Abstract
Neuropsychiatric adverse events (AEs) significantly impact the quality of life of patients using avapritinib. However, the majority of current data comes from pre-marketing, with limited real-world studies. Our research aimed to explore post-marketing data of avapritinib. We evaluated the signals of avapritinib-related neuropsychiatric AEs by data mining using the FDA Adverse Event Reporting System (FAERS). Reporting odds ratio (ROR) and information component (IC) were employed to quantify the signals from the first quarter of 2020 through the fourth quarter of 2023. Subsequently, stratified analyses were conducted to further explore the effect of different stratification schemes on the association between avapritinib and neuropsychiatric AEs. Finally, a combination medication analysis was conducted to explore the impact of the co-administration of neuropsychiatric AEs. A total of 2029 neuropsychiatric AEs were reported, and 49 signals were detected, of which 5 were determined to be new signals. Avapritinib was significantly associated with the occurrence of neuropsychiatric AEs (ROR: 1.52, 95% CI: 1.44–1.61; IC: 0.43, IC025: 0.35). The stratified analysis found that gender, age and eight preferred terms (PTs), including cerebral haemorrhage, may affect the severity of AEs. Combination medication analysis showed that combining avapritinib with 19 other medications, including prochlorperazine, may increase the risk of neuropsychiatric AEs. The median time-to-onset (TTO) of avapritinib-related neuropsychiatric AEs was 32 (interquartile range [IQR] 2-200) days, with about 65% of cases occurring within the first three months of treatment. An increase in the signal for neuropsychiatric AEs was identified in post-marketing studies of avapritinib. Clinicians are advised to remain vigilant for such events, particularly during the initial stages of treatment with avapritinib.
Similar content being viewed by others
Introduction
Avapritinib (BLU-285) is an orally potent, highly selective tyrosine kinase inhibitor (TKI) targeting KIT and platelet-derived growth factor receptor alpha1,2,3. In January 2020, avapritinib was approved in the United States for treating adult patients with unresectable or metastatic gastrointestinal stromal tumors (GIST) that harbor PDGFRA exon 18 mutations, including the PDGFRA D842V mutation4. Avapritinib is the first precision therapy approved for GIST and the first drug with high activity against GIST with exon 18 mutations in the PDGFRA4. It is specifically engineered to selectively bind and inhibit the D816 mutant KIT, a prevalent driver in about 95% of systemic mastocytosis (SM) cases, effectively targeting KIT D816V with minimal off-target effects5. In June 2021, the U.S. Food and Drug Administration (FDA) approved avapritinib for treating adult patients with advanced systemic mastocytosis (AdvSM), including aggressive systemic mastocytosis (ASM), systemic mastocytosis with hematologic neoplasms (SM-AHN), and mast cell leukemia (MCL)5,6,7. Following positive outcomes from the PIONEER clinical trial (NCT03731260), a new indication for indolent systemic mastocytosis (ISM) was added in the United States in May 20238, highlighting avapritinib’s extensive potential applications.
With the widespread use of avapritinib, the issue of its clinical safety application cannot be ignored, and particular attention needs to be paid to adverse events (AEs) that impact patients’ quality of life, e.g., neuropsychiatric AEs. The most common AEs associated with avapritinib are nausea, diarrhoea, anaemia, appetite disorder, and memory impairment, which occur in more than 20% of patients9. Previous clinical trial studies suggest a possible correlation between avapritinib and cognitive impairment, but the triggering mechanism is unknown1,7,10,11. A phase I trial of the long-term efficacy and safety of avapritinib in the treatment of unresectable or metastatic PDGFRA D842V-mutated GIST found that treatment-related AEs leading to discontinuation of the drug were most commonly neuropsychiatric AEs11. Neuropsychiatric AEs have a significant impact on the quality of life of patients using avapritinib. However, most current data are from small pre-marketing randomized controlled trials. Real-world studies of avapritinib-related AEs are fewer, shorter, and focused on overall post-marketing safety analysis of avapritinib without a focus on neuropsychiatric AEs, and one study of neuropsychiatric AEs was limited to patients with GIST, which may have excluded numerous of reports and potentially biased the results12,13. There is a lack of systematic and comprehensive studies of postmarketing neuropsychiatric events associated with avapritinib. Therefore, it is crucial to comprehensively analyze the real-world data on post-marketing neuropsychiatric adverse events of avapritinib.
