Abstract
To determine the five-year changes in intraocular pressure (IOP) and their related factors among individuals aged 40 to 64 years. The present report is part of the Shahroud Eye Cohort study. The sampling process for this study utilized a multi-stage cluster sampling method within Shahroud, Iran. Optometric and ophthalmologic examinations included measurement of uncorrected and best-corrected visual acuity, non-cycloplegic autorefraction and slit-lamp biomicroscope. IOP was measured using a Goldman tonometer. A total of 7384 eyes from 3855 individuals were examined in this report. The average five-year change in IOP was −0.06±2.23 mmHg (95% confidence interval: −0.14 to 0.02). The 25, 75, 95, and 99th percentiles of five-year IOP changes were −1, 1, 4, and 5 mmHg, respectively. According to the multivariate generalized estimating equation (GEE) model, the five-year changes in IOP were positively associated with female gender (coefficients(β)=0.139; P=0.036), blood pressure (β=0.40; P<0.001), diabetes (β=0.539; P<0.001), body mass index (β=0.023; P<0.001), axial length (β=0.104;P<0.007), central corneal thickness (β=0.010; P<0.001), mean keratometry (β=0.0067; P<0.001), and lens thickness at baseline (β=0.182; P<0.047). Moreover, baseline IOP exhibited an inverse association with five-year changes in IOP (β=−0.808; P<0.001). Despite a 4 mmHg increase in IOP observed in 5% of the study participants, the average change in IOP over five years was deemed not clinically or statistically significant. Nevertheless, biometric indices are important for anticipating variations in IOP.
Similar content being viewed by others
Introduction
Approximately 3.54% of individuals between the ages of 40 and 80 years are estimated to have glaucoma, and it is anticipated that the global number of individuals with glaucoma will reach around 111.8 million by the year 20401. After cataracts, glaucoma is the second leading cause of blindness worldwide2, with an estimated 3.6 million people experiencing blindness as a result of this condition3. Abnormal intraocular pressure (IOP) is a crucial risk factor in the development of glaucoma, and monitoring its fluctuations is essential in managing glaucoma patients4. Numerous studies have identified various factors that influence changes in IOP, including age, sex, central corneal thickness (CCT), history of cataract surgery, body mass index (BMI), and systemic diseases5. Several cross-sectional studies have investigated the changes in IOP with age, a topic of considerable importance. Nevertheless, the results of these studies frequently exhibit discrepancies among each other5,6,7,8.
Various studies have demonstrated varying outcomes across different racial groups when examining the correlation between IOP changes and age. While research indicates a positive association between IOP and age in Caucasian and African populations in cross-sectional studies9, findings suggest that IOP tends to decrease with age among Asians10. There is a scarcity of longitudinal studies focusing on the elderly population; Han et al. conducted a study on Chinese individuals aged over 40 years, revealing an average five-year IOP change of 0.43 mm Hg., their findings suggested a lack of linear correlation between IOP changes and age11. Additionally, the mentioned study highlighted that individuals with higher blood pressure, higher BMI, increased fasting glucose levels, and those who experienced myopia progression were at a higher risk of developing elevated IOP11.
Various studies have provided evidence that the production of aqueous humor decreases as individuals grow older, leading to a decline in IOP; On the other hand, age-related alterations in the trabecular meshwork’s structure, which heighten resistance to the outflow of aqueous humor, can ultimately elevate IOP12. It is worth noting that the equilibrium between these two phenomena may vary across different populations. Changes in IOP with age may be different across different racial groups, highlighting the influence of race on these alterations13. However, it is important to acknowledge that existing studies have serious limitations in that they have not thoroughly investigated the changes in IOP within a healthy population. Furthermore, the impact of demographic, ocular, and systemic factors on IOP changes has not been thoroughly explored. Limited longitudinal studies have investigated the changes in IOP in normal and healthy populations.
Given the significance of glaucoma and its primary risk factor, the present study aims to explore the alterations in IOP over a period of five years among individuals aged between 40 and 64 years in Shahroud, Iran. Additionally, the study seeks to establish any potential associations between these changes and various systemic, demographic, and ocular parameters.
