Abstract
Phase angle (PhA) is an indicator of nutritional status. The association between PhA and all-cause mortality has been confirmed in older adults, but no relevant studies have been conducted in adults aged 18–49 years. This study aimed to investigate the relationship between PhA and all-cause mortality in adults aged 18–49 years. Our study included 6338 adults aged 18–49 from the National Health and Nutrition Examination Survey (1999–2004). Kaplan-Meier (KM) curve, multiple Cox regression and stratified analyses were performed. These adults were divided into two groups based on the median PhA value (the low PhA group: PhA < 6.96°; the high PhA group: PhA ≥ 6.96°). During a median follow-up of 214 months, 307 all-cause deaths were recorded. The all-cause mortality rate was 5.07% in the low PhA group and 3.98% in the high PhA group. After full adjustment, a higher PhA value was significantly associated with reduced risk of all-cause mortality (HR = 0.73,95CI%:0.59–0.91, P = 0.005). Similarly, compared with those in the low PhA group, individuals in the high PhA group had a lower risk of all-cause mortality (HR = 0.67, 95% CI 0.46–0.98, P = 0.041). There was an interaction between smoking status and PhA (P for interaction = 0.029), with a statistically significant negative association between PhA and all-cause mortality in the non-smokers (HR:0.62, 95%CI:0.53–0.88, P = 0.003). In conclusion, there was a significant negative association between PhA and all-cause mortality in adults aged 18–49 years. The negative association was stronger in non-smokers.
Similar content being viewed by others
Introduction
Malnutrition can exacerbate individual vulnerability and incapacitation, thereby amplifying the burden of disease and mortality rates, especially among the older adults with chronic disease1. Common contributory factors to malnutrition include aging, acute and chronic diseases, insufficient dietary intake, sarcopenia, etc2,3,4,5. According to the Global Leadership Initiative on Malnutrition (GLIM) guidelines, a range of tools and methods are used for nutritional assessment, including body composition measurements, questionnaires, biomarkers, and medical devices such as computed tomography (CT), bioelectrical impedance analysis (BIA) and dual-energy X-ray absorptiometry (DXA)6.
BIA is widely used as an inexpensive, convenient, and non-invasive method for body composition analysis, by measuring the resistance and reactance of the entire body and specific body segments at designated frequencies7. In clinical trials, the prevailing practice involves single-frequency phase detection utilizing a 50 kHz single-frequency device with a minimum of 4 electrodes affixed at the wrist and ankle8. Phase angle (PhA), the most commonly used impedance parameter in clinical practice and a reliable indicator of nutritional status, is calculated using resistance (R) and reactance (Xc) in BIA8,9,10. PhA reflects the integrity of cellular health and membrane integrity and is an important parameter for assessing the state of cellular health11,12. A high PhA usually indicates good cell membrane integrity and function, while a low PhA may indicate reduced cell function and malnutrition13. In addition, PhA was also directly associated with a variety of nutritional indicators such as body mass index (BMI) and grip strength, which further supports its validity as a nutritional assessment tool14,15. Age differences may affect the PhA level in the body8. With advancing age, a reduction in muscle mass is associated with a decline in Xc, whereas a decrease in body water content is linked to an increase in R, ultimately resulting in a reduction in the PhA level. PhA gradually grows from birth to age 18, stabilizes between the ages of 19 and 48, and then gradually diminishes in older subjects and the elderly16,17.
Our study aimed to investigate the association between PhA and all-cause mortality in adults aged 18–49 years. Much of the existing literature has focused on older adults and chronically ill patients9,16,18,19,20, leaving a gap in understanding nutritional influences on mortality outcomes in younger, generally healthier populations. Additionally, the sample size was large in our study, enhancing the robustness of our findings and providing a reliable assessment of the impact of nutritional status on all-cause mortality in this demographic.
