Abstract
Among the factors influencing relapse after clinical cure of chronic hepatitis B(CHB). There is no standardization of baseline HBsAg levels and end-of-treatment HBsAb levels. This multicenter, retrospective study enrolled 136 patients who achieved functional cure from June 2019 to December 2023, and a total of 48 weeks of follow-up was conducted after treatment cessation according to the CHB guidelines. Baseline characteristics of patients were analyzed using univariates. Multifactorial logistic regression was used to analyze the different levels of HBsAg at baseline and HBsAb at the end of treatment in CHB recurrence. The working characteristic curve of the subject was constructed and observed by the column line graphical prediction model. Our data showed the cumulative recurrence rate using Kaplan-Meier survival analysis. At baseline, the level of HBsAg was significantly greater in the group with recurrence than in the group without recurrence (P = 0.038). At EOT, HBsAb levels were lower in the relapsed group than in the nonrelapsed group (P = 0.014). Multivariate logistic regression analysis revealed that a baseline serum HBsAg concentration ≥ 100 IU/mL was a risk factor for recurrence, and an EOT serum HBsAb concentration ≥ 500 mIU/mL was a protective factor for recurrence. Kaplan-Meier survival analysis showed relapse rates of 3.8% and 12.2% for HBsAg ≤ 100 IU/mL at baseline and HBsAb ≥ 500 mIU/mL at the end of treatment, respectively. Functionally cured patients with CHB when baseline HBsAg ≤ 100 IU/mL and HBsAb ≥ 500 mIU/mL at the end of treatment have a low relapse rate.
Similar content being viewed by others
Introduction
The optimal outcome of antiviral therapy for CHB patients is generally regarded as a functional cure. This situation is characterized by a lack of hepatitis B surface antigen (HBsAg) and serum undetectable hepatitis B virus (HBV) DNA, regardless of whether hepatitis B surface antibody (HBsAb) seroconversion has occurred1. The functional cure of CHB represents an important achievement, as it can help to overcome the stigma that can limit social life and professional opportunities. However, the recurrence of CHB after a functional cure also places a considerable financial burden and psychological stress on patients. A real-world study of 2579 patients with HBeAg-negative CHB treated with 48 weeks of combination therapy reported an HBsAg clearance rate of 36.7% (95% CI: 34.9-38.6%)2. Several other studies have reported HBsAg clearance rates ranging from 8.5–33.3%3,4,5. The main analysis of the meta-analysis revealed that baseline HBsAg, baseline HBV DNA, age, sex, genotype and treatment strategy were independently associated with HBsAg clearance achieved with PegIFNα-based therapy6. Novel biomarkers such as lipoprotein(a) or sPD-L1 may serve as important predictors of clinical cure in patients with chronic hepatitis B treated with polyethylene glycol interferon alpha (PEG IFNα)7. Scientists have begun to focus on the phenomenon of functional cure followed by relapse and to point out the problems that exist.
Most studies have attempted to increase the functional cure rate; however, some reports of recurrence in patients with a functional cure exist, and the reasons, predictors and mechanisms are unclear. Recently, numerous studies have been performed to determine factors that can predict recurrence after a functional cure for CHB8,9,10,11,12. Some studies have reported that elevated levels of HBsAb and hepatitis B core antibody (HBcAb) at the EOT are correlated with reduced recurrence8. Multivariate analyses revealed that HBcrAg and HBV RNA at EOT were independent predictors of recurrence9. Baseline HBsAb is an independent predictor of HBsAg seroconversion and viremia recurrence in PEG-interferon (IFN)-administered patients10. In addition, it has been suggested that the quantitative level of HBcAb may serve as a potential predictor of recurrence after the elimination of HBsAg11.The level of baseline HBsAg is predictive of a functional cure for CHB, there are no standardized criteria for specific baseline levels in patients with relapsed CHB, and the level of HBsAb at the end of treatment is still under investigation.
In this study, we established a follow-up cohort at 48 weeks after the functional cure of CHB. To assess the optimal baseline HBsAg and EOT HBsAb levels that influence relapse after a clinical cure for CHB.
Methods
Patients and study design
We retrospectively searched for patients with CHB who achieved a functional cure with peginterferon (PEG-IFN) alone or in combination with nucleos(t)ide analogs (NAs) antiviral treatment at the First Affiliated Hospital of Anhui Medical University and the Provincial Hospital of the University of Science and Technology of China between June 2019 and December 2023.