Materials and methods
The first phase of the Shahroud Eye Cohort Study was carried out in a cross-sectional manner in 2009, while the second phase took place in 2014. The focus of this study encompassed individuals between the ages of 40 and 64 years residing in the city of Shahroud, northeast Iran. The details of the methodology and sampling have already been reported in detail14, however, they are briefly mentioned in this section. During the first phase, a total of 300 clusters were chosen using a multi-stage cluster sampling method, with a minimum of 20 individuals from each cluster being systematically incorporated into the research. All examinations were conducted at a single location. In total, 6311 people were invited in the first phase, out of which 5190 individuals participated in the study. The second phase was carried out five years later in 2014, where all participants from the first phase were invited to partake in the second phase. In the second phase, the examinations were administered at the identical location and setting as the initial phase. Upon the participation of each subject in the study, demographic information, smoking history, and medical and ocular records were gathered. Subsequently, all participants underwent optometric and ophthalmological examinations as well as imaging procedures.
Similar to the first phase, during the second phase, the Nidek ARK-510A auto-refractometer (Nidek ARK-510A, Fremont, CA, USA) was used to measure non-cycloplegic refraction, followed by the measurement of uncorrected distance and near visual acuity. Subsequently, an optometrist utilized the Heine Beta 200 retinoscope (Heine Beta 200 retinoscope, HEINE Optotechnic, Hersching, Germany) to refine the auto-refraction results. Using the information obtained up to this stage, individuals with uncorrected visual acuity worse than 20/20 underwent subjective refraction at distance and near to determine the best-corrected visual acuity. Biometry was then performed with the Allegro BioGraph (Allegro Biograph, WaveLight AG, Erlangen, Germany). In the next step, a slit-lamp examination (Slit Lamp BM900, HAAG STREIT USA) was performed by an ophthalmologist, and IOP was measured with a Goldman applanation tonometer. Lens examination and cataract diagnosis and classification were performed by the ophthalmologist according to the Lens Opacities Classification System III. In the next step, the fundus photographs were taken (AFC 230 Fundus Camera, Nidek, Chiyoda-ku, Japan), and finally, OCT imaging was performed under dilated pupillary conditions using Tropicamide 1% drops.
Exclusion criteria
This report excluded individuals who had a previous diagnosis of glaucoma, had undergone glaucoma surgery, used glaucoma medications, or had prior ocular surgeries.
Definitions
Refractive errors were determined based on the spherical equivalent (SE) of cycloplegic refraction in the first phase of the study15. Myopia and hyperopia were defined as SEs worse than −0.50 and +0.50 diopter (D), respectively. Lens opacity was graded at the slit lamp according to the LOCS III. Similar to some other studies, nuclear opacity of grade 4 or higher, cortical opacity of grade 2 or higher, and posterior subcapsular (PSC) opacity of grade 2 or higher were considered cataracts.
Statistical analysis
This report provided information on the mean, standard deviation (SD), and 95% confidence interval (CI) of IOP separately in the first and second phases of the study, as well as the changes in IOP between these phases (Phase 2-phase1). The five-year changes in IOP were also reported by age, sex, refractive errors, cataracts, and some demographic and systemic variables. The cluster sampling method was considered in calculating the standard error. Due to the low correlation of fellow eyes in IOP, the results of both eyes were analyzed, univariate and multivariate generalized estimating equation (GEE) analyses were also used to search for the association between five-year IOP changes and other variables.
GEE is an extension of generalized linear models (GLM) designed for analyzing correlated data, particularly in longitudinal studies where repeated measures are taken from the same subjects. In our analysis, we employed GEE to account for the paired nature of intraocular pressure measurements from both eyes of each participant, allowing us to handle correlations within subjects while estimating population-averaged effects effectively. We specified a Gaussian distribution with an identity link function for our continuous outcome variable, IOP. The working correlation structure was chosen based on prior knowledge and exploratory data analysis, often utilizing an exchangeable correlation structure to model within-subject correlations. Importantly, GEE provides robust estimates even if the working correlation structure is misspecified.
Factors influencing IOP changes were identified after assessing collinearity and interaction between variables in the final model.
Ethical considerations
Shahroud Eye Cohort Study was approved by the ethics committee of Shahroud University of Medical Sciences (Reference numbers: IR.SHMU.REC. 1398.039). All methods were carried out in accordance with the Helsinki tenets and other relevant guidelines and regulations. Written informed consent was obtained from all participants.