Materials and methods
Data sources and study population
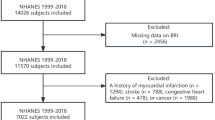
The National Health and Nutrition Examination Survey (NHANES) is a nationwide survey administered by the National Center for Health Statistics (NCHS). NHANES employs a complex multistage probability sampling design, which divides the U.S. into primary sampling units that are further organized into strata and neighborhoods, from which households and participants are randomly selected, with each participant representing approximately 50,000 non-institutionalized civilians in the population. It aims to monitor the health and nutritional status of individuals across the United States, serving as a foundational resource for the development of health policies. The survey is conducted every two years with questionnaire interviews, physical and laboratory examinations of participants. Our study population was derived from three cycles of the NHANES (including 1999–2000, 2001–2002, 2003–2004). The detailed screening process is shown in Fig. 1. The exclusion criteria for the study included the following: (1) failure to complete BIA; (2) lack of data on resistance and reactance, making it impossible to calculate the PhA value; (3) participants younger than 18 years of age; (4) without mortality outcomes. This survey was approved by the NCHS Ethics Review Board and written informed consent has been obtained from all participants, specific details can be found at https://www.cdc.gov/nchs/nhanes/irba98.htm. All methods were performed in accordance with the relevant guidelines and regulations.
Assessment of PhA
BIA is a method used to measure the electrical impedance of body tissues, enabling the assessment of fluid volumes, total body water, fat mass, and fat-free mass. In NHANES, BIA was administered to eligible survey participants 8–49 years of age. A portion of the participants were not eligible for the BIA examination, including (1) pregnant females; (2) individuals with amputated limbs; (3) those with artificial joints, pins, metal plates, or other types of metal objects in their bodies, pacemakers or automated defibrillators, coronary stents or metal suture material in the heart; (4) individuals weighing more than 300 pounds.
The quality of the collected raw frequency data was evaluated through an external Hydra modeling program provided by Xitron Technologies, Inc. The program measured R, Xc, and calculated the PhA value at each measured frequency. The PhA measured at 50 kHz is the most widely utilized9,21, calculated by the following formula: PhA (°) = arctangent (Xc/R) * (180/π)21.
All-cause mortality
NCHS has linked data from the NHANES survey with death certificate records obtained from the National Death Index (NDI). The Linked Mortality Files (LMF) have been regularly updated with mortality follow-up data through December 31, 2019. By referencing the participants’ unique respondent sequence number, we can ascertain their survival status. We accessed and downloaded relevant data at https://www.cdc.gov/nchs/data-linkage/mortality-public.htm.
Covariates
Our study incorporated sociodemographic variables, including age, gender, race, education level, marital status, and income information. Body measurements were collected, including fat-free mass, fat mass, and BMI. Information on participants’ comorbidities (including asthma, chronic obstructive pulmonary disease, cardiovascular disease, hypertension, diabetes, and cancer) and lifestyle habits, such as smoking and drinking, were also collected.
Statistical analysis
The weighted analysis was conducted following NHANES guidelines to account for the complex survey design, and to ensure that our estimates are representative of the US general population. The study population was stratified into two groups based on the median PhA value (the low PhA group: PhA < 6.96°; the high PhA group: PhA ≥ 6.96°). Continuous variables were presented as mean values and the first and third quartiles (Q1, Q3), while categorical variables were presented as percentages and 95% confidence intervals (CI). The survey-weighted linear regression was utilized for continuous variables, and the survey-weighted chi-square test was employed for categorical variables to evaluate differences. To initially assess the survival rate between the groups, Kaplan-Meier curves were plotted. Subsequently, we constructed several different Cox regression models: unadjusted model; Model 1 was adjusted for age, gender, race, education level, marital status, and income information; Model 2 was adjusted for age, gender, race, education level, marital status and income information, fat mass, fat-free mass, BMI, drinking and smoking; Model 3 was additionally adjusted for comorbidities based on Model 2. Additionally, stratified analyses were conducted across different demographic groups. Statistical analyses were conducted using R4.2.3 software and a two-tailed P < 0.05 was considered statistically significant.
