Abstract
Bruch´s membrane (BM) is firmly connected posteriorly to the optic nerve head through the peripapillary choroidal border tissue, and anteriorly through the longitudinal ciliary muscle to the scleral spur. We assessed, whether a difference in the contractile state of the ciliary muscle influences the position of the posterior BM by lifting the posterior BM pole, i.e., induces changes in the subfoveal choroidal thickness (SFCT). Healthy young adult individuals received one drop of cyclopentolate 1% into their right eyes and one drop of pilocarpine 1% into their left eyes. Using optical coherence tomography (OCT), three examiners measured independently SFCT and choroidal thickness in the fundus midperiphery at baseline and 30 min after eye drop instillation. The study included 21 healthy individuals (age:21.9 ± 2.6 years; range:15.7–25.8 years; axial length:24.4 ± 1.2 mm). In the right eyes, SFCT changed by 8.7 ± 34.9 μm (examiner 1), -2.9 ± 18.6 μm (examiner 2), and 10.5 ± 21.8 μm (examiner 3), respectively, and the midperipheral choroidal thickness changed by -10.6 ± 25.9 μm (examiner 1), 0.9 ± 17.5 μm (examiner 2), and 4.2 ± 24.7 μm (examiner 3), respectively, without significant differences between the measurements taken before and after eye drop application (all P > 0.05). In the left eyes, SFCT changed by 5.8 ± 22.2 μm (examiner 1), 5.5 ± 36.5 μm (examiner 2), and 3.9 ± 29.5 μm (examiner 3), respectively, and the midperipheral choroidal thickness changed by -6.9 ± 47.9 μm (examiner 1), -3.5 ± 28.7 μm (examiner 2), and 16.0 ± 28.2 μm (examiner3), respectively, without significant differences between baseline and study end (all P > 0.05). Application of cyclopentolate 1% and of pilocarpine 1% did not result in a statistically significant change in choroidal thickness in young healthy adults.
Similar content being viewed by others
Introduction
Accommodation occurs through a contraction of the ciliary muscle and results in an increase in the refractive power of the lens through a change in the lens shape. The longitudinal part of the ciliary muscle is anteriorly firmly attached to the scleral spur and is posteriorly connected with Bruch´s membrane (BM) in the posterior part of the pars plana region1,2,3,4. Any shortening of the longitudinal ciliary muscle may thus result in an anterior movement of the pars plana and of the BM5,6,7. Since BM forms the anterior border of the choroidal compartment, an anterior movement of BM may change the thickness of the choroid at the posterior pole and in other fundus regions. Previous studies have examined the choroidal thickness-related effect of drugs applied topically and influencing the status of the ciliary muscle8,9,10,11,12,13,14,15,16,17,18,19,20.
Based on the results of the previous studies mentioned above, and considering the anatomical unit between BM and the longitudinal ciliary muscle, inserted at the scleral spur anteriorly and at the peripapillary choroidal border tissue posteriorly, a hypothesis is formulated that an accommodation-related contraction of the longitudinal ciliary muscle may be forwarded to BM, pull BM slightly anteriorly, and may thus indirectly affect the posterior choroidal thickness and the length of the optical axis. We conducted this study to examine the effect of topically applied drugs, influencing the contractile status of the ciliary muscle, on posterior choroidal thickness and axial length. As drugs we used pilocarpine, which is a non-selective muscarinic receptor agonist and which leads to a contraction of the ciliary muscle, and cyclopentolate, which is a muscarinic antagonist and leads to a relaxation of the ciliary muscle.
Methods
The clinical study included healthy young adult individuals with a best corrected distant and near visual acuity of at least 20/20, normal intraocular pressure, a cylindrical refractive error and an anisometropia of one diopter or less, and an unremarkable ophthalmological state. Amblyopia and strabismus were exclusion criteria. The study was conducted in accordance with the tenets of the Declaration of Helsinki and it was approved by the Ufa Eye Research Institute Ethics Committee. All study participants signed an informed consent form.
Upon slit lamp-based biomicroscopy and ophthalmoscopy, the anterior and posterior ocular segments including the optic nerve, macula and peripheral retina were examined. We performed ocular biometry using laser interferometric biometry (AL-Scan, Nidek Co, Ltd, Gamagory, Japan). Axial length was measured in millimeter and was defined as the length of the optical axis as distance between the corneal outer surface and the retinal pigment epithelium / BM in the foveola. The measurements were performed 5 times, and the mean of the readings was taken for further statistical analysis. The range of accommodation was measured by the push-away method, push-up method, and minus-lens method21. Images of the posterior ocular segment were taken by optical coherence tomography (SS-OCT (DRI-OCT, Triton; Topcon Inc., Tokyo, Japan). The foveola was imaged by a horizontal scan line comprising 100 single B scans. The scan length was 9 mm. The depth resolution of the images was 2.6 μm pixel. The foveal center was defined as the thinnest central point of the retina.
