Abstract
Deep vein thrombosis (DVT) is one of the important factors leading to death in patients undergoing fracture surgery. This study aims to investigating the predictive value of the Caprini score combined with thrombus molecular markers for the risk of DVT in patients after traumatic fracture surgery. A total of 342 patients who underwent surgery for traumatic fractures were included in the study. The patients were divided into two groups based on the occurrence of DVT after surgery: the DVT group (n = 57) and the non-DVT group (n = 285). A univariate analysis and logistic regression analysis were conducted on clinical factors and laboratory indicators that might be associated with DVT in patients with traumatic fractures. A predictive model for DVT risk was then constructed by combining thrombus molecular markers with the Caprini score. The median age of all patients was 65 years (54–75 years), the postoperative Caprini score was 9 (6–11), and the length of hospital stay was 11 days (8–16 days). In univariate analysis, age (P = 0.029), postoperative Caprini score (P < 0.001), and length of hospital stay (P = 0.009) were significantly associated with the occurrence of DVT. Logistic regression analysis showed that the risk of developing DVT increased with higher postoperative Caprini scores (P < 0.001), longer hospital stays (P = 0.024), and higher PIC levels (P = 0.046). Among these, the postoperative Caprini score was the most effective factor for diagnosing DVT, with an area under the curve (AUC) of 0.814 (P < 0.001) and a diagnostic cutoff of 11 points. The overall diagnostic efficacy of individual thrombus molecular markers from highest to lowest was TM, DD, PIC, t-PAIC, and TAT, with all except TAT showing statistical significance. The combined diagnostic efficacy of the postoperative Caprini score and PIC also showed statistical significance (AUC = 0.869, P < 0.001). Thrombus molecular markers combined with the postoperative Caprini score have potential predictive value for the risk of DVT in patients after traumatic fracture surgery.
Similar content being viewed by others
Introduction
Venous thromboembolism (VTE) is one of the third most common cardiovascular diseases globally and one of the major factors contributing to postoperative mortality and unexpected in-hospital deaths in hospitalized patients1, with an annual incidence of 1–2‰ in Europe and the United States, while the incidence in Asia and other regions is generally lower than 1‰. Fifty to sixty% of VTE cases are caused by surgery or hospitalization, and approximately 20% of VTE patients die within one year2,3.VTE includes deep vein thrombosis (DVT) and pulmonary embolism (PE) with the former accounting for two-thirds4.DVT refers to the pathological process where blood abnormally coagulates and obstructs the vein lumen in the deep veins of the lower limbs, commonly occurring in the lower extremities. Twenty-five to forty% of DVT patients experience varying degrees of functional impairment and reduced quality of life due to post-thrombotic syndrome (PTS) after thrombus formation. If the thrombus dislodges, pulmonary embolism (PE) can occur, which can lead to sudden death in severe cases. Therefore, DVT poses a significant burden on human health.
Traumatic fractures can impair limb function, affecting patients’ daily lives and work, and surgical intervention is currently the primary clinical treatment for such fractures5. Despite the widespread use of physical and pharmacological prophylaxis, the incidence of DVT remains high among hospitalized patients6,7. A study on hip and knee replacement surgeries reported that the postoperative incidence of DVT exceeded 60%8. Another prospective study on patients undergoing foot and ankle fracture surgery revealed that the prevalence of clinically asymptomatic DVT was significantly higher than previously expected9. Based on extensive research data in orthopedic surgery patients, the association between traumatic fractures and VTE has been widely recognized. Recent studies indicate that the first two weeks after orthopedic surgery is a high-risk period for DVT occurrence10, and early identification of risk factors helps in the individualized risk assessment and intervention for DVT11. Currently, there is no standardized method for early prediction of DVT, making it particularly important to develop a reliable DVT risk prediction scoring system.
The Caprini scoring system is a venous thromboembolism risk assessment scoring system originally developed by Professor Caprini’s team and first published in 199112. As a personalized DVT risk assessment model, it has been validated in over 100 clinical trials worldwide, encompassing more than 250,000 patients13. Among the various venous thromboembolism risk assessment models that have been developed, the Caprini score is currently the most widely used tool for assessing DVT risk14,15,16. Research shows that its applications span across orthopedics, burn care, plastic surgery, gynecology, oncology, and other fields17,18,19,20,21. However, it’s important to note that the predictive value of the Caprini score varies depending on the surgical population, and a certain threshold may not be suitable for all surgical patients22,23. Therefore, further optimization of the application of this score is needed in traumatic fracture surgery patients with a higher risk of DVT.