Results
The baseline characteristics of participants in the low and high PhA groups (weighted) were detailed in Table 1, and the total population (unweighted and weighted) can be seen in Supplementary Table 1. In contrast to the low PhA group (PhA < 6.96°), individuals in the high PhA group (PhA ≥ 6.96°) were younger and had a higher proportion of males. The low and high PhA groups predominantly consisted of non-Hispanic white and individuals with higher levels of education. Compared to the low PhA group, the high PhA group had a higher fat-free mass, lower fat mass, and a higher BMI. Regarding comorbidities, statistically significant differences were observed between the two groups, except for asthma and diabetes. The average follow-up time for individuals in the high PhA group was 212.14 months, with a mortality rate of 3.98%. The low PhA group exhibited a mean follow-up time of 211.58 months, with a mortality rate of 5.07%.
Kaplan-Meier survival plot and the log-rank test were used to compare the survival differences between the two groups (Fig. 2). The survival probability of the population in the high PhA group was higher than that of the population in the low PhA group (P = 0.047).
The results of the Cox regression model were summarized in Table 2. In the analysis of continuous PhA, a statistically significant association with all-cause mortality was observed in the unadjusted model, showing a hazard ratio (HR) of 0.83 (95% CI: 0.71–0.98, P = 0.032). After adjustments, the risk of all-cause mortality decreaseed further in Model 1, Model 2, and Model 3, with HRs of 0.72 (95% CI: 0.58–0.88, P = 0.002), 0.66 (95% CI: 0.51–0.86, P = 0.002), and 0.73 (95% CI: 0.59–0.91, P = 0.005), respectively. For categorized PhA, individuals with low PhA (PhA < 6.96°) are treated as the reference group. In the unadjusted model, those with high PhA (PhA ≥ 6.96°) exhibited a HR of 0.78 (95% CI: 0.60–1.02, P = 0.074). This relationship strengthened in the adjusted models, with HRs of 0.66 (95% CI: 0.48–0.91, P = 0.011), 0.66 (95% CI: 0.45–0.98, P = 0.041), and 0.67 (95% CI: 0.46–0.98, P = 0.041) in Model 1, Model 2 and Model 3, respectively, indicating a statistically significant lower risk of all-cause mortality.
Finally, stratified analyses were performed (Fig. 3). Except for Mexican Americans and non-Hispanic whites and blacks, the negative association between PhA and all-cause mortality was statistically significant in other Hispanic (HR: 0.41, 95% CI: 0.18–0.94, P = 0.035) and other races (HR:0 .38, 95% CI: 0.22–0.66, P = 0.001). Additionally, there was a statistically significant negative association between PhA and all-cause mortality among those with lower level of education (HR: 0.72, 95% CI: 0.56–0.92, P = 0.009), without partner (HR: 0.75, 95% CI: 0.58–0.97, P = 0.026), and lower level of income (HR: 0.69, 95% CI: 0.52–0.92, P = 0.012). Moreover, PhA was similarly negatively associated with all-cause mortality in high adiposity (HR: 0.62, 95% CI: 0.45–0.85, P = 0.003) and overweight (HR: 0.62, 95% CI: 0.41–0.92, P = 0.019) and obese populations (HR: 0.65, 95% CI: 0.46–0.92, P = 0.015). Interestingly, we also observed an interaction between smoking status and PhA (P for interaction = 0.029), with a statistically significant negative association between PhA and all-cause mortality in the non-smokers (HR: 0.62, 95% CI: 0.53–0.88, P = 0.003) and no association in the smokers (HR: 1.04, 95% CI: 0.74–1.47, P = 0.813). As shown in Fig. 3, the negative association between PhA and all-cause mortality can be seen in terms of comorbidities.
Discussion
To our knowledge, this is the first study to investigate the association between PhA and all-cause mortality within a substantial sample of adults aged 18–49 years. Our study demonstrated a significant negative association between PhA and all-cause mortality in adults aged 18–49 years, and the negative association was stronger in non-smokers.
