patient subgroups due to the non-normal distribution of antibody titres and clinical parameters. Spearman rank-order correlation coefficient was applied for continuous variables. A multivariable logistic regression model was performed to assess prognostic variables on damage. The variables included in the multivariable logistic regression were smoking, disease activity, levels of ACPA, RF and anti-CarP. We used R Studio 4.3.0 for statistical analysis. Statistically significant differences were considered values of p < 0.05.
Results
A total of 69 RA patients were recruited. The mean age of patients with RA was 58 ± 11.22 (ranging from 28 to 70) years. The women were 56 (81%). In the HC group, the average age was 56 ± 4.28 (ranging from 30 to 66) years and 81% were women. Thirty-six patients (52%) were smokers (current + former) with a mean of 22 ± 2.15 packs/year (P/Y). In HC group there were 10 (62%) smokers with a mean P/Y of 20 ± 2.59.
The mean RA duration was 8.0 ± 0.9 (ranging from 2 to 33) years. Firty-four patients (78%) were on methotrexate (MTX), of whom 27 (50%) on MTX monotherapy, 15 (28%) on combined therapy with MTX + a second conventional synthetic DMARD (13 hydroxychloroquine, 2 sulfasalazine), 12 (22%) on MTX + biologic DMARD (4 etanercept, 2 adalimumab, 4 tocilizumab and 2 rituximab). Eleven (16%) patients were on therapy only with antimalarials, 3 (4%) on leflunomide and 1 (2%) on sulfasalazine. Fifty-six (81%) patients were also on glucocorticoids with a mean dose of 7.5 ± 3.38 mg (ranging from 5 to 15 mg of prednisone or equivalent).
Erosions were detected in forty-six (67%) patients. The mean value of DAS 28-CRP was 3.7 ± 0.13 (ranging from 1.3 to 6.4): 9 (13%) patients were in remission, 14 (20%) with low disease activity, 36 (52%) with moderate disease activity, and 10 (15%) with high disease activity. Average HAQ-DI value was 0.7 ± 0.04 (ranging from 0.3 to 2): low to moderately impaired functional status was observed in 49 (71%) patients, and 20 (29%) patients had moderate to severe impairment. Out of 69 patients, 17 (25%) were negative for both, RF and ACPA. All HC were negative for both ACPA and RF. Demographic, lifestyle and clinical characteristics of RA and HC are summarized in Table 1.
Presence of anti-CarP
Cut-off value for positivity of anti-CarP antibodies, calculated on the basis of the 95thpercentile HC, was 6 ng/ml. Youden’s index for cut-off value was 0.285, sensitivity 35%, specificity 94%. Based on the obtained cut-off value, 24/69 (35%) patients with RA had positive anti-CarP antibodies. In the HC group, none was positive for anti-CarP antibodies.
No statistically significant correlation was found neither between the circulating levels of anti-CarP antibodies and gender (p = 0.15), nor between the concentration of anti-CarP antibodies and the disease duration (p = 0.93; Spearman r = − 0.0097).
Correlation of anti-Carp with disease features
One quarter (4/17) of seronegative RA patients were anti-CarP positive. Moreover, these patients had a moderate disease activity (DAS28-CRP 3.60 ± 0.58) and a high degree of disability (HAQ-DI 0.70 ± 0.13) and were all characterized by X-ray evidence of erosions. Anti-CarP circulating levels were compared between seropositive and seronegative RA patients, however there was no significant difference.
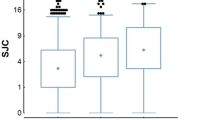
In all RA patients, the circulating levels of anti-CarP antibodies were significantly higher in smokers than in non-smokers (6.7 ± 0.83 vs 3.9 ± 0.55 ng/ml, p = 0.005) (Fig. 1), and a positive correlation between anti-CarP circulating levels and DAS28 CRP (p = 0.0003; Spearman r = 0.4829) was found. (Fig. 2).