Results
The first phase of the study recruited 6311 individuals, with 5190 individuals ultimately participating in the first phase. In the second phase, 453 individuals didn’t participate due to various reasons such as death, migration, and unwillingness, resulting in a total of 4737 individuals participating, yielding a response rate of 91.3%. After applying exclusion criteria, a total of 7384 eyes from 3855 individuals were analyzed in this report. Among these, 2242 (58.2%) were female, and the average age of these individuals in the second phase was 50.36 ± 6.07 years (range: 45 to 69 years).
The data in Table 1 illustrates the mean, SD, and 95% CI of IOP during the first phase, second phase, and the five-year changes in IOP by various study variables. In the overall sample, the mean five-year change in IOP was −0.06 ± 2.23 mmHg with a 95% CI ranging from − 0.14 to 0.02. The distribution of IOP changes is visually represented in (Fig. 1). The skewness and kurtosis values for the five-year changes in IOP were −0.066 and 0.335, respectively. Percentile values for the five-year changes in IOP were determined as follows: 25th percentile at −1 mmHg, 75th percentile at 1 mmHg, 95th percentile at 4 mmHg, and 99th percentile at 5 mmHg.
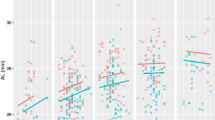
The average five-year change in IOP was − 0.07 ± 2.22 mmHg (95% CI −0.18 to 0.05) in males and −0.06 ± 2.24 mmHg (95% CI −0.16 to 0.04) in females. The IOP changes exhibited a non-linear association with age. Figure 2 displays the IOP changes over five years according to the IOP levels at the baseline (phase 1). It is evident from Fig. 2 that higher baseline IOP was linked to a greater reduction in IOP after five years.
Table 2 shows the association between the five-year changes in IOP with various demographic, systemic, and ocular variables in the first study phase, as analyzed through univariate and multivariate GEE models. The model’s results indicate that women, individuals with higher blood pressure, and those with diabetes experienced a higher increase in IOP after five years. Additionally, BMI, axial length, CCT, mean keratometry, and lens thickness at baseline were directly associated with the five-year increase in IOP. Furthermore, as seen in Table 2, the baseline IOP had an inverse association with the five-year changes in IOP.
Discussion
The present study is among a limited number of studies that investigated the alterations in IOP over five years in individuals aged 40 years and above. According to the results, the average IOP during the first and second phases of the study were 12.83 and 12.73 mmHg, respectively (with an average five-year change of 0.06 mmHg); these changes are deemed to be of minimal clinical and statistical significance.
Evaluation of prior research indicates that age is one of the most important factors influencing IOP changes, with a notable increase in IOP levels observed after the fourth decade of life. Wong et al. demonstrated a rise in IOP between the ages of 40 and 60 years, followed by a decline thereafter8. Chan et al. found that IOP increases with age from 40 to 69 years16. However, Ghanbarnia et al.’s research indicated a decrease in IOP between the ages of 60 and 90 years17. Besides, a study by Hashemi et al. revealed a non-linear trend in IOP changes among individuals aged 60 to 95 years, with an increase in the rate of IOP change observed in those above 75 years of age5. This variability in results can be attributed to the differences in age and race among the populations being examined, as well as the varying levels of control over confounding variables. Moreover, the majority of studies have investigated these changes in a cross-sectional manner which emphasizes the importance of conducting longitudinal cohort studies. For example, in the Lingtou Eye Cohort study conducted by Han et al., the initial cross-sectional report from phase I showed lower IOP values in older individuals compared to younger individuals whereas a follow-up assessment after two years indicated an increase in IOP7.
Chua et al. examined the alterations in IOP over six years among individuals aged 40 to 80 years. Their findings showed that six years of advancing age was associated with a decrease in the average IOP by 0.50 mmHg18. Kashiwagi et al. also reported that individuals aged 40 years and older experienced a reduction in IOP of approximately 0.6 mmHg over a ten-year period19. Asaoka et al. conducted an eight-year study to assess alterations in IOP among individuals with an average age of 52.7 years. Their findings revealed a decrease of 0.08 mmHg in IOP for each year of advancing age20. These findings align with those of the current research, which showed a reduction of 0.06 mmHg in average IOP over a five-year period. In most longitudinal studies, including the present study, a slight decrease in IOP with advancing age has been consistently observed. The exclusion of individuals with pathological conditions, glaucoma, or suspected glaucoma from our study sample supports the notion that this decline in IOP is to be expected. Consequently, it is unlikely for IOP to rise in the absence of any ocular pathology or predisposition to glaucoma; Although the observed mean changes in the present study show a statistical decrease in the amount of IOP, nevertheless, we can ignore the above changes from a clinical point of view in total population in absence of ocular pathologies.