In our study, the mean age was 34 years, and the mean PhA was 6.86°. A meta-analysis of 249,844 participants showed that the mean PhA for men and women aged 18–38 years were 7.3° and 6.4°, respectively; over the age of 80 years, the values dropped to 5.3° and 5.4°, respectively16. Compared with the low PhA group, the high PhA group had a 33% lower risk of all-cause mortality, while the total population had a 27% lower risk of all-cause mortality for every 1° increase in the PhA value. Similarly, Young Eun Kwon et al. indicated that low PhA was significantly associated with mortality in older adults (mean age 83 years), with a 54% reduction in the risk of death for every 1° increase in PhA value22. In addition, lower individual-standardized PhA increased the risk of prolonged hospitalization by 7%, along with a 7.87-fold increase in the risk of death within 12 months23. Moreover, García-García, C et al. noted that PhA was one of the prognostic factors for cancer patients, and high PhA was significantly associated with a lower risk of mortality (HR:0.42, P = 0.014)24. In general, our study demonstrated that PhA is associated with all-cause mortality in adults aged 18–49 years.
PhA is an indicator measured by BIA and is commonly used to assess the nutritional status and mortality risk of individuals8,25. The results of our study indicate a significant association between PhA and all-cause mortality in adults aged 18–49 years. The lower in PhA, the higher the risk of all-cause mortality. In adults, lower PhA is associated with an increased subsequent risk of dying prematurely and cardiovascular disease26. This finding may be related to the sensitivity of PhA to malnutrition. Studies indicate that PhA is sensitive to changes in body composition and can detect the shift from intracellular to extracellular water early in disease-associated malnutrition27,28. Malnutrition is typically accompanied by a shift in intracellular to extracellular water, which results in a reduction in cell mass. These changes can be reflected by alterations in PhA29. Therefore, PhA, as a marker of nutritional status, may account for some of the observed association with mortality.
In subgroup analyses, we found that the relationship between PhA and all-cause mortality was consistent across different subgroups, including sociodemographic variables, body measurements, comorbidities, and lifestyle habits. It’s worth noting that the negative association between PhA and all-cause mortality existed among non-smokers (P = 0.003) and a significant interaction was observed between PhA and smoking status (P for interaction = 0.029). To the best of our knowledge, this study is the first to indicate that smoking status may have an impact on the association between PhA and all-cause mortality. Nevertheless, the mechanisms underlying the association remain unclear and need more research. Some studies have pointed out that the PhA value is lower in smokers due to oxidative stress and inflammation disrupting the normal cell structure30,31,32. The PhA value was lower in smokers than in non-smokers (6.6 ± 0.13° vs. 7 ± 0.06°, P = 0.038)30. Cellular damage caused by unhealthy lifestyle, and smoking-induced systemic oxidative stress and inflammation are the main causes of low PhA. Harmful substances in tobacco, such as nicotine, can disrupt mitochondrial endoplasmic reticulum crosstalk, interfering with normal redox signaling in the body and thus leading to dysfunctions in cell structure and function31. In addition, smoking leads to low-grade inflammation and makes inflammatory factors rise significantly32. Overall, our study demonstrated that the all-cause mortality of non-smokers may decrease with the increase of PhA in adults aged 18–49 years.
In clinical practice, PhA is a simple, non-invasive, and easily obtainable measurement with significant utility; regular monitoring can help identify individuals at risk of nutritional deficiencies or declining health25,33. Additionally, PhA can assist in identifying nutritional health issues across diverse populations, particularly in resource-limited or low-income settings8. Integrating PhA assessment into public health policies can enhance health monitoring and intervention strategies, ultimately reducing the burden of malnutrition-related diseases and lowering mortality rates25.
Strengths and limitations
There are some strengths in our study. Firstly, our study population was characterized by a large sample size and complex sampling, ensuring representation and enhancing the reliability of our conclusions. Secondly, we adjusted for as many confounders as possible in constructing the regression model, including sociodemographic variables, body measurements, comorbidities, and lifestyle habits, and also performed stratified analyses to demonstrate the robustness of our findings. Thirdly, our study benefited from its foundation on a high-quality research study with a prolonged follow-up duration, reinforcing the veracity and dependability of our conclusions. However, several limitations warrant acknowledgment. Firstly, NHANES facilitated a single measurement of PhA in the study population, thus potentially overlooking the significance of examining dynamic trends in the PhA. Secondly, information on the presence of underlying diseases and smoking status is based on self-reporting by respondents in the questionnaire. Even though the questionnaire was based on a procedural approach and was also administered by well-trained staff, recall bias on the part of respondents could not be ruled out. Thirdly, we focused primarily on individuals aged 18 to 49 years. This age group generally tends to be healthier, which may limit our ability to fully capture the relationship between nutritional status and all-cause mortality across different age groups. Future research could benefit from including a broader age range to gain a more comprehensive understanding of the relationships we investigated.