In addition, in all patients a positive correlation was also found between anti-CarP antibody circulating levels and HAQ-DI (p = 0.0003; Spearman r = 0.4253) (Fig. 3).
Erosions were present in 67% of RA patients and in 87% of anti-CarP positive patients. Moreover, the circulating levels of anti-CarP antibodies were significantly higher in patients with erosions comparing to those without erosions (6.6 ± 0.67 vs 2.7 ± 0.41 ng/ml, p = 0.0002) (Fig. 4).
Multivariable regression analysis
The multivariable logistic regression model suggested that disease activity (OR 1.31, 95% CI [1.14, 1.63]) and higher circulating levels of anti-CarP antibodies (OR 1.66, 95% CI [1.28, 2.37]) were independent predictors for erosions. Although ACPA and RF were included in the multivariable model, neither was identified as a significant predictor of erosions.
Discussion
Our study confirms that in RA patients anti-CarP antibodies not only may have a diagnostic role especially in RF and ACPA seronegative patients, but its levels are associated with a higher disease activity, disability and presence of erosions.
RA is a heterogeneous inflammatory joint disease with an outcome that can range from mild impaired functional state to complete disability18,19. It is acknowledged that the identification of RA-specific autoantibodies is crucial for a better understanding of the disease and for patients’ stratification20. Two autoantibodies, RF and ACPA, are pivotal for diagnostic and prognostic importance. In some studies, RF and especially ACPA were associated with high disease activity, higher degree of disability and increased mortality20,21,22.
Recently, new autoantibodies directed against carbamylated proteins have been identified in RA patients3. So far, it has been shown that anti-CarP antibodies can precede the first symptoms of the disease up to 10 years and may occur before and after the development of ACPA23,24. Moreover, if present in patients with arthralgias, they are predictive for the development of RA independently from ACPA positivity, with which they were partially cross-reactive9,10,11,23,24. More recently, Ricchiuti et al.25 showed that anti-CarP antibodies can be detected in ACPA, RF and 14-3-3 eta both positive and negative patients. Anti-CarP may predict the development of a more severe form of the disease and radiological damage in total and ACPA negative population of RA patients3,9,12. Moreover, anti-CarP antibodies were associated with increased mortality, mainly caused by complications of respiratory diseases, in Spanish RA patients26. In addition, anti-CarP antibodies were associated with interstitial lung disease (ILD) in RA27. While it has been proven their predictive significance and the association with radiological damage3,12,13,23,24, their diagnostic value in seronegative patients and the link with disease activity, disability and risk factors is still a matter of research. A possible limitation for the progress of these researches is the consequence of the absence of a standardized method to detect their positivity and circulating levels.
Given that the clinical impact of these antibodies is still under investigation, our aim was to determine the frequency of positive anti-CarP antibody in a real-life cohort of RA patients on synthetic and biologic DMARds and the association between anti-CarP antibodies with radiological damage, disease activity and disability.
In a recent meta-analysis of 12 relevant studies, anti-CarP antibodies were detected in 34–80% of RA patients, and their isolated positivity was found in approximately one-third of seronegative RA patients28,29,30. Our data are in agreement with the literature, showing the presence of these antibodies in a significant number of patients with RA (35%), with one quarter of our seronegative RA patients positive for anti-CarP. Of note, all anti-CarP positive ACPA seronegative patients in our cohort had radiological evidence of erosions, a moderate disease activity (DAS28 CRP 3.60 ± 0.58) and a high degree of disability (HAQ-DI 0.70 ± 0.13) thus suggesting that anti-CarP antibodies may have a fundamental clinical impact on RA seronegative patients.