In populations at risk of DVT, laboratory testing of thrombotic molecular markers can assist in monitoring the risk of DVT. D-dimer is a small protein fragment produced during the process of clot dissolution. Due to its excellent sensitivity, guidelines from the American Society of Hematology recommend it as an important screening indicator for PE24. Ma et al.25 found that D-dimer has good predictive value for DVT associated with foot fracture surgery. Recently, Hojker et al.26 discovered a series of inflammatory coagulation markers with predictive value for DVT in COVID-19 patients, among which the overall diagnostic efficacy of D-dimer is 0.85, with an optimal cutoff value of 2677 mg/L. In recent years, with the development and application of novel thrombotic molecular markers in the coagulation system, markers such as thrombin-antithrombin complex(TAT), thrombomodulin(TM), tissue plasminogen activator-plasminogen activator inhibitor complex(t-PAIC) and plasmin-antiplasmin complex(PIC) have gradually shown their potential value in predicting DVT risk27,28. However, research on molecular markers for predicting thrombosis is still lacking, especially in terms of combining them with thrombotic risk scores.
Therefore, exploring the predictive value of thrombotic molecular markers combined with the Caprini score for the risk of deep vein thrombosis (DVT) in patients after traumatic fracture surgery can help in the earlier and more accurate prevention of DVT and other severe thromboembolic events. This study aims to validate the molecular markers associated with DVT and develop a model for effectively predicting DVT risk by analyzing the combination of thrombotic markers and the Caprini score, thereby improving clinical outcomes for patients (Supplementary Fig. 1).
Methods
Population selection
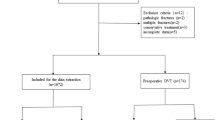
This study reviewed a total of 1,797 patients who were admitted for surgical treatment of traumatic fractures from June 2022 to December 2023. After excluding patients with missing clinical data and those who did not undergo vascular ultrasound examination and thrombotic molecular marker testing during hospitalization, 584 patients were identified. Further exclusions included patients under 18 years of age, those with a history of thrombosis, and those on long-term anticoagulant or antiplatelet therapy, resulting in a final cohort of 342 patients for analysis. Basic medical history, such as smoking, alcohol consumption, hypertension, and diabetes, was collected. Surgical details, including ASA (American Society of Anesthesiologists) classification, intraoperative blood transfusion, preoperative anticoagulant therapy, and the use of erythropoietin (EPO), were recorded. The patients were divided into a DVT group (n = 57) and a non-DVT group (n = 285) based on the occurrence of DVT after surgery (Fig. 1). This study was approved by the Institutional Review Board of Zhejiang Provincial People’s Hospital and strictly complied with the Declaration of Helsinki. Due to the retrospective nature of the study and the anonymous processing of data prior to analysis, the Ethics Committee waived the requirement for informed consent.
Variable definitions
The patients were divided into two groups based on the occurrence of postoperative DVT, with the gold standard for DVT diagnosis being color Doppler ultrasound of the lower extremity vessels. In addition to age and sex, the study variables included individual factors such as smoking, alcohol consumption, diabetes, and a history of hypertension. Surgery-related variables included the postoperative Caprini score, intraoperative blood transfusion, ASA classification, prophylactic anticoagulation, and the use of EPO before surgery. The postoperative Caprini score was assessed within 24 h after surgery, and prophylactic anticoagulation refers to heparin anticoagulation therapy initiated before surgery.
Caprini score scale
The Caprini score scale is based on Caprini’s VTE risk assessment model, which primarily relies on the characteristics of surgical patients and assigns different weight scores to various risk factors29. In this study, we referenced the 2013 version of the Caprini risk assessment model for thrombotic risk assessment13, and all scores were completed by clinical physicians. The scale includes 40 thrombosis risk factors, with each factor assigned a score of 1–5 based on the severity of the risk. In general, based on the cumulative score, patients’ DVT risk is classified into four levels: very low risk (0 point), low risk (1–2 points), moderate risk (3–4 points), and high risk (≥ 5 points), and with higher scores indicating a greater risk of thrombosis formation. However, the patients included in this study were all patients who underwent traumatic fracture surgery, and the conventional stratification criteria were not applicable30. Based on previous research and data from this study, patients with scores ≥ 10 points are considered high-risk groups17,22.