Inclusion and exclusion criteria
The inclusion and selection criteria for this study were in accordance with the Guidelines for the Avoidance and Control of Chronic Hepatitis B (2022 Edition) developed as part of the Infectious Diseases Branch of the Chinese Medical Association13,14. The exclusion criteria included cirrhosis or hepatocellular carcinoma, other viral (hepatitis C or D virus, HIV, Epstein–Barr virus, and cytomegalovirus) infections, comorbidities (autoimmune and alcoholic hepatitis, genetic metabolic hepatitis), steatohepatitis, drug-induced liver disease, and other hepatic disorders), other malignant neoplasms, immune-modulating therapy for other diseases, and pregnancy or breastfeeding. This clinical research was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University (approval number PJ 2022-08-12) and conducted in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients.
Data collection
We collected basic information about the participants, such as sex, age, and family history of hepatitis B virus (HBV) infection. Hepatitis B virus (HBV) measures (including HBsAg, HBsAb, HBeAg, HBeAb, and HBcAb), blood biochemistry (ALT and AST) and HBV DNA were measured every 12 weeks. The HBV markers were determined via Abbott Ireland’s Chemiflex technology, and the biochemical indices were determined via a Beckman BX800 automatic biochemistry instrument and its corresponding reagents. HBV DNA was quantified via real-time quantitative PCR (polymerase chain reaction). Patients treated with NAs for CHB were defined as those who had been prescribed one or more NAs during any one course of treatment, and those receiving IFN were defined as those who had been given PEG-IFN α-2a/2b.
Definition of events
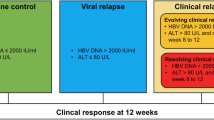
Chronic HBV infection was defined as the presence of HBsAg in the blood or serum over 24 weeks, with or without evidence of persistent HBV infection. “HBsAg loss” refers to the transition from a positive to negative HBsAg result in a single test. Confirmed HBsAg loss was considered to have occurred if at least one further result was negative for HBsAg after HBsAg loss, with a 6-month interval between the two tests. A functional cure is defined as the sustained disappearance of HBsAg (lasting more than 6 months after treatment has ended) with or without seroconversion to anti-HBs, also known as a clinical cure. Recurrence is considered to be the re-emergence of HBsAg, HBV DNA or both on one of two occasions within 4–8 weeks of stopping treatment15.
Statistical analyses
Statistics were carried out via SPSS 26.0 (IBM SPSS Statistics for Windows, version 26) and R, version 4.2.1. (R Foundation, Vienna, Austria). Comparisons were made via the t test for normally spaced variables, and the data are presented as the means ± standard deviations. Nonnormally adjusted data are shown as medians (25th and 75th percentiles), and nonparametric tests were used for group comparisons. Qualitative differences between groups were calculated via Fisher’s approximate test, and quantitative differences were calculated via Student’s t test and the Mann‒Whitney U test. Cumulative recurrence was estimated via Kaplan‒Meier survival analysis, with the log-rank test used to evaluate between-group differences. Multifactor logistic and regression analyses were applied to construct nomogram plots, develop a receiver operating characteristic (ROC) prediction model, construct decision curve analysis curves to assess clinical applicability, and construct calibration curves to assess model calibration. The significance level was P < 0.05.
Results
Characteristics of recurrent and nonrecurrent patients
The participant selection procedure is shown in Fig. 1. Altogether, 1386 patients with CHB received PEG-IFN alone or PEG-IFN in combination with NA. Among these patients, 416 patients achieved a negative HBsAg result ≥ 1, and HBsAg loss was confirmed in 394 patients. Among the 371 patients who completed treatment, 136 completed 48 weeks of follow-up. Among these patients, 27 patients relapsed, and 109 did not. The cohort consisted of 95 men and 41 women; 16 were younger than 30 years, and 120 were older than 30 years. Among these patients, 53 received PEG-IFN alone, and 83 received a combination of NAs and PEG-IFN (Table 1). At EOT, all patients had a level of HBV DNA less than 300 IU/mL and HBsAg less than 0.05 IU/mL. Twenty-seven of the patients who completed the 48-week observation experienced recurrence: 13 experienced recurrence within 12 weeks, 7 experienced recurrence within 24 weeks, 5 experienced recurrence within 36 weeks, and 2 experienced recurrence within 48 weeks (Table 1).