However, the 25, 75, 95, and 99th percentiles of the changes in IOP over the same five-year period were 1, 1, 4, and 5 mmHg, respectively. It became evident that there exists a distinct pattern of IOP changes in certain individuals as opposed to the average trend observed across the entire sample. In other words, approximately a quarter of the participants in the present study exhibited at least a 1 mmHg rise in IOP, while 5% of participants encountered an increased IOP of 4 mmHg. These findings indicate that a small proportion of individuals aged 40 years and older are at risk of experiencing a significant increase in IOP, highlighting the importance of regular glaucoma monitoring for this group. Han et al. demonstrated a 0.43 mmHg increase in the average IOP over four years in individuals aged 40 years and above11. The mentioned study highlighted that performing analysis with the assumption of non-linear changes of IOP across various age groups, as well as factors like differences in aqueous humor secretion and ocular anatomy among different populations may have contributed to the observed findings11. In fact, it can be said that the mean changes do not represent the changes in the whole society and there is a need for regular evaluation of IOP in all people in this age range.
In the multivariate GEE analysis, the amount of IOP in both investigated phases was higher in women than in men; Although the mean changes in both genders were almost equal to each other and about the same as the overall changes in the studied population. The impact of sex on alterations in IOP has been a subject of varying conclusions across different studies. For instance, Han et al. found a higher rate of IOP changes in women11, while Chan et al.,16 Hashemi et al.,5 and Zhao et al.21 reported that IOP tends to be higher in men than in women. However, Baek et al. demonstrated that the rate of change in IOP in female eyes was lower than in male eyes6. Various reasons are mentioned regarding the observed results in different studies, the cross-sectional and cohort nature of the conducted studies, the difference in the age range of the investigated participants, the ocular structural differences between the two genders22, the difference in biometric dimensions in different races23, the effect of estrogen hormone on the secretion of aqueous humor24, the difference in IOP before and after menopause25, the effect of hemoglobin, red blood cells, white blood cells and other related components Blood in the amount of IOP20, the difference in these parameters like globin between the two sexes26 and also the influence of blood parameters to the geographical location27 can be among the reasons affecting the difference in the results observed in different studies.
The association between BMI and IOP has been examined in various cross-sectional studies. The outcomes of the multivariate GEE model in the present study indicated that higher BMI is linked to elevated IOP. Numerous cross-sectional studies have explored this particular relationship. Han et al.,11 Nakano et al.28 Ghanbarnia et al.17 and Chua et al.18 have demonstrated a positive association between BMI and IOP. Conversely, Hashemi et al. found an inverse relationship between BMI and IOP5. Li et al. reported no significant association between IOP and BMI29. Chan et al. reported an inverse relationship between height and IOPg, while a direct relationship was found between height and IOPcc16. Prior research has demonstrated a connection between height and BMI with ocular biometric indices30. Most studies pointed to a direct relationship between BMI and IOP, implying that heightened oxidative stress due to higher levels of adiposity could play a role in the degeneration of the trabecular meshwork, ultimately causing an increase in IOP31.
According to the results, diabetic individuals experienced a significant increase in IOP. This finding has already been supported by Ghanbarnia et al.17 Chua et al.,18 Hashemi et al.5 and Hanyuda et al.32 also. Several studies have proposed various factors to explain this phenomenon, including the elevation of transforming growth factor (TGF)-β2 in the aqueous humor of diabetics. This increase has been linked to dysfunction of the trabecular meshwork and the impaired discharge of aqueous humor from the anterior chamber33. Furthermore, the increased accumulation of advanced glycation end-products (AGEs) within the trabecular meshwork of diabetic individuals could potentially contribute to the elevation of IOP in this population34. An increase in IOP has been observed in patients with diabetes while controlling the effect of corneal thickness32.