Thrombus-related coagulation testing
This study includes novel thrombotic molecular markers TM, TAT, t-PAIC, PIC, detected using a fully automated chemiluminescent immunoassay analyzer (Shine i2900, IncreCare). Thromboelastography (TEG) testing in this study was performed using a thromboelastograph instrument (BVCA-VIII, Bring Biology), with the following parameters: R-value representing clot initiation time, α-angle representing clotting rate, K-value representing blood clotting time, MA-value representing maximum clot strength, LY30 for clot dissolution rate within 30 min, EPL for clot dissolution percentage within 30 min, and CI for clotting index. Plasma coagulation parameters were measured using the Japanese Sysmex CA7000 fully automated coagulometer, with the following parameters: PT representing prothrombin time, APTT representing activated partial thromboplastin time, FIB representing fibrinogen, and DD representing D-dimer. Additionally, routine blood parameters were measured using the Japanese Sysmex XE-5000 fully automated hematology analyzer. All coagulation tests were conducted within one hour after specimen collection.
Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD) or median (interquartile range), while categorical variables are presented as percentages (%). The chi-square test is used to compare differences in categorical variables between two groups. The t-test is used to compare continuous variables that follow a normal distribution, while the Mann-Whitney U test is used for continuous variables that do not follow a normal distribution. Logistic regression analysis is employed for analyzing related factors and constructing predictive models. The predictive performance and goodness of fit of the model are evaluated using ROC curves and the Hosmer-Lemeshow test, respectively. All P values are two-tailed, and P < 0.05 is considered statistically significant. All statistical analyses are performed using SPSS version 24.0.
Results
General clinical characteristics
This study reviewed patients (n = 1,797) who were admitted for surgical treatment of traumatic fractures from June 2022 to December 2023. After excluding patients with missing clinical data and those who did not undergo vascular ultrasound examination and thrombotic molecular marker testing during hospitalization, 584 patients were identified. Further exclusions included patients under 18 years of age, those with a history of thrombosis, and those on long-term anticoagulant or antiplatelet therapy, resulting in a final cohort of 342 patients for analysis, of which 57 were in the DVT group and 285 in the non-DVT group (Table 1).
The median age of the overall population was 65 years (range 54–75), with males accounting for 50.0%, and the median BMI was 23.8 kg/m². The most common pre-existing conditions were hypertension (23.7%), alcohol consumption (17.3%), smoking (16.7%), and diabetes (16.1%). All patients had a postoperative Caprini score of 9 (range 6–11), with 32.2% of patients receiving intraoperative blood transfusion and 38.0% having an ASA grade > 2. Preoperative use of EPO and prophylactic anticoagulation was noted in 21.6% and 35.7% of patients, respectively. Additionally, the median length of hospital stay for all patients was 11 days (range 8–16).
Univariate analysis of DVT-related factors
All patients were divided into a DVT group (n = 57) and a non-DVT group (n = 285) based on whether they developed DVT postoperatively. Univariate analysis results (Table 1) showed that age (P = 0.029), postoperative Caprini score (P < 0.001), and length of hospital stay (P = 0.009) were significantly associated with the occurrence of DVT. Additionally, an ASA grade greater than 2 (P = 0.013) and prophylactic anticoagulation therapy (P = 0.021) were also correlated with DVT incidence. Among coagulation-related laboratory indicators, RBC count (P = 0.016), D-dimer (P = 0.002), TM (thrombomodulin) (P < 0.001), t-PAIC (tissue plasminogen activator inhibitor complex) (P < 0.001), and PIC (plasminogen inhibitor complex) (P = 0.007) were all significantly associated with the occurrence of DVT (Table 1).
Risk prediction model of DVT
The regression analysis results that included age (Table 2) showed that the postoperative Caprini score (P < 0.001) was an independent factor associated with the occurrence of DVT. Additionally, the risk of developing DVT increased with longer hospital stays (P = 0.024) and higher preoperative PIC (plasminogen inhibitor complex) levels (P = 0.046).
OR: odds ratio; CI: confidence interval; ASA, American society of Aneshesiologists; EPO, erythropoietin; DD, d-dimer; FIB, fibrinogen; PT, prothrombin time; TM, thrombomodulin; PIC, plasmin-antiplasmin complex.
As shown in Fig. 2A, the postoperative Caprini score was the most effective single variable for diagnosing DVT, with an AUC of 0.863 (P < 0.001). Based on these results, we developed a model to predict the risk of DVT in patients with traumatic fractures, incorporating variables such as the postoperative Caprini score, length of hospital stays, and PIC (plasminogen inhibitor complex). The Omnibus and Hosmer-Lemeshow tests indicated that the model was generally significant and well-fitted to the data, with an AUC of 0.878 (P < 0.001) (Fig. 2B). Based on the Youden index for each variable, the diagnostic cut-off points were set at 11 points for the postoperative Caprini score, 12 days for the length of hospital stay, and 1.39 µg/ml for PIC.