HBsAg and HBsAb levels in the recurrence and nonrecurrence groups
To evaluate the relationship between HBsAg and HBsAb results and recurrence, we compared these results between patients with and without recurrence at baseline and at the EOT (Fig. 2). At baseline, the level of HBsAg was significantly greater in the group with recurrence than in the group without recurrence (P = 0.038). At EOT, HBsAb levels were lower in the relapsed group than in the nonrelapsed group (P = 0.014).
Cumulative probability of recurrence
The 136 patients who completed follow-up were assigned to the following two groups according to their baseline HBsAg results: HBsAg ≤ 100 IU/mL and HBsAg > 100 IU/mL. Kaplan–Meier curve analysis revealed that the cumulative recurrence probabilities during 48 weeks of follow-up were 12.2% and 29% in the two groups, respectively (Fig. 3B). Patients were assigned to three outcome groups: HBsAb < 100 mIU/mL, 100 ≤ HBsAb < 500 mIU/mL, and HBsAb ≥ 500 mIU/mL, depending on the HBsAb level at the EOT. The cumulative probabilities of recurrence were 28.4%, 16.3%, and 3.8% in the three groups, respectively (Fig. 3C). The cumulative rates of EOT recurrence at 12, 24, 36, and 48 weeks were 9.7%, 14.7%, 18.4%, and 19.8%, respectively (Fig. 3A). Clinical recurrence occurred most commonly at week 12 (n = 13 [48.15%]). During the 48-week follow‐up, none of the patients experienced hepatocellular carcinoma (HCC) or developed cirrhosis decompensation.
(A) Cumulative recurrence rate at the EOT with a complete follow-up of 48 weeks. (B) Cumulative relapse rates for HbsAg ≥ 100 IU/mL and HbsAg < 100 IU/mL at the EOT with a complete 48-week follow-up. (C) Cumulative recurrence rates for HBsAb < 100 mIU/mL, 100–500 mIU/mL and ≥ 500 mIU/mL at the EOT with a complete follow-up of 48 weeks.
Predictors of recurrence after a functional cure in CHB patients
Among the 136 patients who completed the 48-week follow-up after the EOT, 27 and 109 patients experienced recurrence and no recurrence, respectively. In the multifactorial logistic regression analyses, there were no differences in sex, age, or treatment (Table 1). However, there were significant differences in the baseline HBsAg and EOT HBsAb levels (P < 0.05).
Multivariate logistic regression analysis revealed that a baseline serum HBsAg concentration ≥ 100 IU/mL (OR = 3.74, 95% CI: 1.47–9.52, P = 0.006) was a risk factor for CHB recurrence. This shows that patients with baseline HBsAg levels greater than or equal to 100 IU/mL are likelier to suffer a recurrence than patients with baseline HBsAg levels below 100 IU/mL. An EOT serum HBsAb concentration ≥ 500 mIU/mL (OR = 0.08, 95% CI: 0.01–0.63, P = 0.017) was a protective factor for CHB recurrence.This implies that patients with EOT serum HBsAb concentrations greater than or equal to 500 mIU/mL are less likely to relapse than those with less than 500 mIU/mL(Table 2). A nomogram was constructed to represent the predictive model from the results of the multivariable logistic regression analysis (Fig. 4). The AUC of the nomogram was 0.74 (95% CI, 0.65–0.83) (Fig. 5A). The sensitivity was 42%, and the specificity was 93%. The predictive model was well calibrated by bootstrapping (number of resamples = 1000) (Fig. 5B), and the decision curve (Fig. 5C) indicated that the predictive model could be effectively applied in a clinical setting.
Discussion
The objective of CHB treatment, as determined via the National and International guidelines, is to enhance long-term outcomes by optimizing the sustained inhibition of HBV16,17,18.CHB treatment aims to achieve a cure. Cures for hepatitis B can be classified according to the existence or lack of specific biomarkers and the possibility of long-term results. Partial healing, functional healing and complete healing are included in this classification system19. A complete cure for CHB is not currently achievable owing to the difficulty in removing CCCDNA from the host. A functional cure can be achieved in some patients following IFN therapy alone or in combination with NAs. However, there is still a risk of recurrence after a functional cure, and there is no consensus on the factors affecting recurrence. This study investigated the effects of baseline HBsAg and EOT HBsAb levels on CHB recurrence after functional cure.