Currently, other studies have linked anti-CarP antibodies to disease activity, but the bulk of data is still limited and additional studies are needed to extend our knowledge about the association of anti-CarP antibodies with disease activity in RA. In the work of Kumar et al.31 the presence of anti-CarP antibodies was not significantly associated with DAS28 in RA patients, while Yee et al.12 argued that biomarkers (RF, ACPA and anti-CarP antibodies) were not separately associated with disease activity, and that anti-CarP antibodies combined with ACPA fine specificities correlated with DAS28. In patients with early inflammatory arthritis (Norfolk Arthritis Register), Humphreys et al.32, found a higher disease activity in anti-CarP positive patients. In 2019, Elsayed et al.33 found a significant correlation between anti-CarP titer and DAS28 in RA patients, and more recently, Zhang et al.34 showed that the level of anti-CarP antibody positively correlated with DAS28. Recently, Elsawy et al.35 found that anti-CarP antibodies are associated with higher disease activity, increased disability, fatigability and decreased physical activity in premenopausal RA women. In our study a positive correlation between anti-CarP antibody circulating levels and DAS28-CRP in all RA patients (p = 0.0003; Spearman r = 0.4829) was found, confirming that anti-CarP antibodies are associated with higher disease activity. We find that is important to note that although data on ACPA were available, we did not explore whether the presence of both anti-CarP and ACPA correlates with worse clinical outcomes. This remains an important area for future research, particularly in the context of antibody cross-reactivity.
Examining the association of anti-CarP antibodies with functional status, Othman et al.30 showed that anti-CarP positive patients were more incapacitated than anti-CarP negative ones, as already suggested by two other studies32,35. Our results clearly show a positive correlation between the circulating levels of anti-CarP antibodies and HAQ-DI in all RA patients (p = 0.0003; Spearman r = 0.4253).
Smoking can theoretically increase myeloperoxidase activity, and by doing this can promote the carbamylation of the protein in animal models36. Jiang et al.37 and Koppejan et al.38 did not find any association between smoking and anti-CarP antibodies. One multicenter cohort study found that smoking is related with the coexisting presence of multiple autoantibodies associated with RA and that represents the risk factor for breaking tolerance to multiple autoantigens in RA39. Verheul et al.40 found that anti-CarP antibody levels are not increased in heavy smokers. On the other hand, in our study a significantly higher circulating levels of anti-CarP antibodies were found in smokers (p = 0.005).
Radiological damage was associated with anti-CarP antibodies, both as radiological progression and as damage at certain stages of the disease course3,12,13. We observed that circulating levels of anti-CarP antibodies are significantly higher in patients with bone erosions. This evidence may further corroborate the hypothesis that higher circulating levels of anti-CarP antibodies may predict joint damage. This result is consistent with previous studies and confirms the association between anti-CarP antibodies and radiological damage, thus suggesting that anti-CarP might be of fundamental importance for prognosis. Furthermore, the multivariable logistic regression model suggested that the disease activity and the higher circulating levels of anti-CarP antibodies were significant predictors for the presence of erosions. In patients with active disease (OR 1.31, 95% CI [1.14, 1.63]) and with a higher circulating levels of anti-CarP (OR 1.66, 95% CI [1.28, 2.37]) in fact a greater occurrence of erosions was detected. Interestingly, ACPA and RF were not significant predictors of radiographic erosions in our cohort, whereas anti-CarP levels and disease activity (DAS28) were. This suggests that anti-CarP may provide additional prognostic value beyond traditional serological markers.
In addition to anti-CarP antibodies, several other biomarkers have been associated with joint erosion in RA, including 14-3-3η protein, anti-PAD4 antibodies, MMP-3, and anti-RA33 antibodies (41, 42, 43, 44). These findings underscore the multifactorial nature of erosive disease progression in RA and the need for comprehensive biomarker profiling.