The FDA Adverse Event Reporting System (FAERS) database, managed by the FDA, supports post-market safety monitoring of drugs and is one of the largest pharmacovigilance databases globally14. This study aims to comprehensively evaluate the neuropsychiatric AEs related to avapritinib by collecting, screening, and statistically analyzing the data from FAERS database and providing a comprehensive and valuable reference for its rational clinical application.
Methods
Data collection and definition
This study is an observational, retrospective pharmacovigilance analysis of the FAERS database, which, as of December 31, 2023, had collected more than 20 million reports of suspected adverse reactions covering virtually the entire global population. FAERS database includes reports of AEs and medication error reports submitted by healthcare professionals, consumers, and drug manufacturers15. Each report is assigned a unique identification number (primaryid), case ID (caseid), and the date the case was received by the FDA (fda_dt). Additionally, the report includes information regarding the patient (age, gender, and weight), the country or region of the report, the type of reporter, the suspected and concomitant medications, and their respective indications. It also contains information regarding the AEs, the time-to-onset (TTO) of AEs, and the severity of the events.
This study obtained the ASCII files (covering the period from the first quarter of 2020 to the fourth quarter of 2023) from the “FAERS Quarterly Data Extract Files” (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html). To ensure the integrity of the data, only data collected between January 9, 2020 (the date of first approval for avapritinib) and December 31, 2023, was utilized. The data in the DEMO table was initially de-duplicated by the official documentation. For reports with the same caseid, the largest fda_dt value was retained, and for those with both the same caseid and fda_dt, the largest primaryid value was retained. Subsequently, reports with identical caseid as those in the DELETE table were removed. The effects of drugs reported in FAERS were categorized as primary suspect drug (PS), secondary suspect drug (SS), concomitant (C), and interacting (I). Only reports in which the drug was classified as a PS were analyzed. To evaluate the difference between avapritinib and other TKIs regarding neuropsychiatric AEs, we also collected reports of neuropsychiatric AEs for 15 other TKIs, as outlined in Supplementary Table S1. The “drugname” field in the FAERS database contains the reported drug’s trade name or active ingredient. The “drugname” field in FAERS contains the reported drug’s trade name or active ingredient, which was subsequently normalized using RxNorm.
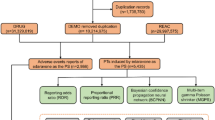
Furthermore, the “prod_ai” field also contains the drug’s active ingredient. Thus, we employed both “drug name” and “prod_ai” to identify the target drug. AEs documented in the FAERS were classified according to the preferred terms (PTs) codes defined in the Medical Dictionary for Regulatory Activities (MedDRA). This classification system is organized into five hierarchical levels: The System Organ Class (SOC), High-Level Group Terms (HLGT), High-Level Terms (HLT), PTs, and Lower-Level Terms (LLT) were utilized. For this study, reports were selected as “cases” if they included at least one AE falling within the SOC of either “nervous system disorders” or “psychiatric disorders.” In the absence of any AEs falling within the categories of “nervous system disorders” or “psychiatric disorders,” the reports were classified as “non-cases.” In the event of multiple neuropsychiatric AEs within a single report, only a single instance was counted. This study was conducted using MedDRA version 26.1. The process of data extraction and cleaning is illustrated in Fig. 1.
The process of selecting avapritinib-related neuropsychiatric AEs from FAERS database. AEs, adverse events; TKIs, Tyrosine kinase inhibitors; MedDRA, Medical Dictionary for Regulatory Activities; PS, primary suspect drug; DELETE, FDA or Manufacturers may delete cases for various reasons including combining cases; DEMO contains patient demographic and administrative information, a single record for each event report; DRUG contains drug/biologic information for as many medications as were reported for the event; REAC contains all MedDRA terms coded for the AEs.