DVT risk prediction model based on logistic regression. (A) The ROC of each single variable (Postoperative Caprini score, hospitalization days, and PIC). (B) The ROC of total three variables. ROC: receiver operating characteristic curve; AUC: area under the curve; CI: confidence interval; PIC, plasmin-antiplasmin complex.
The DVT risk prediction model combining thrombotic molecular markers with postoperative Caprini score
In this study, the overall diagnostic performance of each thrombotic molecular marker in univariate analysis ranked from high to low as follows: TM, DD, PIC, TAT, t-PAIC, with TM and DD exhibiting statistically significant AUCs (Fig. 3A). The predictive models combining postoperative Caprini score with each thrombotic molecular marker showed good overall diagnostic performance (Fig. 3B-G). Among these, the combination with TM demonstrated the highest performance (AUC = 0.869, P < 0.001), followed by the combination with PIC (AUC = 0.867, P < 0.001). The AUC reached 0.868 (P < 0.001) when postoperative Caprini score was combined with all markers (Table 3).
ROC curves for DVT with the combination of thrombotic molecular markers and postoperative Caprini score (A). The ROC of postoperative Caprini score and five thrombotic biomarkers (DD, TAT, TM, t-PAIC and PIC). (B). The ROC of postoperative Caprini score plus DD; (C). The ROC of postoperative Caprini score plus TAT; (D). The ROC of postoperative Caprini score plus TM; (E). The ROC of postoperative Caprini score plus t-PAIC; (F). The ROC of postoperative Caprini score plus PIC; (G). The ROC of postoperative Caprini score plus five thrombotic biomarkers. ROC: receiver operating characteristic curve; CI: confidence interval; DD, d-dimer; TAT, thrombin-antithrombin complex; TM, thrombomodulin; t-PAIC, tissue plasminogen activator-plasminogen activator inhibitor complex; PIC, plasmin-antiplasmin complex.
Relationship between Caprini score and thrombotic molecular markers with DVT
All patients underwent thrombotic molecular marker testing upon admission, while only 21 patients underwent retesting after surgery, with 8 in the DVT group and 13 in the non-DVT group. The comparison results (Fig. 4A) showed that both in the DVT and non-DVT groups, the postoperative Caprini score was generally higher than the preoperative score, and the difference in postoperative Caprini scores between the two groups was statistically significant (P = 0.031). Additionally, no significant differences were found in thrombotic molecular markers TAT, TM, PIC, and t-PAIC between the two groups (Fig. 4B-E).
Comparison of Caprini scores and thrombotic molecular markers before and after surgery in DVT group (n = 8) and non-DVT group (n = 13) patients (A). The comparison of changes in Caprini score before and after operation. (B). The comparison of changes in TAT before and after operation. (C). The comparison of changes in TM before and after operation. (D). The comparison of changes in t-PAIC before and after operation. (E). The comparison of changes in PIC before and after operation. TAT, thrombin-antithrombin complex; TM, thrombomodulin; t-PAIC, tissue plasminogen activator-plasminogen activator inhibitor complex; PIC, plasmin-antiplasmin complex.
Discussion
The pathophysiological process of DVT involves three elements: venous stasis, endothelial damage, and hypercoagulability of the blood31. Previous studies have shown that the clinical risk factors for DVT are multifaceted, including obesity, ischemic heart disease, infection, tumors, and surgical factors, especially orthopedic surgeries32,33,34. Research has indicated that the duration of surgery is an important risk factor for DVT in patients with lower limb fractures35,36. The data from this study show that surgical duration > 4 h (P < 0.001) is an independent risk factor for postoperative DVT (data was not shown). Therefore, combining the scoring of various risk factors is the primary method for clinically predicting the risk of DVT in patients.
Over the past decade, clinical scoring systems used to predict the risk of DVT in patients have included the Padua, Caprini, and Wells DVT scores37,38. Based on the tendencies of different scoring systems, the Caprini score is commonly used in surgery to assess the perioperative risk of DVT in patients. Kitchai et al.39 found that the Caprini score in patients undergoing hip fracture surgery was significantly higher in the DVT group compared to the non-DVT group (P < 0.05), and the cutoff value has reached 12 points. This study also showed (Fig. 4) that the postoperative Caprini score increased more in the DVT group compared to the non-DVT group (P < 0.001) and the cutoff value was 11 points. As reported previously17,22,39,40, it is valuable and necessary to adjust the scoring criteria in a specific population for more accurate predictions. As noted in Table 2, the postoperative Caprini score (P < 0.001) is an independent factor associated with DVT and is the most effective single variable for diagnosis. However, in the combined risk model with thrombotic molecular markers, there was only a slight improvement in diagnostic efficacy (Fig. 3). This suggests that while the Caprini score has been validated and adjusted extensively and demonstrates strong predictive performance, incorporating molecular markers into the assessment has potential for achieving more personalized and precise prevention of DVT in clinical practice.