HBsAg levels at baseline have been well studied for predicting improved clinical cure rates and for screening populations at risk for CHB. One study enrolled 292 patients who were HBsAg less than 1500 IU/ml and negative for both HBeAg and HBV DNA, all of whom were treated with PEG-IFNa-2b. The lower the HBsAg level is, the greater the HBsAg clearance and functional cure rate20. Some studies have recruited 135 patients with HBsAg less than 100 IU/ml who were treated with NAs. The patients were followed for 5 years after the end of treatment. Patients with HBsAg levels less than 40 IU/ml at the end of treatment had significantly lower virological and clinical recurrence rates than those with HBsAg levels greater than 40 IU/ml (P < 0.001)21. A meta-analysis revealed that age, duration of consolidation therapy, and baseline and EOT HBsAg levels are predictors of recurrence in CHB patients with HBeAg-negative disease who discontinue NA therapy12. Among the above studies, few relate to low baseline HBsAg levels, as relapse after CHB has been treated with PEG-IFN alone or in combination with NAs, and a functional cure has been achieved. On the basis of univariate analysis, patients in this study were assigned to two groups depending on baseline HBsAg levels, with the recurrence group having a higher baseline HBsAg level than the nonrecurrence group. Multiple logistic regression identified a baseline HBsAg concentration ≥ 100 IU/mL as a predictor of recurrence following functional cure in CHB patients. The cumulative recurrence rate according to the Kaplan‒Meier curve was greater in patients with HBsAg ≥ 100 IU/mL than in patients with HBsAg < 100 IU/mL. These results suggest that patients with a baseline HBsAg level < 100 IU/mL have a low recurrence rate.
HBsAb is an antibody with specific protective properties. By neutralizing HBsAg, HBsAb prevents further HBsAg infection.Furthermore, HBsAb can predict CHB recurrence after a functional cure. A retrospective study revealed that in 32 patients who experienced recurrence after functional cure, HBsAb and HBcAb levels at the EOT were predictors of recurrence (P < 0.001, P = 0.023)8. Gao et al.. reported that HBsAb levels ≥ 100 mIU/mL at the time of drug discontinuation (OR 0.110, 95% CI: 0.034–0.353; P < 0.001) were a predictor of HBsAg seroreversion22. Xie et al.. used multifactorial analysis and reported that EOT HBsAb levels and ≥ 12 weeks of consolidation were independent predictors of sustained HBsAg clearance, with an HBsAb threshold of 62 mIU/mL23. Another cross-sectional study revealed that recurrence after functional cure of CHB is associated with a high HBsAb level at the EOT, with 36-month cumulative recurrence rates of 2.8% and 59.1% in patients with HBsAb levels ≥ 1.3 log10 IU/L and < 1.3 log10 IU/L, respectively (P < 0.001)24. In previous studies, there has been no uniform indication of HBsAb levels that should be maintained at EOT to reduce recurrence. In our study, patients were classified into three groups according to their EOT HBsAb level, and HBsAb ≥ 500 mIU/mL was found to be a predictor of functional cure. The cumulative relapse rate after 48 weeks of follow-up in the Kaplan–Meier analysis was only 3.8%, which was considerably lower than that of the HBsAb < 100 mIU/mL and 100 ≤ HBsAb < 500 mIU/mL groups. Thus, our study suggests that the recurrence rate is low in patients with HBsAb levels ≥ 500 mIU/mL at EOT.
The cumulative rates of virologic relapse at 12, 24, 36 and 48 weeks were 40.2%, 54.3%, 57.6% and 63.0%, respectively, in a prospective study of 97 NA-treated patients who completed 48 weeks of follow-up at the end of treatment9. Zhiliang Gao’s team enrolled 222 patients who completed 48 weeks of follow-up after the end of PEG-IFN therapy, and the cumulative HBsAg seroreversal and virologic relapse rates were 13.5% and 1.8%, respectively. At 48 weeks, the majority of patients with HBV relapse (86.6%) maintained undetectable HBV DNA levels after HBsAg seroreversion22.