All examinations were performed in the morning between 9:00 am and 12:00 am, to decrease the risk of effects of diurnal factors. In addition, the study participants had to have refrained from smoking or drinking coffee or tea for the whole morning of the examination day, and they had refrained from any physical exercise including climbing staircases for at least one hour before start of the examinations.
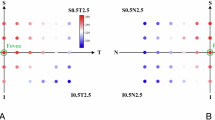
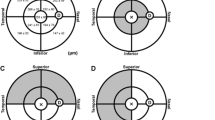
We measured manually the subfoveal choroidal thickness (SFCT) in µm on the horizontal OCT section running through the foveal center. It was defined as the vertical distance between BM line and the inner scleral surface (Fig. 1). We additionally measured manually the choroidal thickness on OCT images obtained from the fundus midperiphery at approximately 45° peripheral to the foveola (Fig. 2). The reason for measuring choroidal thickness also in the fundus midperiphery was that any change in choroidal thickness in the foveal region may be associated with a change in regions closer to the equator of the globe, although associations between peripheral choroidal thickness and axial elongation have been started to be explored only recently22,23,24,25. The reason for choosing the location of approximately 45° peripheral to the fovea for determining the peripheral choroidal thickness in our study was that that region was still roughly assessable and visualizable by the OCT device used in our study. The images obtained at study end were taken in follow-up mode based on the images taken before the eye drops had been applied. The choroidal thickness measurements were performed in a masked manner by three examiners independently of each other and without knowledge whether the image was taken at baseline of the study and at study end. We did not correct the choroidal thickness measurements for axial length and refractive power of cornea and lens of the individual eyes, since according to a physical principle of OCT and its basis, low-coherence interferometry, magnification of a fundus structure imaged by OCT applies only in the transverse dimension but not in the axial dimension26,27,28,29. As also discussed and explored by Salmon and colleagues, axial measurements, such as choroidal thickness determinations, may therefore not needed to be adapted to the optical characteristics of the eyes examined26,27,28,29,30. The variability in the SFCT measurements was assessed by measuring the SFCT on the OCT images of 5 individuals 10 times by 5 examiners independently of each. The coefficient of variation was calculated as the ration of the mean of the mean values divided by the mean of the standard deviations.
The right eyes of the study participants received one drop of cyclopentolate 1% and the left eyes one drop of pilocarpine 1%. The OCT examination and ocular biometry were performed at baseline and at 30 min after the instillation of the eye drops.
The statistical analysis was conducted using a statistical software program (IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY). We calculated the mean values and their standard deviations of the main outcome parameters, i.e. change in axial length and change in SFCT. We assessed the normal distribution of the values of the measured parameters using the Kolmogorov-Smirnov test. For the parameters with a normal distribution, we used the paired t-test to examine the statistical significance of differences in the measurements obtained at baseline and measurements obtained at study end. For parameters without a normal distribution of their values, we applied the Wilcoxon test.
Results
The clinical study included 21 healthy individuals (6 men, 15 women) with a mean age of 21.9 ± 2.6 years (range: 15.7–25.8 years) and a mean axial length of 24.4 ± 1.2 mm (range: 22.18–26.02 mm) in the right eyes and 24.4 ± 1.2 mm (range: 22.12–26.14 mm) in the left eyes. Best corrected visual acuity ranged between 20/20 and 30/20 for both eyes. Mean intraocular pressure was 14.9 ± 2.8 mm Hg (range: 8–19 mm Hg) and 15.2 ± 3.1 mm Hg (range: 10–21 mm Hg) for the right eyes and left eyes, respectively. In the right eyes and left eyes, the mean spherical refractive error was − 2.73 ± 0.62 diopters (D) (range: +0.25 D to -4.75 D) and − 2.95 ± 1.68 D (range: 0.00 to -5.00 D), respectively, with a mean cylindric refractive error of -0.25 ± 0.14D and − 0.32 ± 0.19 D, respectively. Upon slit lamp-based biomicroscopy and ophthalmoscopy, the anterior and posterior ocular segments including the optic nerve, macula and peripheral retina were unremarkable. The range of accommodation was 7.8 ± 1.5 cm for the pull-away method, 8.6 ± 1.4 cm for the push-up method, and 6.2 ± 1.4 diopters for the minus-lens method. As measured by OCT, SFCT was at baseline of the study 277.1 ± 84.6 μm (range: 174–460 μm) and 272.1 ± 70.5 μm (range: 179–425 μm) in the right eyes and left eyes, respectively (as measured by examiner #2).