Signal mining
In pharmacovigilance studies, disproportionality analyses are primarily employed to investigate potential correlations between specific AEs and medications16. Disproportionality analysis were conducted using the reporting odds ratio (ROR) to assess the potential associations between avapritinib and neuropsychiatric AEs. A valid signal was defined as at least three reports of neuropsychiatric AEs for which the lower limit of the 95% confidence interval (CI) of the ROR was more significant than one. To reduce false positive signals, we also used a Bayesian confidence propagation neural network (BCPNN) to confirm the signals of the detected AEs17. The signal is significant if the lower limit of the 95% CI of the information component (IC) exceeds zero. (Supplementary Table S2).
Furthermore, the stratified analyses were performed by gender, age (18–64 years and ≥ 65 years), reporter type (healthcare professional and consumer), and report outcome (serious report and non-serious report) to investigate the impact of different stratification schemes on the association between avapritinib and psychiatric and neurological AEs.
In addition, a medication combination analysis was performed to investigate whether the coadministration of avapritinib with other drugs affects the occurrence of neuropsychiatric AEs.
Finally, we analyzed the TTO of AEs following avapritinib administration. TTO is calculated as the difference between the date of the AE (event_dt in the DEMO table) and the date of the drug use initiation (start_dt in the THER table), excluding reports with missing or unusual values. Kaplan-Meier curves and box plots were plotted to illustrate the occurrence of avapritinib-related neuropsychiatric AEs and other AEs, and the Wilcoxon rank sum test was employed to compare the differences in the occurrence of neuropsychiatric AEs and other AEs at different periods. Moreover, the Weibull shape parameter (WSP) test was conducted to evaluate alterations in the occurrence of AEs18.
Statistical analysis
Descriptive statistics were employed to present the clinical characteristics of neuropsychiatric AEs associated with avapritinib. Categorical variables were expressed as frequencies (percentages), while continuous variables were expressed as medians (interquartile range [IQR]). The differences between the groups were analyzed using the Wilcoxon rank sum test, the chi-square test, and Fisher’s exact test. All data were processed and analyzed using PostgreSQL (version 15.3) and R software (version 4.3.2), and p < 0.05 was considered statistically significant.
Results
Baseline characteristics of avapritinib
A total of 5,896 individual case safety reports involving avapritinib as the primary suspect drug were recorded between January 9, 2020, and December 31, 2023. Of the total reports, 34.41% (2029/5896) were related to neuropsychiatric AEs. The median age of patients who reported AEs was 68.00 years (IQR 57.00–74.00), with 62.50% being over 65 years. Slightly more female than male cases were reported (1059 vs. 937). The majority of cases originated from the United States (n = 1948, 96.01%) and China (n = 58, 2.86%), with the majority of reports being submitted by consumers (n = 1891, 94.31%). A total of 699 cases of serious neuropsychiatric AEs were recorded. Of these cases, 3.72% (26/699) resulted in mortality, and the most commonly reported severe outcomes were other serious (important medical events) and hospitalization, which accounted for 89.13% (623/699) and 32.76% (229/699), respectively. The most significant number of cases was reported in 2022, comprising 35.4%. The five indications with the highest reported cases were gastrointestinal stromal tumor, systemic mastocytosis, hematopoietic neoplasm, indolent systemic mastocytosis, and advanced systemic mastocytosis. Further details can be found in Table 1.