The findings of the present study demonstrated the impact of blood pressure on alterations in IOP, with individuals in both the hypertensive and prehypertensive categories exhibiting elevated IOP compared to individuals with normal blood pressure. This observation aligns with previous studies conducted by Han et al.11 Nakano et al.28 Asaoka et al.20 Ghanbarnia et al.17 and Wong et al.8 The suggested mechanism is that individuals with high blood pressure, especially those with systolic blood pressure above the normal range, undergo an increase in ocular ultrafiltration as the capillary pressure increases. As a result, there is a reduction in the drainage of aqueous humor from the eye because of the heightened pressure in the episcleral veins11.
Our findings showed a statistically significant direct relationship between the baseline AL and the rise in IOP. Several studies with a cross-sectional design have investigated the association between AL and IOP. It has been suggested that extended periods of near work can lead to the contraction of the ciliary muscles, causing changes in the curvature of the crystalline lensx35,36. These alterations then apply pressure on the eyeball, resulting in axial elongation36. Moreover, excessive convergence during near work results in thickening of the extraocular muscles and subsequently elevated IOP37. Eyes with elongated AL are more prone to mechanical damage from increased IOP, possibly due to the thinness of the sclera and lamina cribrosa38. Previous studies have also noted the increase in AL due to accommodation during near work, may leading to a subsequent rise in IOP36,39. However, it is important to highlight that this issue cannot be raised in the current study due to the lack of accommodation in the age group under investigation. Hence, based on the demographic composition of the sample and the biometric features of the study participants, varying outcomes may be anticipated regarding the influence of AL on IOP changes.
The present study, along with study carried out by Hoffmann et al.40, which indicate the direct influence of crystalline lens thickness on IOP; Besides, it has been shown that an increase in the crystalline lens thickness during the accommodation process39 is associated with an increase in IOP36. This relationship can be attributed to the reduction in anterior chamber depth due to rise in crystalline lens thickness22, subsequently decreasing the anterior chamber angle41. Consequently, the increased crystalline lens thickness emerges as a crucial factor hindering the outflow of aqueous humor from the anterior chamber, ultimately resulting in an increase in IOP. Kader et al. found that performing phacoemulsification, along with replacing the crystalline lens with a thin intraocular lens, results in a notable deepening of the anterior chamber and a reduction in IOP, particularly in individuals with angle closure glaucoma42.
Corneal thickness was observed to have a positive association with changes in IOP, a finding that was similarly documented in the studies by Wong et al.8 and Hashemi et al.5 The impact of corneal thickness on IOP can be analyzed from two different perspectives; firstly, some stududies indicate that corneal thickness decreases with age43, and if the corneal thickness is not controlled, the measured IOP tends to rise with age. On the other hand, Chan et al.’s study revealed a direct correlation between age and IOPcc16, suggesting that despite controlling for corneal effects, IOP still rises with age. The multivariate GEE model in the present study showed that a rise in corneal thickness leads to an increase in IOP. However, it is important to consider that the nature of the method used to assess IOP in this study, specifically the applanation contact method, could have influenced the outcomes observed.
The current study’s multivariate GEE model also demonstrated that an increase in corneal curvature leads to a rise in IOP. This observation aligns with the results reported in the studies by Hashemi et al.5 and Matsuura et al.44 Based on the results presented in the study by Wang et al.,45 a decrease of one millimeter in the corneal radius of curvature (mm) leads to a 0.76 mmHg increase in IOP. In a different study, Sedaghat et al.46 argued that an increase in myopia levels (including spherical equivalent, mean keratometry, and AL is linked to a decrease in corneal stiffness and biomechanics. Hashemi et al., reported an inverse relationship between the mean keratometry and CCT47. Therefore, it is anticipated that as corneal curvature increases, there will be a decrease in the measured IOP. Nevertheless, the exact cause for the rise in IOP as a result of increased corneal curvature remains unclear. This is a matter that should be investigated in forthcoming studies.