Previous studies have identified the following factors as influencing relapse after functional cure of chronic hepatitis B (CHB): sex, age, family history, alanine transaminase (ALT), aspartate transaminase (AST), HBV-DNA, hepatitis B genotype, HBV-RNA, hepatitis C core antibody (HBcAb), hepatitis B core antigen (HBcAg), baseline and end-of-treatment levels of HBsAg, end-of-treatment levels of HBsAb, and so on6,7,8,9,11,12,13. Given the above factors, the ability of a single factor to predict recurrence after functional cure of CHB is usually limited, and a comprehensive prediction model incorporating multiple factors is usually required to provide more accurate predictions6. Why are patients with baseline HBsAg > 100 IU/mL and EOT HBsAb < 500 mIU/mL more likely to relapse after clinical cure? The main factors are HBV replication and host HBsAg production (cccDNA and integrated HBV DNA), high viral loads (HBV DNA and HBsAg), and host innate and adaptive immune responses to HBV19.Any HBV therapy must target both cccDNA and integrated HBV DNA25.Interferon and nucleoside analogs have been shown to act synergistically in the treatment of CHB to achieve a functional cure for the disease26,27. Interferon has been shown to enhance the antiviral activity of natural killer (NK) cells and lead to the depletion of CD8 + T-cell function, thus limiting the restorative effect on HBV-specific CD8 + T-cell function. Interferon has been shown to promote the degradation of intracellular cccDNA in an in vitro study28.Nucleoside analogs, in turn, have been shown to inhibit HBV replication and directly potentiate the effects of interferon-induced activation of the innate immune system29,30. Consequently, a combination of these two factors has been shown to promote functional restoration of HBV-specific T cells and innate and adaptive immune cells31,32. The combination therapy of PEG-IFN and NAs yields higher rates of HBsAg seroclearance compared to monotherapy and achieves a functional cure of CHB. Higher levels of HBsAg in CHB infection create a tolerant intrahepatic and extrahepatic environment. High levels of circulating HBsAg are thought to promote HBV persistence through several mechanisms33,34. That’s why patients with chronic hepatitis B who achieve functional cure with HBsAg levels above 100 IU/mL at baseline are more likely to relapse.HBsAb levels reflect the body’s ability to mount an immune response to the HBV.Lower levels of HBsAb may indicate that a patient’s immune system is less effective at controlling the hepatitis B virus (HBV). Therefore, patients with functionally cured chronic hepatitis B (CHB) who have HBsAb levels below 500 mIU/mL at the end of treatment are at a greater risk of experiencing a relapse.Several studies referenced above have demonstrated that functionally cured chronic hepatitis B (CHB) patients who have higher levels of hepatitis B surface antibodies (HBsAb) at the end of treatment experience lower relapse rates22,23,24. In contrast, patients with low or negative HBsAb levels at the conclusion of treatment tend to have higher relapse rates. These findings align with the results presented in this paper.
This study constructed a nomogram plot to represent the prediction model on the basis of multivariate logistic regression results. The AUC of the nomogram model was 0.74 (95% CI: 0.65–0.83), indicating a sensitivity of 42% and a specificity of 93%. The construction of a nomogram plot model and the generation of ROC curves for the prediction of recurrence following clinical cure of CHB have been uncommon procedures in previous studies. Our proposed prediction model is suitable for application in clinical practice. However, the short follow-up, loss to follow-up and relatively small cohort size were some of the limitations of this study. Future studies of longer durations and larger scales are needed, and the use of highly sensitive methods could aid in the detection of patients with low viral loads to gain insight into the factors influencing the recurrence of CHB in functionally cured patients.
Conclusions
In summary, in our study, we found that patients who were functionally cured of CHB had a low incidence of recurrence among patients with baseline HBsAg levels less than 100 IU/mL and HBsAb levels greater than or equal to 500 mIU/mL at the end of treatment.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due [REASON WHY DATA ARE NOT PUBLIC] but are available from the corresponding author on reasonable request.
Abbreviations
- CHB:
-
Chronic hepatitis B
- HBV:
-
Hepatitis B virus
- HBsAg:
-
Hepatitis B surface antigen
- HBsAb:
-
Hepatitis B surface antibody
- HBeAg:
-
Hepatitis B e antigen
- HBeAb:
-
Hepatitis B e antibody
- HBcAb:
-
Hepatitis B core antibody
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- HBV DNA:
-
Hepatitis B virus DNA
- NAs:
-
Nucleos(t)ide analogs
- EOT:
-
End-of-treatment
- IFN:
-
Interferon
- OR:
-
Odds ratio
- PEG-IFN:
-
Pegylated interferon
- ROC:
-
Receiver operating characteristic curve
- HCC:
-
Hepatocellular carcinoma
References
Howell, J. et al. Pathway to global elimination of hepatitis B: HBV cure is just the first step. Hepatology 78 (3), 976–990 (2023).