Signal detection results
Avapritinib was significantly associated with neuropsychiatric AEs (ROR: 1.52, 95% CI: 1.44–1.61; IC: 0.43, IC025: 0.35). There were no potential safety signals for other TKIs (ROR: 0.79, 95% CI: 0.77–0.80; IC: -0.26, IC025: -0.29), and avapritinib had a greater signal value than other TKIs. Specifically, dasatinib, erdafitinib, fostamatinib, imatinib, lenvatinib, midostaurin, pazopanib, ponatinib, ripretinib, sunitinib had no potential safety signals, and avapritinib had a smaller ROR value than pexidartinib and regorafenib, and a larger ROR value than tivozanib, sorafenib, and nilotinib. See Supplementary Figure S1 for details. At the PT level, the FAERS database reported 220 avastinib-related neuropsychiatric AEs in PTs, of which 49 PTs had potential safety signals, including primary progressive aphasia (n = 7, ROR: 555.34, 95% CI: 22 1.48-1392.41; IC: 3.85, IC025: 2.55), repetitive speech (n = 5, ROR: 47.29, 95%CI: 19.29-115.95; IC: 3.17, IC025: 1.61), patient elopement (n = 6, ROR: 36.18, 95%CI: 16.02–81.70; IC: 3.27, IC025: 1.86), executive dysfunction (n = 5, ROR: 34.14, 95%CI: 14.00-83.24; IC: 3.08, IC025: 1.52), and hypogeusia (n = 11, ROR: 24.31, 95%CI: 13.36–44.22; IC: 3.58, IC025: 2.55). Five signals not reported in the FDA instructions were found, including parosmia(n = 17, ROR: 9.05, 95% CI: 5.61–14.61; IC: 2.87, IC025: 2.05), sciatica (n = 16, ROR: 3.53, 95% CI: 2.16–5.77; IC: 1.71, IC025: 0.86), neuralgia (n = 16, ROR: 2.16, 95% CI: 1.32–3.53; IC: 1.06, IC025: 0.22), tremor (n = 54, ROR: 1.44, 95% CI: 1.10–1.88; IC: 0.51, IC025: 0.06), and eating disorder (n = 13, ROR: 2.16, 95% CI:1.25–3.72; IC: 1.04, IC025: 0.11). Compared to other TKIs, avapritinib has a more significant effect on ageusia (ROR: 8.52 vs. 3.06, IC: 2.98 vs. 1.57), hypersomnia (ROR: 4.69 vs. 1.51, IC: 2.14 vs. 0.58), hypogeusia (ROR: 24.31 vs. 3.56, IC: 3.58 vs. 1.70), taste disorder (ROR: 10.91 vs. 3.17, IC: 3.35 vs. 1.62), brain fog (ROR: 19.62 vs. 1. 43, IC: 4.05 vs. 0.50), aphasia (ROR: 16.34 vs. 1.29, IC: 3.89 vs. 0.36), headache (ROR: 1.49 vs. 1.22, IC: 0.55 vs. 0. 27) signal values were greater; on eating disorder (ROR: 2.16 vs. 2.87, IC: 1.04 vs. 1.48), cerebral haemorrhage (ROR: 2.08 vs. 2.31, IC: 1.00 vs. 1.18) signal values were smaller. See Table 2 for details.
Stratification analysis
We used four different stratification strategies to increase the reliability of the findings. After separately assessing neuropsychiatric AEs by gender, age, reporter type, and serious reports, the lower limit of the 95% CI of ROR value for all stratifications except for healthcare professionals were greater than one, indicating that the observed association between avapritinib and neuropsychiatric AEs was not due to chance (Fig. 2). At the PTs level, including aphasia, brain fog, cognitive disorder, and memory impairment, have potential safety signals at all levels (Supplementary Figure S2).
Serious versus non-serious cases
There were significant differences between serious and non-serious cases of neuropsychiatric AEs in terms of age (69 vs. 67, p < 0.001) and gender (male: 50.22% vs. 45.22%, p = 0.037). confusional state (χ2 = 4.50, p = 0.034), cognitive disorder (χ2 = 4.89, p = 0.027), cerebral haemorrhage (p < 0.001), sciatica (p = 0.031), mental impairment (p < 0.001), dementia (p < 0.001), disorientation (p = 0.003), primary progressive aphasia (p = 0.001). The remaining p-values were more significant than 0.05. (Table 3).
Combination medication analysis
Considering that each patient uses multiple medications, combined medication use may affect the occurrence of AEs. We analyzed the combined medication use in cases of avapritinib-related neuropsychiatric AEs. It was found that the combined use of avapritinib with 19 medications, including prochlorperazine, can lead to an increased risk of neuropsychiatric AEs, as shown in Fig. 3.