The findings of the present study did not indicate an association between economic status and changes in IOP, the impact of economic status on IOP appears to vary across different studies. Ahmed et al. observed lower economic statue increases the IOP48. Yip et al. also noted that low income is a risk factor for increasing IOP49. Although, Tamm et al. mentioned that economic level does not affect the rate of glaucoma progression50. Yip et al. contend that individuals with lower incomes exhibit elevated blood pressure levels49, considering the established connection between blood pressure and IOP11, the role of economic status can be attributed secondary to high blood pressure49. It is found that individuals with a lower socioeconomic status tend to have shorter eyes, even after adjusting for age and sex and this may contribute to the association between lower economic status and lower IOP51,52. On the other hand, considering the effect of nutrition and food53, the economic status is likely to have a direct relationship with the amount and variety of consumed nutrients and thus lead to changes in IOP. In the present study, although the level of low economic status compared to high level was accompanied by a positive coefficient towards the increase the IOP, nevertheless, its effect was not statistically significant.
The present study included some limitations, the lack of evaluation of the changes made during the five-year period in the structure of ocular system and IOP was one of them; It is recommended that the next cohort studies be carried out sequentially and multi-stage evaluations which can evaluate the process of changes in more detail. Besides, the used term in this study “IOP changes” may be confused with changes in the IOP during the day or in different seasons of the year; in the present study, changes over a 5-year period were considered as “IOP change”.
Conclusion
The findings of the present study indicate that the five-year changes in IOP among the participants were statistically insignificant but not clinically. However, it is worth noting that a minority of individuals experienced a noteworthy elevation in IOP, which warrants attention. Certain systemic conditions, including diabetes, hypertension, and obesity, were found to be significantly associated with increased IOP. Furthermore, individuals with longer AL, thicker corneas, steeper mean keratometry, and greater lens thickness showed a more substantial elevation in IOP; it is recommended to use these findings in the clinical examinations, any patients with these situations need to be evaluate about the IOP changes.
Data availability
Data are available from corresponding author (afotouhi@tums.ac.ir) on reasonable request.
References
Tham, Y. C. et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121 (11), 2081–2090 (2014).
Kingman, S. Glaucoma is second leading cause of blindness globally. Bull. World Health Organ. 82 (11), 887–888 (2004).
Collaborators, G. & Rawal, L. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the right to Sight: an analysis for the global burden of Disease Study. (2021).
Birhanu, G. & Tegegne, A. S. Predictors for elevation of intraocular pressure (IOP) on glaucoma patients; a retrospective cohort study design. BMC Ophthalmol. 22 (1), 254 (2022).
Hashemi, H. et al. Distribution and associated factors of intraocular pressure in the older population: Tehran geriatric eye study. Int. J. Ophthalmol. 16 (3), 418–426 (2023).
Baek, S. U., Kee, C. & Suh, W. Longitudinal analysis of age-related changes in intraocular pressure in South Korea. Eye (Lond,) 29 (5), 625–629 (2015).
Han, X. et al. Age-related changes of intraocular pressure in elderly people in Southern China: Lingtou eye cohort study. PLoS One 11 (3), e0151766 (2016).
Wong, T. T. et al. The relationship of intraocular pressure with age, systolic blood pressure, and central corneal thickness in an Asian population. Invest. Ophthalmol. Vis. Sci. 50 (9), 4097–4102 (2009).
Astrom, S., Stenlund, H. & Linden, C. Intraocular pressure changes over 21 years—A longitudinal age-cohort study in northern Sweden. Acta Ophthalmol. 92 (5), 417–420 (2014).
Belamkar, A. et al. Asian race and primary open-angle glaucoma: where do we stand? J. Clin. Med. 11 (9). (2022).
Han, X. et al. Longitudinal changes in intraocular pressure and association with systemic factors and refractive error: Lingtou eye cohort study. BMJ Open 8 (2), e019416 (2018).
Vranka, J. A., Kelley, M. J., Acott, T. S. & Keller, K. E. Extracellular matrix in the trabecular meshwork: intraocular pressure regulation and dysregulation in glaucoma. Exp. Eye Res. 133, 112–125 (2015).
Grant, A. et al. Exploring ethnic and racial differences in intraocular pressure and glaucoma: the Canadian longitudinal study on aging. Heliyon 10 (7), e28611 (2024).
Fotouhi, A. et al. Cohort profile: Shahroud eye cohort study. Int. J. Epidemiol. 42 (5), 1300–1308 (2013).
Hashemi, H. et al. Association between refractive errors and ocular biometry in Iranian adults. J. Ophthal. Vis. Res. 10 (3), 214–220 (2015).
Chan, M. P. et al. Associations with intraocular pressure in a large cohort: results from the UK biobank. Ophthalmology 123 (4), 771–782 (2016).