Our study has some limitations. The fact that standardized antigen assay is not yet available as well as the cut-off values are still to be defined may limit the clinical impact of our results. Another limitation is posed by the use of a fetal calf serum-based antigen without subtraction of native signal, which may impact specificity. Moreover, our cross-sectional study cannot establish a temporal relationship between anti-CarP antibodies, radiological progression, therapy, disease activity and functional status. Indeed, the sample size is relatively small and heterogeneous, and therefore does not allow a better comparison between subgroups (therapy, disease activities, functional status). Another potential weakness is the limited number of healthy controls. Our findings are based on a monocentric cohort with a relatively small sample size, which may limit the generalizability of the results to other populations. Differences in genetic background, environmental exposures, and disease management strategies across geographic regions may influence the distribution and clinical relevance of anti-CarP antibodies. Therefore, further validation in larger and more diverse cohorts is warranted. However, our data are obtained from consecutive patients and may therefore reflect the real life we experience every day in our clinics.
Our data confirm in RA the diagnostic and prognostic role of anti-CarP antibodies. However further and larger studies are warranted to better determine the clinical significance of anti-CarP antibodies.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
References
Scott, D. L., Wolfe, F. & Huizinga, T. W. Rheumatoid arthritis. Lancet 376, 1094–1108 (2010).
Conigliaro, P. et al. Autoantibodies in inflammatory arthritis. Autoimmun Rev. 15(7), 673–683 (2016).
Shi, J., Knevel, R., Suwannalai, P., van der Linden, M. P., Janssen, G. M., van Veelen, P. A. et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc. Natl. Acad. Sci. USA. 2011;108(42):17372–7.
Derksen, V. F. A. M., Huizinga, T. W. J. & van der Woude, D. The role of autoantibodies in the pathophysiology of rheumatoid arthritis. SeminImmunopathol. 39(4), 437–446 (2017).
Wynckel, A. et al. Kinetics of carbamylated haemoglobin in acute renal failure. Nephrol Dial Transplant. 15, 1183–1188 (2000).
Wang, Z. et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 13, 1176–1184 (2007).
Mydel, P. et al. Carbamylation-dependent activation of T cells: A novel mechanism in the pathogenesis of autoimmune arthritis. J Immunol. 184, 6882–6890 (2010).
Pecani, A. et al. Prevalence, sensitivity and specificity of antibodies against carbamylated proteins in a monocentric cohort of patients with rheumatoid arthritis and other autoimmune rheumatic diseases. Arthritis Res Ther. 18(1), 276 (2016).
Shi, J. et al. Anti-carbamylated protein antibodies are present in arthralgia patients and predict the development of rheumatoid arthritis. ArthritisRheum. 65, 911–915 (2013).
Shi, J. et al. Recognition of citrullinated and carbamylated proteins by human antibodies: Specificity, cross-reactivity and the “AMC-Senshu” method. Ann Rheum Dis 72, 148–150 (2013).
Brevet, P. et al. Anti-carbamylated fibrinogen antibodies might be associated with a specific rheumatoid phenotype and include a subset recognizing in vivo epitopes of its γ chain one of which is not cross reactive with anti-citrullinated protein antibodies. Front Immunol 12, 733511 (2021).
Yee, A. et al. Anti-CarP antibodies as promising marker to measure joint damage and disease activity in patients with rheumatoid arthritis. Immunol Res. 61(1–2), 24–30 (2015).
Brink, M. et al. Anti-carbamylated protein antibodies in the presymptomatic phase of rheumatoid arthritis, their relationship with multiple anticitrulline peptide antibodies and association with radiological damage. Arthritis Res Ther. 17, 25 (2015).
Aletaha, D. et al. 2010 Rheumatoid arthritis classification criteris: An American College of Rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis 69, 1580–1588 (2010).
Prevoo, M. L., van Riel, P. L. et al. Validity and reliabilitiy of joint indices. A longitudinal study in patients with recent onset rheumatoid arthritis. Br. J. Rheumatol. 1993;32(7):589–594.
Bruce, B. & Fries, J. F. The Stanford Health Assessment Questionnaire: Dimensions and practical applications. Health Qual Life Outcomes. 1, 20 (2003).