Chu, J. H. et al. Real-world study on HBsAg loss of combination therapy in HBeAg-negative chronic hepatitis B patients. J. Viral Hepat. 29 (9), 765–776 (2022).
Boglione, L., Cariti, G., Di Perri, G. & D’Avolio, A. Sequential therapy with entecavir and pegylated interferon in a cohort of young patients affected by chronic hepatitis B. J. Med. Virol. 88 (11), 1953–1959 (2016).
Ning, Q. et al. Switching from entecavir to PegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: a randomised open-label trial (OSST trial). J. Hepatol. 61 (4), 777–784 (2014).
Wursthorn, K. et al. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology 44 (3), 675–684 (2006).
Jiang, S. et al. Predictors of HBsAg seroclearance in patients with chronic HBV infection treated with pegylated interferon-alpha: a systematic review and meta-analysis. Hepatol. Int. 18 (3), 892–903 (2024).
Huang, L. et al. Efficacy of pegylated interferon alpha-2b plus entecavir therapy and predictors of treatment success in children with chronic hepatitis B. Front. Immunol. 14, 1282922 (2023).
Lin, X. et al. Study on the Retreatment, Outcome, and potential predictors of recurrence in patients with recurrence of Hepatitis B after Functional Cure. Front. Immunol. 13, 879835 (2022).
Kaewdech, A. et al. Hepatitis B surface antigen, core-related antigen and HBV RNA: Predicting clinical relapse after NA therapy discontinuation. Liver Int. 40 (12), 2961–2971 (2020).
Pan, C. Q. et al. Outcome of Chinese patients with hepatitis B at 96 weeks after functional cure with IFN versus combination regimens. Liver Int. 41 (7), 1498–1508 (2021).
Wu, Y., Wang, X., Lin, X., Shen, C. & Chen, X. Quantitative of serum hepatitis B core antibody is a potential predictor of recurrence after interferon-induced hepatitis B surface antigen clearance. J. Microbiol. Immunol. Infect. 54 (2), 238–244 (2021).
Liu, Y., Jia, M., Wu, S., Jiang, W. & Feng, Y. Predictors of relapse after cessation of nucleos(t)ide analog treatment in HBeAg-negative chronic hepatitis B patients: a meta-analysis. Int. J. Infect. Dis. 86, 201–207 (2019).
Xu, W. X. & Peng, L. Response to: Comment on 48-Week Outcome after Cessation of Nucleos(t)ide Analogue Treatment in Chronic Hepatitis B Patient and the Associated Factors with Relapse. Can J Gastroenterol Hepatol 2019:2970510. (2019).
You, H. et al. Guidelines for the Prevention and Treatment of Chronic Hepatitis B (version 2022). J. Clin. Transl Hepatol. 11 (6), 1425–1442 (2023).
Lok, A. S., Zoulim, F., Dusheiko, G. & Ghany, M. G. Hepatitis B cure: from discovery to regulatory approval. Hepatology 66 (4), 1296–1313 (2017).
European Association for the Study of the Liver. Electronic address eee, European Association for the study of the L: EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J. Hepatol. 67 (2), 370–398 (2017).
Cornberg, M., Lok, A. S., Terrault, N. A., Zoulim, F. & Faculty, E-A-H-T-E-C. Guidance for design and endpoints of clinical trials in chronic hepatitis B - Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference(double dagger). J Hepatol 72(3):539–557. (2020).
Chinese Society of Infectious Diseases CMA, Chinese Society of Hepatology CMA. [The guidelines of prevention and treatment for chronic hepatitis B (2019 version)]. Zhonghua Gan Zang Bing Za Zhi. 27 (12), 938–961 (2019).
Wong, G. L. H., Gane, E. & Lok, A. S. F. How to achieve functional cure of HBV: stopping NUCs, adding interferon or new drug development? J. Hepatol. 76 (6), 1249–1262 (2022).