Ghanbarnia, M. J. et al. Age-specific distribution of intraocular pressure in elderly Iranian population and its associated factors. Casp. J. Intern. Med. 14 (1), 112–120 (2023).
Chua, J. et al. Inter-relationship between ageing, body mass index, diabetes, systemic blood pressure and intraocular pressure in asians: 6-year longitudinal study. Br. J. Ophthalmol. 103 (2), 196–202 (2019).
Kashiwagi, K., Shibuya, T. & Tsukahara, S. De novo age-related retinal disease and intraocular-pressure changes during a 10-year period in a Japanese adult population. Jpn J. Ophthalmol. 49 (1), 36–40 (2005).
Asaoka, R. et al. The association between age and systemic variables and the longitudinal trend of intraocular pressure in a large-scale health examination cohort. Invest. Ophthalmol. Vis. Sci. 63 (11), 22 (2022).
Zhao, D. et al. A longitudinal study of age-related changes in intraocular pressure: the Kangbuk Samsung health study. Invest. Ophthalmol. Vis. Sci. 55 (10), 6244–6250 (2014).
Jamali, A. et al. Ocular biometry characteristics in cataract surgery candidates: a cross-sectional study. Med. Hypothesis Discov Innov. Ophthalmol. 10 (1), 11–17 (2021).
Ip, J. M. et al. Ethnic differences in refraction and ocular biometry in a population-based sample of 11-15-year-old Australian children. Eye (Lond.) 22 (5), 649–656 (2008).
Gupta, P. D., Johar, K., Sr., Nagpal, K. & Vasavada, A. R. Sex hormone receptors in the human eye. Surv. Ophthalmol. 50 (3), 274–284 (2005).
Panchami, Pai, S. R., Shenoy, J. P. & Kole, J. S. Postmenopausal intraocular pressure changes in south Indian females. J. Clin. Diagn. Res. 7 (7), 1322–1324 (2013).
Murphy, W. G. The sex difference in haemoglobin levels in adults-mechanisms, causes, and consequences. Blood Rev. 28 (2), 41–47 (2014).
Shaheen, N. A. et al. Hematological indices in the adult Saudi population: reference intervals by gender, age, and region. Front. Med. (Lausanne) 9, 901937 (2022).
Nakano, T., Tatemichi, M., Miura, Y., Sugita, M. & Kitahara, K. Long-term physiologic changes of intraocular pressure: a 10-year longitudinal analysis in young and middle-aged Japanese men. Ophthalmology 112 (4), 609–616 (2005).
Li, Y. et al. Intraocular pressure of adults in a coastal province in southern China: the Fujian cross-sectional eye study. Ann. Palliat. Med. 10 (12), 12390–12402 (2021).
Kolacko, S. et al. Do gender, age, body mass and height influence eye biometrical properties in young adults? A pilot study. Int. J. Environ. Res. Public. Health 18 (21). (2021).
Zhao, D. et al. A longitudinal study of association between adiposity markers and intraocular pressure: the Kangbuk Samsung health study. PLoS One 11 (1), e0146057 (2016).
Hanyuda, A. et al. Relationships of diabetes and hyperglycaemia with intraocular pressure in a Japanese population: the JPHC-NEXT eye study. Sci. Rep. 10 (1), 5355 (2020).
Shepard, A. R. et al. Adenoviral gene transfer of active human transforming growth factor-β2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Investig. Ophthalmol. Vis. Sci. 51 (4), 2067–2076 (2010).
Park, C. H. & Kim, J. W. Effect of advanced glycation end products on oxidative stress and senescence of trabecular meshwork cells. Korean J. Ophthalmol. 26 (2), 123–131 (2012).
Kaur, S., Sukhija, J., Khanna, R., Takkar, A. & Singh, M. Diplopia after excessive smart phone usage. Neuroophthalmology 43 (5), 323–326 (2019).
Zhang, D. et al. A review of intraocular pressure (IOP) and axial myopia. J. Ophthalmol. 2022, 5626479 (2022).
de Jong, P. Myopia: its historical contexts. Br. J. Ophthalmol. 102 (8), 1021–1027 (2018).