Time-to-onset analysis
The TTO of avastinib-related AEs was collected from the FAERS database. A total of 1128 reports (55.60%, 1128/2029) were included in the analysis after excluding any reports with missing and abnormal time values. The results demonstrated that the median time to onset of neuropsychiatric AEs associated with avapritinib was significantly shorter in comparison to other AEs (days: 32[2-200] vs. 58[8-270], p < 0.001). Additionally, approximately 65% of avapritinib-related neuropsychiatric AEs occurred within the initial three months of treatment initiation (Fig. 4). The shape parameter β for avapritinib-related neuropsychiatric AEs WSP test was 0.48 (95% CI: 0.46–0.51). The upper limit of the 95% CI of β was less than one, indicating a decrease in the incidence of neuropsychiatric AEs over time, which suggests an early failure type (Table 4). The upper 95% CI of β for 40 AEs, including insomnia, confirmatory state, brain fog, cognitive disorder and aphasia, was less than one in the significant signal, indicating that these AEs are of the early failure type (see Supplementary Table S3). Furthermore, additional analysis comparing the timing of neuropsychiatric AEs to other AEs at varying intervals demonstrated a statistically significant difference between 60 and 90 days and between 180 and 360 days (p < 0.05) (Supplementary Figure S3).
Discussion
Based on the data from the first quarter of 2020 to the fourth quarter of 2023 in FAERS database, this study conducted a comprehensive and systematic analysis of avapritinib-related neuropsychiatric safety profiles. Through disproportionality analysis, we identified 2029 reports of avapritinib-related neuropsychiatric AEs and detected 49 types of neuropsychiatric AEs. Notably, the correlation between avapritinib and neuropsychiatric AEs conditions persist even after stratification by gender, age, and serious reports. Overall, there are several exciting key findings that deserve our special attention and further discussion.
New adverse event signals
After obtaining the signal results of avapritinib-related neuropsychiatric AEs, the PT signals were ranked according to the number of reported cases and the significance of the signals. Subsequently, we found that most PT signals were consistent with AEs reported in the instructions, including memory impairment, cognitive disorder, taste disorder, confidential state, speech disorder, etc. Notably, we also identified new signals not reported in the instructions, including parosmia, sciatica, neuralgia, tremor and eating disorder.
The cause of parosmia is unknown. Van Elst et al.19 reported that 23% of patients treated with TKIs (without avapritinib) for GIST experienced parosmia. In addition, chemotherapy may lead to decreased salivation and oral mucositis, potentially affecting taste and smell20. Neuralgia may be related to factors such as inflammation, vascular disease, trauma, and muscle tension and may also be related to tumour compression21. Sciatica is a type of neuralgia encompassing a range of musculoskeletal, connective tissue disorders, lumbar segmental spinal cord and nerve root disorders, in which nerve root compression and inflammation may play an important role in its pathogenesis22. In our analysis, parosmia (ROR = 9.05, IC = 2.87) and sciatica (ROR = 3.53, IC = 1.71) had strong signals; stratified analysis showed that sciatica seemed to be more likely to be reported in severe cases. Remind us to pay more attention to these AEs when using avapritinib. Both the AEs reported in the instructions and the new AEs we identified indicate that the use of avapritinib has a great influence on the patient’s neuropsychiatric systems. In fact, the incidence of neuropsychiatric AEs associated with avapritinib was as high as 48%4. It appeared to be more prone to neuropsychiatric AEs than other TKIs with KIT target genes, which may be related to the different mechanism of action of avapritinib from other type II TKIs1,23. Type I kinase inhibitors can bind to the active conformation of the kinase, while type II kinase inhibitors can only bind to the inactive conformation24,25. Avapritinib is a highly selective type I inhibitor that can specifically bind to the active conformation of the KIT kinase domain and inhibits all activation loop mutations, leading to secondary mutations in other regions of the kinase (i.e., ATP-binding pocket and gatekeeper mutations), which have been hypothesized to be involved26. In addition, cognitive-related AEs were considered to be unique neuropsychiatric AEs of avapritinib, except kinase inhibitors (e.g., lorlatinib and larotrectinib), which can penetrate the blood-brain barrier and interact with unknown targets in the brain, leading to cognitive-related AEs11,26. Therefore, clinicians need to thoroughly evaluate patients’ treatment needs and monitor patients closely. If neuropsychiatric AEs occur, it may be necessary to reduce the dose, suspend or stop the treatment.