After the instillation of cyclopentolate into the right eyes, the measurements of subfoveal choroidal thickness (SFCT) changed by 8.7 ± 34.9 μm (examiner 1), -2.9 ± 18.6 μm (examiner 2), and 10.5 ± 21.8 μm (examiner 3), respectively, without a significant difference between the measurements taken at baseline and the measurement performed after the eye drop instillation (P = 0.27, P = 0.55, and P = 0.12), respectively (student´s t-test for paired samples) (Table 1). Correspondingly, the difference between the SFCT measurements obtained before and after the drop instillation did not vary significantly between examiner 1 and examiner 2 (P = 0.20), between examiner 2 and examiner 3 (P = 0.06), and between examiner 3 and examiner 1 (P = 0.60 (student´s t-test for paired samples)).
Axial length measured in the right eyes at baseline 24.38 ± 1.19 mm and at study end 24.38 ± 1.19 mm, without a significant difference (0.00 ± 0.01 mm; P = 0.08 (student´s t-test for paired samples) (Table 2).
After the instillation of cyclopentolate into the right eyes, the measurements obtained in the fundus midperiphery changed by -10.6 ± 25.9 μm (examiner 1), 0.9 ± 17.5 μm (examiner 2), and 4.2 ± 24.7 μm (examiner 3), respectively, without a significant difference (P = 0.07, P = 0.84, and P = 0.44, respectively (student´s t-test for paired samples)). Correspondingly, the difference between the measurements obtained before and after the drop instillation did not vary significantly between examiner 1 and examiner 2 (P = 0.20), between examiner 2 and examiner 3 (P = 0.06), and between examiner 3 and examiner 1 (P = 0.60) (student´s t-test for paired samples).
After the instillation of pilocarpine into the left eyes, the measurements of subfoveal choroidal thickness (SFCT) changed by 5.8 ± 22.2 μm (examiner 1), 5.5 ± 36.5 μm (examiner 2), and 3.9 ± 29.5 μm (examiner 3), respectively, without a significant difference (P = 0.25, P = 0.57, and P = 0.55, respectively (student´s t-test for paired samples)). Correspondingly, the difference between the SFCT measurements obtained before and after the drop instillation did not vary significantly between examiner 1 and examiner 2 (P = 0.89), between examiner 2 and examiner 3 (P = 0.80), and between examiner 3 and examiner 1 (P = 0.80) (student´s t-test for paired samples).
After the instillation of pilocarpine into the left eyes, the measurements of choroidal thickness obtained in the fundus midperiphery changed by -6.9 ± 47.9 μm (examiner 1), -3.5 ± 28.7 μm (examiner 2), and 16.0 ± 28.2 μm (examiner 3), respectively, without a significant difference (P = 0.53, P = 0.65, and P = 0.06 (after Bonferroni correction), respectively (student´s t-test for paired samples)). Correspondingly, the difference between the SFCT measurements obtained before and after the drop instillation did not vary significantly between examiner 1 and examiner 2 (P = 0.22), between examiner 2 and examiner 3 (P = 0.06), and between examiner 3 and examiner 1 (P = 0.05) (student´s t-test for paired samples).
Axial length measured in the left eyes at baseline 24.36 ± 1.24 mm and at study end 24.37 ± 1.24 mm, without a significant difference (0.01 ± 0.01 mm; P = 0.10).
The coefficient of variation of the re-measurements of the SFT was 16.3 / 349.3 or 0.047.
Discussion
In our study on young individuals, neither medical activation of the ciliary muscle by pilocarpine 1% nor its relative relaxation by cyclopentolate 1% led to a measurable, statistically significant change in the thickness of the choroid, determined at the posterior pole beneath the foveola, nor in the fundus midperiphery. It suggests that the medical influence of the muscle tone of the longitudinal part of the ciliary muscle as influenced by pilocarpine 1% or cyclopentolate 1% was not significantly associated with a detectable change in the position of the posterior BM pole as determining by choroidal thickness.