Prior research has established the value of D-dimer in predicting thrombotic risk, and studies have also found that FDP can enhance the diagnostic accuracy of DVT in fracture patients. However, traditional coagulation function tests have always failed to fully reflect their predictive value for DVT, and laboratories still lack molecular markers for assessing thrombotic risk. With the rise of novel thrombotic molecular markers detection, clinical assessment of DVT risk no longer solely relies on risk factor scoring but has garnered more attention towards thrombotic molecular markers. In this study, from the perspective of combining with the Caprini score, we focused on analyzing the predictive value of thrombotic molecular markers for DVT risk.
D-dimer is a specific degradation product of cross-linked fibrin produced under fibrinolysis and is an important thrombotic marker widely used in DVT screening for many years. Research has confirmed that D-dimer levels have a sensitivity for thrombus formation exceeding 95%, but its specificity is only 20–40%41,42. Some studies have found that FDP (fibrin degradation products) can also improve diagnostic accuracy for DVT in fracture patients43. Chen et al.44 found that incorporating both D-dimer and FIB (fibrinogen) into a predictive model for DVT in patients with femoral neck fractures effectively assesses thrombus formation risk. The results of this study similarly show that D-dimer has some predictive ability for DVT risk in postoperative patients (AUC = 0.633, P = 0.002), and when combined with the Caprini score, the AUC improves to 0.865 (P < 0.001) (Table 3).
Since changes in D-dimer are influenced by both thrombus formation and fibrinolytic degradation, and its specificity for thrombus diagnosis is lacking, traditional coagulation tests have not fully reflected its predictive value for DVT. There is still a lack of sensitive molecular markers for assessing thrombus risk in laboratories. With the advent of new thrombotic molecular markers, clinical practice is shifting away from relying solely on risk factor scoring to evaluating DVT risk and is focusing more on thrombotic molecular markers. This study emphasizes the value of thrombotic molecular markers in predicting DVT risk from the perspective of combining them with the Caprini score.
In addition to D-dimer, thrombotic molecular markers include novel markers such as TM, TAT, PIC, and t-PAIC, which also have important reference value in clinical assessment of DVT risk45. TM is a glycoprotein mainly expressed in vascular endothelial cells and serves as a marker of endothelial damage, playing a role in regulating thrombin activity46. Cheng et al.47 found that plasma TM levels were significantly higher in DVT patients compared to healthy controls, and animal studies suggest that TM may be involved in DVT progression through its effects on the NF-κB signaling pathway, making it a dynamic biomarker for disease activity. Luo et al.48 also reported a significant positive correlation between TM and DVT after total hip replacement surgery.
In this study (Table 3), TM demonstrated a good predictive ability for DVT risk in postoperative patients, with an AUC of 0.687 (P < 0.001).
PIC also holds predictive value for thrombus formation. As a marker that reflects the activation of the fibrinolytic system, PIC indicates the level of plasmin activation and is commonly used to guide antifibrinolytic treatment. Guo et al.49 found that PIC levels were significantly higher in DVT patients compared to non-DVT patients, and DVT risk increased with higher PIC levels, showing a strong correlation with the Caprini score (r = 0.408, P < 0.001).In this study, the predictive ability of PIC for DVT was second only to TM among single variables, with an AUC of 0.867 (P < 0.001) when combined with the Caprini score.
consists of thrombin and its inhibitor, and its elevation indicates ongoing thrombin generation and continuous consumption of antithrombin, making it a sensitive marker of thrombin activation and a key indicator of the activation of the coagulation system50. Lin et al.27 found that TAT levels on the first postoperative day were significantly higher in patients undergoing joint replacement surgery compared to preoperative levels and non-DVT groups, highlighting its value in predicting early postoperative DVT. Cheng et al.28 reported that the TAT/PIC ratio was significantly elevated on the 6th postoperative day in VTE patients compared to non-VTE patients (P < 0.0001), suggesting that the TAT/PIC ratio could be a potential early prognostic marker for VTE following joint replacement surgery.