Serious versus non-serious cases
In this study, there was a significant difference between severe and non-serious cases of the eight PTs (p < 0.05), including cognitive effects (cognitive disorder, confusional state, mental impairment, dementia, disorientation, primary progressive aphasia), cerebral haemorrhage, and sciatica. Cognitive effects and cerebral haemorrhage are AEs that have been the focus of particular attention in previous studies9. Previous studies have shown that most cognitive effects associated with avapritinib treatment are not severe, with grades 1–2 and approximately 5% ≥ grade 31,2,7,11,27. Our study also showed that the number of reported non-serious was higher than serious (1330 vs. 699). Further stratified analysis showed that cognitive disorder and confusional state were more likely to be reported as non-serious cases; however, mental impairment, dementia, disorientation, and primary progressive aphasia were more likely to be reported as severe cases. It should be noted that even low-level (including grade I) cognitive-related effects may also hurt patients27. Previous reports have suggested that patients treated with avapritinib may experience severe intracranial bleeding, including intracranial haemorrhage, subdural haematoma and cerebral haemorrhage, with an overall incidence of 3.0%11. Intracranial bleeding may require permanent discontinuation of the drug. As with the results of previous studies, all the cerebral haemorrhages reported in this study were severe cases and, therefore, require special attention.
In addition, patient gender (p = 0.034) and age (p < 0.001) may be associated with an increased risk of neuropsychiatric AEs. Descriptive analysis in Table 1 showed that females (53.06%) appeared to be more inclined to report neuropsychiatric AEs. The stratified analysis revealed a different spectrum of neuropsychiatric AEs between females and males, with females being associated with more neuropsychiatric AEs categories than males. This result was different from the results of a previous pharmacovigilance study, which showed that males reported a slightly higher proportion of neuropsychiatric AEs after receiving avapritinib than females13. One clinical study found that the incidence of cognition-related AEs in GTST patients treated with avapritinib was independent of gender27. However, further comparison of serious with non-serious cases in this study showed that the proportion of serious AEs in males was numerically higher than that in females, which was statistically significant (50.22% vs. 49.78%, p = 0.037), and females mainly reported non-serious cases. The reason for this phenomenon is not yet clear. Few studies have evaluated the influence of gender on avapritinib-related side effects, and the exact mechanism by which gender affects avapritinib-related neurotoxicity has not been explored. Further prospective studies are needed to determine the possible reasons. In a previous study, Barbieri et al.13 found that the number of neuropsychiatric AEs reports in elderly patients (≥ 65 years) was higher than that in young patients based on a retrospective analysis of the EudraVigilance database, which is consistent with the results of our current study. Further stratified analysis found that the median age of patients reporting serious cases was significantly higher than that of patients reporting non-serious cases (median age 69 vs. 67 years, p < 0.001). In addition, this study observed differences in the spectrum of neuropsychiatric AEs between different age stratifications. Patients aged ≥ 65 years reported more neuropsychiatric AE categories than those aged < 65 years (Supplementary Figure 2). This is consistent with a previous study, which showed that common cognitive-related AEs were reported more frequently in patients aged ≥ 65 years than in patients aged < 65 years after avapritinib use27. However, our results should be interpreted cautiously; further investigation is required to confirm this.
Combination medication analysis
It is worth noting that the combination of 19 medications with avapritinib may lead to an increased risk of neuropsychiatric AEs compared to non-combinations. The increased risk of neuropsychiatric AEs associated with avapritinib in combination with other medications may be related to themselves, such as alprazolam and prochlorperazine28,29. Secondly, avapritinib is a substrate of CYP3A, and co-administration with CYP3A inhibitors may increase plasma concentrations, leading to an increase in neuropsychiatric AEs30. Interestingly, there is controversy regarding the neuropsychiatric AEs associated with proton pump inhibitors (PPIs). One cross-sectional study found that PPIs reduced cognitive function31, but a recent prospective cohort study did not confirm this effect32. There is minimal evidence of an association between an increased risk of neuropsychiatric AEs related to cognitive impairment and PPIs. Previous studies on angiotensin-converting enzyme inhibitors and statins have shown that these drugs can reduce the risk of cognitive impairment33,34,35,36,37. Renin-angiotensin blockers that can cross the blood-brain barrier are more conducive to reducing the risk of cognitive impairment than those that cannot38. However, severe agitation has been reported with statins39. The reason for the neuropsychiatric AEs with the combination of avapritinib and the above medications increased is not clear. However, our results should be interpreted cautiously and need to be verified by further investigation, as the number of reported cases in patients was small.