The present study fits into a series of previous investigations which have addressed a potential association between SFCT and topically applied drugs influencing the ciliary muscle and accommodation. Zhang and colleagues applied atropine 1% twice daily for a week in children and found a significant increase in subfoveal choroidal thickness, while there was no significant association between the changes in choroidal thickness and changes in axial length8. Öner et al. used topical tropicamide and cyclopentolate applied three times in healthy adult individuals. Choroidal thickness increased in the cyclopentolate group while the tropicamide group did not show a significant change9. Chiang and associates reported that in young adult moderately myopic individuals, topical atropine abolished a choroidal thinning induced by a hyperopic defocus. The authors discussed that atropine reduced the effect of a hyperopic defocus on choroidal thickness, e.g., by preventing accommodation10. In another study, Chiang et al. observed in children that myopic or hyperopic defocus caused a thickening and thinning of the choroid, respectively12. When the children had additionally received 0.3% atropine eye drops daily for a period of 6 months, choroidal thickening had further increased in eyes with a myopic defocus, while choroidal thinning decreased in eyes with a hyperopic defocus. Sander et al. observed a mean subfoveal choroidal thinning of 11 ± 2 μm after imposing a hyperopic defocus, while the application of 0.01% atropine was associated with a reduction in the hyperopic defocus-related choroidal thinning11. Eyes receiving 0.01% atropine eye drops without resenting a defocus showed a thickening of the choroid by 6 ± 2 μm. Changes in axial length were inversely correlated with the changes in SFCT. Maltsev and colleagues found in healthy young adult individuals after application of 1% pilocarpine eye drops a choroidal thinning, more marked in eyes with shorter axial length and thicker choroid13. Also in other studies, topical application of atropine eye drops was associated with choroidal thickening in children14,15,17,18. In contrast, Bahar et al. reported that the topical application of pilocarpine 2% increased choroidal thickness in young adult individuals16, Ye reported that topically applied cyclopentolate (in contrast to atropine eye drops) was associated with a choroidal thinning17, Zhou found that topical atropine eye drops were not associated with a significant change in choroidal thickness in hyperopic children18, and Kobia-Acquah observed a choroidal thinning after application of 1% cyclopentolate eyedrops19. In an experimental investigation, Mathis and associates reported that atropine injected intravitreally was associated with a transient increase in choroidal thickness in chicken, concurrent with an increase in the intraretinal dopamine concentration20. In recent investigations conducted by Croft and colleagues, eye drops of homatropine 5% applied to one eye and pilocarpine 4% given to the contralateral eye of 12 individuals with an age of 18 to 51 years led to a thickening of the lens and a thinning of the choroid, moving away from the optic nerve head6,7. Correspondingly, SFCT was negatively associated with lens thickness. Interestingly, the accommodative change in SFCT and the accommodation-related choroidal movement did not decline with age. Croft et al. discussed that the age-related choroidal thinning (observed in their investigations and in other studies) might have been due to changes in the geometry of the accommodative apparatus to which it is attached, such as the ciliary muscle and the lens complex. It could explain the reverse relationship between accommodative lens thickening and subfoveal choroidal thinning. An accommodative decrease in choroidal thickness and stretch of the retina/choroid might suggest that accommodation-associated forces of stress and strain in the region of the optic nerve may have implications for glaucoma.
In view of the potential anatomical relationship between the scleral spur, longitudinal ciliary muscle and BM the parapapillary end of which eventually connects to the peripapillary choroidal border tissue, any contraction of the longitudinal ciliary muscle may lead to an anterior movement of BM. It could cause a widening of the subfoveal choroidal space (i.e., choroidal thickening). As a corollary, relaxation of the longitudinal ciliary muscle could be associated with a backward movement of the posterior BM, i.e. subfoveal choroidal thinning, as observed in the study performed by Croft et al.5. While Croft et al. considered the choroid as the structure conveying the ciliary muscle force to the posterior ocular segment, the anatomical relationships discussed above may suggest BM as the main biomechanical structure connecting the ciliary body with the posterior pole. The anatomical connection between BM and the longitudinal ciliary muscle may also lead to a re-discussion of considering a thickening or swelling of the choroid as part of the process of accommodation31,32,33.
From a clinical point of view, the findings of our study may indicate that medical mydriasis may not markedly influence the results of the axial length measurements. It may have importance for cataract surgery. The results of our study may also be helpful for the interpretation of the subfoveal choroidal thickness measurements in patients with a pachychoroid-related or leptochoroid-related diseases, such as central serous choroidopathy or myopic macular degeneration.