Conclusion
In adults aged 18–49 years, there was a significant negative association between PhA and all-cause mortality. The negative association was stronger in the non-smoking population. It is therefore important to monitor and manage PhA in order to reduce the risk of all-cause mortality. Further studies are required to validate these findings and determine how PhA can be optimally utilised in clinical practice to enhance quality of life.
Data availability
The data supporting the findings of this study are available from the NHANES database, which can be accessed at https://www.cdc.gov/nchs/nhanes/index.htm.
References
Arosio, B. & Cesari, M. Nutr. Muscle Health Nutrients 13, 797 (2021).
Dent, E., Wright, O. R. L., Woo, J. & Hoogendijk, E. O. Malnutrition in older adults. Lancet Lond. Engl. 401, 951–966 (2023).
Norman, K., Pichard, C., Lochs, H. & Pirlich, M. Prognostic impact of disease-related malnutrition. Clin. Nutr. Edinb. Scotl. 27, 5–15 (2008).
Ye, K. X. et al. Adequacy of nutrient intake and malnutrition risk in older adults: findings from the diet and healthy aging cohort study. Nutrients 15, 3446 (2023).
Sieber, C. C. Malnutrition and sarcopenia. Aging Clin. Exp. Res. 31, 793–798 (2019).
Tang, H. & Yang, M. Nutritional assessment in patients with chronic diseases: Tools, challenges, and future directions. Nutrients 15, 4794 (2023).
Vermeulen, K. M. et al. Bioelectrical impedance vector analysis and phase angle on different oral zinc supplementation in eutrophic children: randomized triple-blind study. Nutrients 11, 1215 (2019).
Bellido, D., García-García, C., Talluri, A., Lukaski, H. C. & García-Almeida, J. M. Future lines of research on phase angle: strengths and limitations. Rev. Endocr. Metab. Disord. 24, 563–583 (2023).
de Blasio, F. et al. Raw bioelectrical impedance analysis variables are independent predictors of early all-cause mortality in patients with COPD. Chest 155, 1148–1157 (2019).
Norman, K., Stobäus, N., Pirlich, M. & Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis–clinical relevance and applicability of impedance parameters. Clin. Nutr. Edinb. Scotl. 31, 854–861 (2012).
Bosy-Westphal, A. et al. Phase angle from bioelectrical impedance analysis: population reference values by age, sex, and body mass index. JPEN J. Parenter. Enter. Nutr. 30, 309–316 (2006).
Gonzalez, M. C., Barbosa-Silva, T. G., Bielemann, R. M., Gallagher, D. & Heymsfield, S. B. Phase angle and its determinants in healthy subjects: influence of body composition. Am. J. Clin. Nutr. 103, 712–716 (2016).
Hu, Y., Liu, J. & Jin, H. Association between serum uric acid and phase angle in patients with type 2 diabetes mellitus: a cross-sectional study. Front. Endocrinol. 14, 1124565 (2023).
Ferreira, G. O. C. et al. Phase angle and its determinants among adolescents: influence of body composition and physical fitness level. Sci. Rep. 14, 13697 (2024).
Sacco, A. M. et al. Raw bioelectrical impedance analysis variables (phase angle and impedance ratio) are significant predictors of hand grip strength in adolescents and young adults. Nutr. Burbank Los Angel Cty. Calif. 91–92, 111445 (2021).
Mattiello, R., Amaral, M. A., Mundstock, E. & Ziegelmann, P. K. Reference values for the phase angle of the electrical bioimpedance: systematic review and meta-analysis involving more than 250,000 subjects. Clin. Nutr. Edinb. Scotl. 39, 1411–1417 (2020).