Additionally, t-PAIC (tissue plasminogen activator inhibitor complex) has been recognized as a risk factor for myocardial infarction51, and recent studies suggest that resistance to tissue plasminogen activator is an early predictor of post-traumatic venous thromboembolism52. Yang et al.53 found that in DVT patients following knee arthroplasty, PAI-1 and TAT were more valuable than D-dimer. However, this study did not confirm the predictive value of TAT and t-PAIC for DVT.
Currently, research on the predictive value of new thrombotic molecular markers for DVT in patients with traumatic fractures is still limited. In our study, we did not find a significant relationship between changes in thrombotic molecular markers before and after surgery (Fig. 4) or changes in TEG parameters before and after surgery and the occurrence of DVT (Supplementary Fig. 2). It requires a larger sample size study in the perioperative patient population with a unified anticoagulant therapy regimen.
In this study, a DVT risk prediction model for patients undergoing traumatic fracture surgery was constructed based on the Caprini score combined with thrombotic molecular markers; however, several limitations remain. First, as a retrospective case-control study, the sample size is limited, and larger-scale randomized controlled trials are needed to provide more robust evidence. Secondly, it is necessary to communicate with specialist doctors to clarify the inclusion criteria for patients’ clinical processes, collect and verify data from hospital electronic medical record systems and big data platforms simultaneously, and use statistical methods to evaluate and adjust confounding bias. Then, anticoagulant therapy may affect the changes in molecular markers of thrombus formation to varying degrees. In this study, the detection samples of thrombus molecular markers were mainly collected on the same day or the next morning after admission, and most of the patients used the same treatment regimen, thereby significantly reducing the impact of anticoagulant therapy on the detection results. Lastly, different patient conditions and unquantifiable non-pharmacological DVT prevention measures represent confounding factors that could not be excluded from this study. In conclusion, there is still a need for more advanced studies to develop better DVT risk prediction strategies in the future.
Conclusion
Thrombosis molecular markers combined with postoperative Caprini scores have good predictive value for DVT risk in patients after traumatic bone fracture surgery.
Data availability
The data is available upon a reasonable request from the corresponding author.
Change history
13 May 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-00838-1
References
Maynard, G. A. et al. Optimizing prevention of hospital-acquired venous thromboembolism (VTE): prospective validation of a VTE risk assessment model. J. Hosp. Med. 5(1), 10–18 (2010).
Heit, J. A. The epidemiology of venous thromboembolism in the community. Arterioscler. Thromb. Vasc Biol. 28(3), 370–372 (2008).
Lutsey, P. L. & Zakai, N. A. Epidemiology and prevention of venous thromboembolism. Nat. Rev. Cardiol. 20(4), 248–262 (2023).
Duffett, L. Deep venous thrombosis. Ann. Intern. Med. 175(9), ITC129–ITC144 (2022).
Shen, Y. et al. Effect of predictive trauma nursing on emergency traumatic fracture patients. Minerva Surg. 76(6), 606–608 (2021).
Geerts, W. H. et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and thrombolytic therapy. Chest 126(3 Suppl), 338S–400S (2004).
Gould, M. K. et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 141(2 Suppl), e227S–e277S (2012).
Chotanaphuti, T. et al. The prevalence of thrombophilia and venous thromboembolism in total knee arthroplasty. J. Med. Assoc. Thai. 90(7), 1342–1347 (2007).
Sullivan, M. et al. Prevalence of deep vein thrombosis in low-risk patients after elective foot and ankle surgery. Foot Ankle Int. 40(3), 330–335 (2019).
Read, J. et al. The incidence of symptomatic venous thromboembolism following orthopaedic surgery at Bay of Plenty District Health Board. N Z. Med. J. 136(1571), 41–48 (2023).
Zuo, J. & Hu, Y. Admission deep venous thrombosis of lower extremity after intertrochanteric fracture in the elderly: a retrospective cohort study. J. Orthop. Surg. Res. 15(1), 549 (2020).
Arcelus, J. I. et al. Venous thromboembolism prophylaxis and risk assessment in medical patients. Semin Thromb. Hemost. 17(Suppl 3), 313–318 (1991).
Cronin, M. et al. Completion of the updated Caprini Risk Assessment Model (2013 version). Clin. Appl. Thromb. Hemost. 25, 1076029619838052 (2019).
Liew, N. C. et al. Asian venous thromboembolism guidelines: updated recommendations for the prevention of venous thromboembolism. Int. Angiol. 36(1), 1–20 (2017).
Konstantinides, S. V. & Meyer, G. The 2019 ESC guidelines on the diagnosis and management of Acute Pulmonary Embolism. Eur. Heart J. 40(42), 3453–3455 (2019).
Guyatt, G. H. et al. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 141(2 Suppl), 7S-47S (2012).