Limitations of our study have to be taken into account. First, the concentrations of the applied drugs of cyclopentolate and pilocarpine were relatively low, so that a potential effect of the contractile status of the ciliary muscle on choroidal thickness might have become noticeable if higher concentrations had been used. Second, reproducible choroidal thickness measurements cannot be performed with an accuracy of 5 μm or less µm, so that a potential effect might have been hidden in the noise of the measurements. Third, we did not check whether any participant was a non-responder to a particular drug, e.g., by checking the change in the pupil size after the instillation of the eye drops. Fourth, the number of study participants was relatively low, so that a fine difference might have escaped from statistical significance. Fifth, one may discuss whether the algorithm incorporated into the imaging device was sufficient for correction of the magnification of the fundus image by the optic media of the eye. Since however we compared OCT images obtained before and after the application of the drugs, any systemic error might have compensated itself. Sixth, the study did not have a control group without drug application. Future studies may apply higher doses of the drugs applied and a higher number of participants also including a control group. These studies may re-address the question of a potential association between the ciliary muscle contractile status and choroidal thickness, and they may explore the biomechanical importance of the potential anatomical unit of scleral spur-longitudinal ciliary muscle-BM-peripapillary choroidal border tissue- peripapillary scleral flange- posterior sclera.
In conclusion, the application of cyclopentolate 1% and of pilocarpine 1% did not result in a statistically significant change in choroidal thickness.
Data availability
The data will be available upon reasonable request to the corresponding author.
References
Rohen, J. W. Über den Ansatz der Ciliarmuskulatur im Bereich des Kammerwinkels. Ophthalmologica 131, 51–60 (1956).
Tamm, E., Lütjen-Drecoll, E., Jungkunz, W. & Rohen, J. W. Posterior attachment of ciliary muscle in young, accommodating old, presbyopic monkeys. Invest. Ophthalmol. Vis. Sci. 32, 1678–1692 (1991).
Tamm, E. R. The trabecular meshwork outflow pathways: Structural and functional aspects. Exp. Eye Res. 88, 648–655 (2009).
Jonas, J. B., Jonas, R. A., Jonas, S. B. & Panda-Jonas, S. Bruch´s membrane and Brücke’s muscle in the pars plana region. Acta Ophthalmol. 102 (1), e53–e59 (2024).
Croft, M. A. et al. Accommodative movements of the vitreous membrane, choroid, and sclera in young and presbyopic human and nonhuman primate eyes. Invest. Ophthalmol. Vis. Sci. 54, 5049–5058 (2013).
Croft, M. A. et al. Accommodative movements of the choroid in the optic nerve head region of human eyes, and their relationship to the lens. Exp. Eye Res. 222, 109124 (2022).
Croft, M. A. et al. Intraocular accommodative movements in monkeys; relationship to presbyopia. Exp. Eye Res. 222, 109029 (2022).
Zhang, Z. et al. The effect of topical atropine on the choroidal thickness of healthy children. Sci. Rep. 6, 34936 (2016).
Öner, V., Bulut, A. & Öter, K. The effect of topical anti-muscarinic agents on subfoveal choroidal thickness in healthy adults. Eye 30, 925–928 (2016).
Chiang, S. T. & Phillips, J. R. Effect of atropine eye drops on choroidal thinning induced by hyperopic retinal defocus. J. Ophthalmol. 2018, 8528315 (2018).
Sander, B. P., Collins, M. J. & Read, S. A. Short-term effect of low‐dose atropine and hyperopic defocus on choroidal thickness and axial length in young myopic adults. J. Ophthalmol. 2019, 4782536 (2019).
Chiang, S. T., Turnbull, P. R. & Phillips, J. R. Additive effect of atropine eye drops and short-term retinal defocus on choroidal thickness in children with myopia. Sci. Rep. 10, 18310 (2020).
Maltsev, D. S., Kulikov, A. N. & Vasiliev, A. S. Effect of topical pilocarpine on choroidal thickness in healthy subjects. Optom. Vis. Sci. 97, 457–461 (2020).
Ye, L. et al. Effects of atropine treatment on choroidal thickness in myopic children. Invest. Ophthalmol. Vis. Sci. 61, 15 (2020).
Jiang, Y. et al. Change and recovery of choroid thickness after short-term application of 1% atropine gel and its influencing factors in 6-7-year-old children. Curr. Eye Res. 46, 1171–1177 (2021).