Iacono, D. et al. Low mortality rate in Italian rheumatoid arthritis patients from a tertiary center: Putative implication of a low anti-carbamylated protein antibodies prevalence. Open Access Rheumatol. 10, 129–134 (2018).
van der Helm-van Mil, A. H., Breedveld, F. C., Huizinga, T. W. Aspects of early arthritis. Definition of disease states in early arthritis: remission versus minimal disease activity. Arthritis Res. Ther. 2006;8:216.
Humphreys, J. H., Warner, A., Chipping, J., Marshall, T., Lunt, M., Symmons, D. P., Verstappen, S. M. Mortality trends in patients with early rheumatoid arthritis over 20 years: Results from the Norfolk Arthritis Register. Arthritis Care Res. (Hoboken) 2014;66:1296–301.
De Rycke, L. et al. Rheumatoid factor and anticitrullinated protein antibodies in rheumatoid arthritis: Diagnostic value, associations with radiological progression rate, and extra-articular manifestations. Ann Rheum Dis 63, 1587–1593 (2004).
Shidara, K. et al. Anti-cyclic citrullinated peptide antibody predicts functional disability in patients with rheumatoid arthritis in a large prospective observational cohort in Japan. RheumatolInt 32, 361–366 (2012).
Humphreys, J. H. et al. Rheumatoid factor and anti-citrullinated protein antibody positivity, but not level, are associated with increased mortality in patients with rheumatoid arthritis: results from two large independent cohorts. Arthritis Res Ther. 16, 483 (2014).
Shi, J. et al. Anti-carbamylated protein (anti-CarP) antibodies precede the onset of rheumatoid arthritis. Ann Rheum Dis 73, 780–783 (2014).
Gan, R. W. et al. Anti-carbamylated protein antibodies are present prior to rheumatoid arthritis and are associated with its future diagnosis. J Rheumatol 42, 572–579 (2015).
Ricchiuti, V. et al. Anti-carbamylated protein (anti-CarP) antibodies in patients evaluated for suspected rheumatoid arthritis. Diagnostics (Basel). 12(7), 1661 (2022).
Vidal-Bralo, L. et al. Anti-carbamylated protein autoantibodies associated with mortality in Spanish rheumatoid arthritis patients. PLoS ONE 12(7), e0180144 (2017).
Oka, S. et al. Serum rheumatoid factor IgA, anti-citrullinated peptide antibodies with secretory components, and anti-carbamylated protein antibodies associate with interstitial lung disease in rheumatoid arthritis. BMC Musculoskelet Disord. 23(1), 46 (2022).
Verheul, M. K., Böhringer, S., van Delft, M. A. M., Jones, J. D., Rigby, W. F. C., Gan, R. W. et al. Triple positivity for anti-citrullinated protein autoantibodies, rheumatoid factor, and anti-carbamylated protein antibodies conferring high specificity for rheumatoid arthritis: implications for very early identification of at-risk individuals. Arthritis Rheumatol. 2018;70(11):1721–1731.
Montes, A. et al. Anti-carbamylated protein antibodies as a reproducible independent type of rheumatoid arthritis autoantibodies. PLoS ONE 11, e0161141 (2016).
Othman, M. A., Ghazali, W. S. W., Hamid, W. Z. W. A., Wong, K. K., Yahya, N. K. Anti-carbamylated protein antibodies in rheumatoid arthritis patients and their association with rheumatoid factor. Saudi Med. J. 2017;38(9):934–941.
Kumar, S., Pangtey, G., Gupta, R., Rehan, H. S. & Gupta, L. K. Assessment of anti-CarP antibodies, disease activity and quality of life in rheumatoid arthritis patients on conventional and biological disease-modifying antirheumatic drugs. Reumatologia 55(1), 4–9 (2017).
Humphreys, J. H., Verheul, M. K., Barton, A., MacGregor, A. J., Lunt, M., Toes, R. E. et al. Association of anti-carbamylated protein antibodies with long-term disability and increased disease activity in patients with early inflammatory arthritis: results from the Norfolk Arthritis Register. Lancet. 2015;385Suppl 1:S44.