Krauss, E. S. et al. Implementation and validation of the 2013 Caprini score for risk stratification of Arthroplasty patients in the Prevention of venous thrombosis. Clin. Appl. Thromb. Hemost. 25, 1076029619838066 (2019).
Guo, T. et al. Validation of two risk assessment models for venous thromboembolism in patients undergoing gynecologic surgery. Ann. Transl Med. 10(1), 18 (2022).
Pannucci, C. J. et al. Assessment of postoperative venous thromboembolism risk in plastic surgery patients using the 2005 and 2010 Caprini Risk score. Plast. Reconstr. Surg. 130(2), 343–353 (2012).
Li, Q. et al. Stratification of venous thromboembolism risk in burn patients by Caprini score. Burns 45(1), 140–145 (2019).
Zhang, X. et al. Deep vein thrombosis and validation of the Caprini risk assessment model in Chinese orthopaedic trauma patients: a multi-center retrospective cohort study enrolling 34,893 patients. Eur. J. Trauma. Emerg. Surg. 49(4), 1863–1871 (2023).
Bartlett, M. A. et al. Perioperative venous thromboembolism prophylaxis. Mayo Clin. Proc. 95(12), 2775–2798 (2020).
Krauss, E. S. et al. Utilization of the Caprini score for risk stratification of the arthroplasty patient in the prevention of postoperative venous thrombosis. Semin Thromb. Hemost. 48(4), 407–412 (2022).
Lim, W. et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. Blood Adv. 2(22), 3226–3256 (2018).
Ma, J. et al. Incidence and hematological biomarkers associated with preoperative deep venous thrombosis following foot fractures. Foot Ankle Int. 41(12), 1563–1570 (2020).
Hojker, M. et al. Predictive value of inflammatory and coagulation biomarkers for venous thromboembolism in COVID-19 patients. Clin. Hemorheol Microcirc. 83(4), 387–395 (2023).
Lin, Z. et al. Thrombin antithrombin complex concentration as an early predictor of deep vein thrombosis after total hip arthroplasty and total knee arthroplasty. BMC Musculoskelet. Disord. 23(1), 574 (2022).
Cheng, Y. et al. Clinical impact of coagulation and fibrinolysis markers for predicting postoperative venous thromboembolism in total joint arthroplasty patients. Clin. Appl. Thromb. Hemost. 25, 1076029619877458 (2019).
Caprini, J. Thrombosis risk Assessment as a Guide to Quality Patient care 512–3 (DM DISEASE-A-MONTH, 2005).
Hanh, B. M. et al. Determination of risk factors for venous thromboembolism by an adapted Caprini Scoring System in surgical patients. J. Personalized Med. 9(3) (2019).
Kyrle, P. A. & Eichinger, S. Deep vein thrombosis. Lancet. 365(9465), 1163–1174 (2005).
Keller, K. et al. Venous thromboembolism in patients hospitalized for hip joint replacement surgery. Thromb. Res. 190, 1–7 (2020).
Xu, D. et al. Analysis of risk factors for deep vein thrombosis after spinal infection surgery and construction of a nomogram preoperative prediction model. Front. Cell. Infect. Microbiol. 13, 1220456 (2023).
Gouzoulis, M. J. et al. Risk factors for venous thromboembolism following fractures isolated to the foot and ankle fracture. PLoS One. 17(10), e0276548 (2022).
Zhang, J. et al. Post-operative deep vein thrombosis in patients over sixty years of age diagnosed with closed distal femur fractures undergoing open reduction internal fixation. Int. Orthop. 45(6), 1615–1623 (2021).
Peng, J. et al. Incidence and risk factors of isolated calf muscular venous thrombosis after tibial plateau fractures surgery. BMC Musculoskelet. Disord. 24(1), 625 (2023).
Bakhsh, E. The benefits and imperative of venous thromboembolism risk screening for hospitalized patients: a systematic review. J. Clin. Med. 12(22) (2023).
Stuck, A. K. et al. Risk assessment models for venous thromboembolism in acutely ill medical patients. A systematic review. Thromb. Haemost. 117(4), 801–808 (2017).
Luksameearunothai, K. et al. Usefulness of clinical predictors for preoperative screening of deep vein thrombosis in hip fractures. BMC Musculoskelet. Disord. 18(1), 208 (2017).
Pannucci, C. J. et al. Individualized venous thromboembolism risk stratification using the 2005 Caprini score to identify the benefits and harms of chemoprophylaxis in surgical patients. Ann. Surg. 265(6), 1094–1103 (2017).