Bahar, A. & Pekel, G. The effects of pharmacological accommodation and cycloplegia on axial length and choroidal thickness. Arq. Bras. Oftalmol. 84, 107–112 (2021).
Ye, L. et al. Comparisons of atropine versus cyclopentolate cycloplegia in myopic children. Clin. Exp. Optom. 104, 143–150 (2021).
Zhou, Y. et al. Changes of choroidal thickness in children after short-term application of 1% atropine gel. Ophthalmic Res. 66, 421–430 (2023).
Kobia-Acquah, E., Flitcroft, D. I., Lingham, G., Kerin, E. & Loughman, J. Short‐term effects of cyclopentolate and tropicamide eye drops on macular choroidal thickness in myopic children. Ophthalmic Physiol. Opt. 44, 280–291 (2024).
Mathis, U., Feldkaemper, M. P. & Schaeffel, F. Effects of single and repeated intravitreal applications of atropine on choroidal thickness in alert chickens. Ophthalmic Res. 64, 664–674 (2021).
Majumder, C. & Afnan, H. Amplitude of accommodation among students of a Malaysian private university as assessed using subjective and objective techniques. Korean J. Ophthalmol. 34, 219–226 (2020).
Hoseini-Yazdi, H., Vincent, S. J., Collins, M. J. & Read, S. A. Alonso-Caneiro, D. Wide-field choroidal thickness in myopes and emmetropes. Sci. Rep. 9, 3474 (2019).
Yazdani, N. et al. Wide-field choroidal thickness and vascularity index in myopes and emmetropes. Ophthalmic Physiol. Opt. 41, 1308–1319 (2021).
Funatsu, R. et al. Normal peripheral choroidal thickness measured by widefield optical coherence tomography. Retina 43, 490–497 (2023).
Pusti, D. et al. Peripheral choroidal response to localized defocus blur: influence of native peripheral aberrations. Invest. Ophthalmol. Vis. Sci. 65, 14 (2024).
Kennedy, S. J., Schwartz, B., Takamoto, T. & Eu, J. K. Interference fringe scale for absolute ocular fundus measurement. Invest. Ophthalmol. Vis. Sci. 24, 169–174 (1983).
Izatt, J. A. & Choma, M. A. Theory of optical coherence tomography. in Optical Coherence Tomography: Technology and Applications (eds Drexler, W. & Fujimoto, J. G.) 47–72 (Springer Berlin Heidelberg, 2008).
Häusler, G. & Lindner, M. W. Coherence radar and spectral radar-new tools for dermatological diagnosis. J. Biomed. Opt. 3, 21–31 (1998). (1998).
Wojtkowski, M., Leitgeb, R., Kowalczyk, A., Bajraszewski, T. & Fercher, A. F. In vivo human retinal imaging by Fourier domain optical coherence tomography. J. Biomed. Opt. 7, 457–463 (2002).
Salmon, A. E., Sajdak, B. S., Atry, F. & Carroll, J. Axial scaling is independent of ocular magnification in OCT images. Invest. Ophthalmol. Vis. Sci. 59, 3037–3040 (2018).
Wallman, J. et al. Moving the retina: Choroidal modulation of refractive state. Vis. Res. 35, 37–50 (1995).
Nickla, D. L. & Wallman, J. The multifunctional choroid. Prog. Retin. Eye Res. 29, 144–168 (2010).
Ostrin, L. A. et al. IMI-The dynamic choroid: New insights, challenges, and potential significance for human myopia. Invest. Ophthalmol. Vis. Sci. 64, 4 (2023).
Author information
Authors and Affiliations
Contributions
Design: MMB, GMB, JBJ; Examination of study participants: MMB, GMB, AN, GRM; Providing means: MMB, GMK; Statistical analysis: SPJ, JBJ; Writing of the first manuscript draft: SPJ, JBJ; Improving and final approval of the manuscript: MMB, GMB, SPJ, AN, GRM, JBJ.
Corresponding author
Ethics declarations
Competing interests
Jonas JB, Panda-Jonas S: European patent EP 3 271 392, JP 2021-119187, and US 2021 0340237 A1: „Agents for use in the therapeutic or prophylactic treatment of myopia or hyperopia. All other authors do not have a competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bikbov, M.M., Kazakbaeva, G.M., Panda-Jonas, S. et al. Choroidal thickness under pilocarpine versus cyclopentolate. Sci Rep 15, 2221 (2025). https://doi.org/10.1038/s41598-025-85712-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-85712-w