Jonas, J. B., Berenshtein, E. & Holbach, L. Lamina cribrosa thickness and spatial relationships between intraocular space and cerebrospinal fluid space in highly myopic eyes. Invest. Ophthalmol. Vis. Sci. 45 (8), 2660–2665 (2004).
Yan, L., Huibin, L. & Xuemin, L. Accommodation-induced intraocular pressure changes in progressing myopes and emmetropes. Eye (Lond.) 28 (11), 1334–1340 (2014).
Hoffmann, E. M. et al. Intraocular pressure and its relation to ocular geometry: results from the gutenberg health study. Invest. Ophthalmol. Vis. Sci. 63 (1), 40 (2022).
Schuster, A. K. et al. Distribution of anterior chamber angle width and correlation with age, refraction, and anterior chamber depth-the gutenberg health study. Invest. Ophthalmol. Vis. Sci. 57 (8), 3740–3746 (2016).
Kader, M. A. et al. Lowering of intraocular pressure after phacoemulsification in primary open-angle and angle-closure glaucoma: correlation with lens thickness. Indian J. Ophthalmol. 70 (2), 574–579 (2022).
Galgauskas, S., Juodkaite, G. & Tutkuviene, J. Age-related changes in central corneal thickness in normal eyes among the adult Lithuanian population. Clin. Interv Aging 9, 1145–1151 (2014).
Matsuura, M. et al. Relationship between novel intraocular pressure measurement from Corvis ST and central corneal thickness and corneal hysteresis. Br. J. Ophthalmol. 104 (4), 563–568 (2020).
Wang, Y. X., Xu, L., Wei, W. B. & Jonas, J. B. Intraocular pressure and its normal range adjusted for ocular and systemic parameters. The Beijing eye study 2011. PLoS One 13 (5), e0196926 (2018).
Sedaghat, M. R. et al. Corneal biomechanical properties in varying severities of myopia. Front. Bioeng. Biotechnol. 8, 595330 (2020).
Hashemi, H. et al. The distribution of keratometry in a population based study. J. Curr. Ophthalmol. 33 (1), 17–22 (2021).
Ahmed, M. A. A. & Abdelhalim, A. S. Corrected intraocular pressure variability with central corneal thickness measurement. Clin. Ophthalmol. 14, 4501–4506 (2020).
Yip, J. L. et al. Socioeconomic status, systolic blood pressure and intraocular pressure: the Tanjong pagar study. Br. J. Ophthalmol. 91 (1), 56–61 (2007).
Tamm, B. et al. Progression of glaucoma in patients of low and high socioeconomic status and various age groups. Investig. Ophthalmol. Vis. Sci. 59 (9), 2734–2734 (2018).
Goverdhan, S., Lockwood, A., Fogarty, A., Osmond, C. & Kirwan, J. Hyperopia and small axial length are associated with socio-economic deprivation: a UK cohort study. Investig. Ophthalmol. Vis. Sci. 50 (13), 3962–3962 (2009).
Wong, T. Y., Foster, P. J., Johnson, G. J. & Seah, S. K. Education, socioeconomic status, and ocular dimensions in Chinese adults: the Tanjong pagar survey. Br. J. Ophthalmol. 86 (9), 963–968 (2002).
Al Owaifeer, A. M. & Al Taisan, A. A. The role of diet in glaucoma: a review of the current evidence. Ophthalmol. Ther. 7 (1), 19–31 (2018).
Funding
This project is funded in part by the Noor Ophthalmology Research Center and Shahroud University of Medical Sciences (Project Numbers: 8737 and 9826).
Author information
Authors and Affiliations
Contributions
Design and conduct of the study (HH, AF); collection, management of the data (AF, ME); analysis, and interpretation of the data (HH, MK, AJ, ME); and preparation, review, and approval of the manuscript (HH, MK, AJ, ME, AF).
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Shahroud Eye Cohort Study was approved by the ethics committee of Shahroud University of Medical Sciences (Reference numbers: IR.SHMU.REC. 1398.039). All methods were carried out in accordance with the Helsinki tenets and other relevant guidelines and regulations. Written informed consent was obtained from all participants.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hashemi, H., Khabazkhoob, M., Emamian, M.H. et al. Longitudinal changes in the intraocular pressure and their related factors among adults aged 40 to 64 years. Sci Rep 15, 10918 (2025). https://doi.org/10.1038/s41598-025-85835-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-85835-0