Han, C. et al. The performance of age-adjusted D-dimer cut-off in Chinese outpatients with suspected venous thromboembolism. Thromb. Res. 136(4), 739–743 (2015).
Dempfle, C. E. et al. Sensitivity and specificity of a quantitative point of care D-dimer assay using heparinized whole blood, in patients with clinically suspected deep vein thrombosis. Thromb. Haemost. 96(1), 79–83 (2006).
Yang, S. et al. Risk factors of deep vein thrombosis in adults with Acute Compartment Syndrome following lower extremity fractures. Clin. Appl. Thromb. Hemost. 29, 10760296231165053 (2023).
Chen, W. et al. Risk factors and new diagnostic index for deep venous thrombosis of lower extremities in elderly patients with traumatic femoral neck fracture. Front. Surg. 9, 1050347 (2022).
Li, L. et al. Changes in biomarkers of coagulation, fibrinolytic, and endothelial functions for evaluating the predisposition to venous thromboembolism in patients with Hereditary Thrombophilia. Clin. Appl. Thromb. Hemost. 26, 1076029620944471 (2020).
Loghmani, H. & Conway, E. M. Exploring traditional and nontraditional roles for thrombomodulin. Blood 132(2), 148–158 (2018).
Cheng, X. et al. Identification of thrombomodulin as a dynamic monitoring biomarker for deep venous thrombosis evolution. Exp. Ther. Med. 21(2), 142 (2021).
Luo, H. & Qiao, Y. Correlation analysis of blood TM, TG and D-dimer with deep venous thrombosis formation in patients after total hip arthroplasty. Pak J. Med. Sci. 39(2), 539–543 (2023).
Guo, X. et al. The alpha2-Plasmin inhibitor-plasmin complex is a potential biomarker of venous thromboembolism in orthopedic trauma patients. Clin. Lab., 67(4) (2021).
Lundbech, M. et al. Thrombin generation, thrombin-antithrombin complex, and prothrombin fragment F1 + 2 as biomarkers for hypercoagulability in cancer patients. Thromb. Res. 186, 80–85 (2020).
Nordenhem, A. et al. The complex between tPA and PAI-1: risk factor for myocardial infarction as studied in the SHEEP project. Thromb. Res. 116(3), 223–232 (2005).
Knudson, M. M. et al. Tissue plasminogen activator resistance is an early predictor of posttraumatic venous thromboembolism: a prospective study from the CLOTT research group. J. Trauma. Acute Care Surg. 93(5), 597–603 (2022).
Yang, Y. et al. Plasminogen activator inhibitor-1, thrombin-antithrombin, and prothrombin fragment F1 + 2 have higher diagnostic values than D-dimer for venous thromboembolism after TKA. Clin. Appl. Thromb. Hemost. 28, 10760296221097383 (2022).
Funding
This work was supported by Zhejiang Provincial Medical and Health Science and Technology Project of China (Grant NO. 2025KY583, Grant NO. 2024KY637 and Grant NO. 2024KY020), Zhejiang Traditional Chinese Medicine Science and Technology Program (Grant NO. 2025ZL184 and Grant NO. 2023ZL259), National Natural Science Foundation of China (Grant NO. 82202605), and Zhejiang Provincial Special Support Program for Cultivation of High-Level Innovative Health Talents of China (Grant NO. 2023, Yaoqiang Du).
Author information
Authors and Affiliations
Contributions
ZW and QX designed the study. ZW collected the data. YD drafted the manuscript. YD and XC contributed to the data analyzation. QX supervised the study and revised the manuscript. All authors approved the submitted version.
Corresponding author
Ethics declarations
Ethical approval
Ethical approval of this study was approved by the ethics committee of Zhejiang Provincial People’s Hospital (approval NO. 2024 − 106). Informed consent was waived by Zhejiang Provincial People’s Hospital ethics committee.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this Article contained an error in Affiliation 2, which was incorrectly given as ‘Center for Rehabilitation Medicine, Department of Neurology, Affiliated People’s Hospital), Zhejiang Provincial People’s Hospital, Hangzhou Medical College, Hangzhou, Zhejiang, China’. The correct affiliation is listed here: ‘Center for Rehabilitation Medicine, Department of Neurology, Zhejiang Provincial People's Hospital (Affiliated People's Hospital), Hangzhou Medical College, Hangzhou, Zhejiang, China’.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, Z., Du, Y., Cai, X. et al. Caprini score combined with thrombotic molecular markers for predicting DVT in patients with traumatic fractures. Sci Rep 15, 1847 (2025). https://doi.org/10.1038/s41598-025-85941-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-85941-z