Campa, F. et al. New bioelectrical impedance vector references and phase angle centile curves in 4,367 adults: the need for an urgent update after 30 years. Clin. Nutr. 42, 1749–1758 (2023).
De Benedetto, F., Marinari, S. & De Blasio, F. Phase angle in assessment and monitoring treatment of individuals with respiratory disease. Rev. Endocr. Metab. Disord. 24, 491–502 (2023).
Hirose, S. et al. Phase angle as an indicator of Sarcopenia, malnutrition, and cachexia in inpatients with cardiovascular diseases. J. Clin. Med. 9, 2554 (2020).
Amano, K., Bruera, E. & Hui, D. Diagnostic and prognostic utility of phase angle in patients with cancer. Rev. Endocr. Metab. Disord. 24, 479–489 (2023).
Akamatsu, Y. et al. Phase angle from bioelectrical impedance analysis is a useful indicator of muscle quality. J. Cachexia Sarcopenia Muscle. 13, 180–189 (2022).
Kwon, Y. E. et al. Impact of Sarcopenia and phase angle on mortality of the very elderly. J. Cachexia Sarcopenia Muscle. 14, 279–287 (2022).
Fernández-Jiménez, R. et al. Phase angle and handgrip strength as a predictor of disease-related malnutrition in admitted patients: 12-month mortality. Nutrients 14, 1851 (2022).
García-García, C. et al. Rectus femoris muscle and phase angle as prognostic factor for 12-month mortality in a longitudinal cohort of patients with cancer (AnyVida Trial). Nutrients 15, 522 (2023).
Bellido, D. et al. Phase angle and mortality: a systematic review. Eur. J. Clin. Nutr. 73, 495–508 (2019).
Langer, R. D., Ward, L. C., Larsen, S. C. & Heitmann, B. L. Can change in phase angle predict the risk of morbidity and mortality during an 18-year follow-up period? A cohort study among adults. Front. Nutr. 10, 1157531 (2023).
Selberg, O. & Selberg, D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur. J. Appl. Physiol. 86, 509–516 (2002).
Franco-Oliva, A. et al. Association between phase angle and the nutritional status in pediatric populations: a systematic review. Front. Nutr. 10, 1142545 (2023).
Fernández-Jiménez, R. et al. Phase angle as a marker of outcome in hospitalized pediatric patients. A systematic review of the evidence (GRADE) with meta-analysis. Rev. Endocr. Metab. Disord. 24, 751–765 (2023).
Portugal, M. R. C. et al. Smoking status affects bioimpedance-derived phase angle in men but not in women: the Pró-Saúde Study, Brazil. Nutr. Burbank Los Angel Cty. Calif. 61, 70–76 (2019).
Manevski, M. et al. Cellular stress responses and dysfunctional mitochondrial-cellular senescence, and therapeutics in chronic respiratory diseases. Redox Biol. 33, 101443 (2020).
Shiels, M. S. et al. Cigarette smoking and variations in systemic immune and inflammation markers. J. Natl. Cancer Inst. 106, dju294 (2014).
Raiten, D. J., Combs, G. F., Steiber, A. L., Bremer, A. A. & Perspective Nutritional status as a Biological Variable (NABV): integrating Nutrition Science into Basic and Clinical Research and Care. Adv. Nutr. 12, 1599–1609 (2021).
Acknowledgements
We would like to thank the participants and staff of the National Health and Nutrition Examination Survey (NHANES) for their invaluable contributions to this study. The data used in this research were obtained from the NHANES public database, which provided essential information for our analysis.
Author information
Authors and Affiliations
Contributions
X.X. conducted the formal analysis and data curation and drafted the manuscript. C.L. and Y.C. edited the manuscript. X.X., C.L., X.X., Y.C., F.H., and H.G. conducted the investigation. Y.C. and H.G. supervised the project administration. All authors reviewed and approved the final version of the manuscript. H.G. had primary responsibility for final content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xia, Xx., Li, Cx., Xue, Xx. et al. Association between phase angle and all-cause mortality in adults aged 18–49 years: NHANES 1999–2004. Sci Rep 15, 2785 (2025). https://doi.org/10.1038/s41598-025-86825-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-86825-y