Elsayed, S. A., Esmail, M. A., Ali, R. M. & Mohafez, O. M. Diagnostic and prognostic value of anti-CarP antibodies in a sample of Egyptian rheumatoid arthritis patients. ClinRheumatol. 38(10), 2683–2689 (2019).
Zhang, B. et al. Elevated levels of anti- carbamylated protein antibody in patients with rheumatoid arthritis: association with disease activity and bone destruction. J Investig Med. 68(6), 1186–1192 (2020).
Elsawy, N. A., Mohamed, R. A., Ghazala, R. A., Abdelshafy, M. A., Elnemr, R. Anti-carbamylated protein antibodies in premenopausal rheumatoid arthritis women: relation to disease activity and bone loss. Rheumatology (Oxford). 2020 Sep 30:keaa549.
Pruijn, G. J. Citrullination and carbamylation in the pathophysiology of rheumatoid arthritis. Front Immunol. 6, 192 (2015).
Jiang, X. et al. Anti-CarP antibodies in two large cohorts of patients with rheumatoid arthritis and their relationship to genetic risk factors, cigarette smoking and other autoantibodies. Ann Rheum Dis 73, 1761–1768 (2014).
Koppejan, H. et al. Role of anti-carbamylated protein antibodies compared to anti-citrullinated protein antibodies in indigenous North Americans with rheumatoid arthritis, their first-degree relatives, and healthy controls. Arthritis Rheumatol. 68(9), 2090–2098 (2016).
van Wesemael, T. J. et al. Smoking is associated with the concurrent presence of multiple autoantibodies in rheumatoid arthritis rather than with anticitrullinated protein antibodies per se: A multicenter cohort study. Arthritis Res Ther 18, 285 (2016).
Verheul, M. K. et al. Anti-carbamylated protein antibodies: A specific hallmark for rheumatoid arthritis. Comparison to conditions known for enhanced carbamylation; renal failure, smoking and chronic inflammation. Ann Rheum Dis. 75(8), 1575–1576 (2016).
Maksymowych, W. P. et al. 14-3-3η is a novel mediator associated with the pathogenesis of rheumatoid arthritis and joint damage. Arthritis Res Ther. 16(2), R99 (2014).
Hammam, N., Salah, S., Kholef, E. F., Moussa, E. M. & Marotta, A. 14-3-3η Protein in serum and synovial fluid correlates with radiographic damage and progression in a longitudinal evaluation of patients with established rheumatoid arthritis. Mod Rheumatol. 30(4), 664–670 (2020).
Kor, A. et al. Does eta protein differentiate rheumatoid arthritis from psoriatic arthritis?. Curr Med Chem. 31(39), 6510–6520 (2024).
Šenolt, L., Grassi, W. & Szodoray, P. Laboratory biomarkers or imaging in the diagnostics of rheumatoid arthritis?. BMC Med. 12, 49 (2014).
Acknowledgements
The authors gratefully acknowledge the support of clinical staff and nurses at Department of Rheumatology and Clinical Immunology and of Institute for Medical Research of the Military Medical Academy-Belgrade, Serbia and the patients.
Author information
Authors and Affiliations
Contributions
MM- study conception; acquisition, analysis and interpretation of data. CC- analysis and interpretation of data. BG, MP and GRstudy conception; acquisition, analysis and interpretation of data. IS and DVanalysis and interpretation of data. NV- analysis and interpretation of data. MMC and LD- study conception; analysis and interpretation of data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Markovic, M., Campochiaro, C., Glisic, B. et al. Association of circulating levels of anti-CarP antibodies with disease activity, disability and radiological damage in rheumatoid arthritis patients: an open-label, observational study. Sci Rep 15, 18325 (2025). https://doi.org/10.1038/s41598-025-03464-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-03464-z