Yang, X. et al. Interferon add-on therapy increased clinical cure significantly for interferon-experienced chronic hepatitis B patients with low HBsAg. Front. Immunol. 13, 997608 (2022).
Tseng, T. N. et al. Incidence and factors Associated with HBV Relapse after Cessation of Entecavir or Tenofovir in patients with HBsAg below 100 IU/mL. Clin. Gastroenterol. Hepatol. 18 (12), 2803–2812e2802 (2020).
Gao, N. et al. Role of hepatitis B surface antibody in seroreversion of hepatitis B surface antigen in patients achieving hepatitis B surface antigen loss with pegylated interferon-based therapy. J. Viral Hepat. 29 (10), 899–907 (2022).
Li, M. H. et al. Predictors of sustained functional cure in hepatitis B envelope antigen-negative patients achieving hepatitis B surface antigen seroclearance with interferon-alpha-based therapy. J. Viral Hepat. 26 (Suppl 1), 32–41 (2019).
Guo, Y. et al. End-of-treatment anti-HBs levels and HBeAg status identify durability of HBsAg loss after PEG-IFN discontinuation. Front. Cell. Infect. Microbiol. 13, 1120300 (2023).
Cornberg, M. & Manns, M. P. Hepatitis: no cure for hepatitis B and D without targeting integrated viral DNA? Nat. Rev. Gastroenterol. Hepatol. 15 (4), 195–196 (2018).
Micco, L. et al. Differential boosting of innate and adaptive antiviral responses during pegylated-interferon-alpha therapy of chronic hepatitis B. J. Hepatol. 58 (2), 225–233 (2013).
Xun, Z. et al. Taurocholic acid inhibits the response to interferon-alpha therapy in patients with HBeAg-positive chronic hepatitis B by impairing CD8(+) T and NK cell function. Cell. Mol. Immunol. 18 (2), 461–471 (2021).
Lucifora, J. X. Y. et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science 343, 1221–1228 (2014).
Gill, U. S. & Kennedy, P. T. F. The impact of currently licensed therapies on viral and immune responses in chronic hepatitis B: considerations for future novel therapeutics. J. Viral Hepat. 26 (1), 4–15 (2019).
Pang, X. et al. Combination of pegylated interferon-alpha and nucleos(t)ide analogue treatment enhances the activity of natural killer cells in nucleos(t)ide analogue experienced chronic hepatitis B patients. Clin. Exp. Immunol. 202 (1), 80–92 (2020).
de Niet, A. et al. Restoration of T cell function in chronic hepatitis B patients upon treatment with interferon based combination therapy. J. Hepatol. 64 (3), 539–546 (2016).
Tout, I. et al. Hepatitis B surface antigen seroclearance: Immune mechanisms, clinical impact, importance for drug development. J. Hepatol. 73 (2), 409–422 (2020).
Schuch, A. et al. Phenotypic and functional differences of HBV core-specific versus HBV polymerase-specific CD8 + T cells in chronically HBV-infected patients with low viral load. Gut 68 (5), 905–915 (2019).
Christoph Höner zu Siederdissen MC. The role of HBsAg levels in the current management of chronic HBV infection. Annals Gastroenterol. 27, 105–112 (2014).
Acknowledgements
We thank Prof Yufeng Gao and Prof Jiabin Li for their financial support and all the authors who contributed to this paper.
Funding
The study was supported by the Natural Science Foundation of Anhui Province (No. 2022AH040160), the National Natural Science Foundation of China (No. 82370608), the National Natural Science Foundation of China (No. 81973983), the Translational Clinical Medical Research of Anhui Province (No. 202304295107020040) and the Natural Science Foundation of Anhui Province (No. 2208085MH204).
Author information
Authors and Affiliations
Contributions
Lianxiu Han completed the preliminary research design, data analysis, visualization, and manuscript drafting. Zilong Wang participated in the data acquisition and data entry. Luyang Kang participated in the data entry. Xiaoling Cui participated in the data entry. Yi Li and Huafa Yin provided patient information. Yufeng Gao and Jiabin Li controlled and coordinated the research design. All the authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University.All methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, L., Wang, Z., Kang, L. et al. Predicting relapse after achieving a functional cure for chronic hepatitis B (CHB) using baseline HBsAg and end-of-treatment HBsAb levels. Sci Rep 15, 13873 (2025). https://doi.org/10.1038/s41598-025-86555-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-86555-1