Time-to-onset analysis
We observed that the median TTO of avapritinib-related neuropsychiatric AEs was 32 days (IQR 2-200), significantly earlier than other AEs associated with avapritinib (32 days [2-200] vs. 58 days [8-270], p < 0.001). The reason for this phenomenon is unclear and requires further investigation. Meanwhile, approximately 65% of avapritinib-related neuropsychiatric AEs occurred within the first three months after starting the drug, with nearly 50% occurring within the first month. NAVIGATOR study found that cognitive effects are a common reason for discontinuing avapritinib. When the dosage was adjusted to 200 mg/day or the drug was discontinued, patients’ cognitive function improved, with a median time to improvement of 1.6 weeks for grade ≥ II AEs11. Therefore, it is crucial to closely monitor the occurrence of neuropsychiatric AEs during treatment with avapritinib, especially in the early stages, to allow for timely dose adjustments or discontinuation of the drug and to minimize the occurrence of severe neuropsychiatric AEs.
Limitations and strengths
Firstly, the results of this study based on the FAERS database should be interpreted with caution, considering the limitations of all pharmacovigilance databases, including the possibility of submission of incomplete, inaccurate, untimely and unverified reports, and nearly 90% of the reports in this study were spontaneous reports from consumers. Secondly, the incidence of AEs could not be calculated by disproportionate analysis due to the lack of the total size of the population using avapritinib, and it may also be affected by over-reporting or under-reporting. Thirdly, as with all pharmacovigilance studies, a causal relationship between the drug and the AEs could not be established. Finally, the influence of potential diseases and the dose of the drug on the outcome was not considered in this study. Despite these limitations, the new findings in this study may raise awareness of neuropsychiatric AEs associated with avapritinib. Spontaneous individual safety reports provide valuable information on drug safety and remain the cornerstone of post-marketing safety evaluation.
Conclusion
Our study provides an updated analysis of the relationship between avapritinib and neuropsychiatric AEs. We identified five new signals of avapritinib-related neuropsychiatric AEs. The concomitant use of avapritinib and 19 medications, including alprazolam, may increase the risk of neuropsychiatric AEs. Most of the reported cases occurred within the first 3 months of administration. Therefore, given these potential risks, it is critical for clinicians to monitor patients closely for neuropsychiatric AEs while using avapritinib, especially in the early stages of dosing, to facilitate timely adjustments in dosage or cessation of treatment if necessary.
Data availability
All data were sourced from the publicly accessible FDA Adverse Event Reporting System (FAERS) database.
References
Kang, Y. K. et al. Avapritinib Versus Regorafenib in locally Advanced Unresectable or metastatic GI stromal tumor: a randomized, open-label phase III study. J. Clin. Oncol. 39, 3128–3139 (2021).
Heinrich, M. C. et al. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): a multicentre, open-label, phase 1 trial. Lancet Oncol. 21, 935–946 (2020).
Klug, L. R., Khosroyani, H. M., Kent, J. D. & Heinrich, M. C. New treatment strategies for advanced-stage gastrointestinal stromal tumours. Nat. Rev. Clin. Oncol. 19, 328–341 (2022).
S, D. & Avapritinib First Approval Drugs 80, 433–439 (2020).
Gotlib, J., Reiter, A. & DeAngelo, D. J. Avapritinib for advanced systemic mastocytosis. Blood 140, 1667–1673 (2022).
Pardanani, A. Systemic mastocytosis in adults: 2021 update on diagnosis, risk stratification and management. Am. J. Hematol. 96, 508–525 (2021).
DeAngelo, D. J. et al. Safety and efficacy of avapritinib in advanced systemic mastocytosis: the phase 1 EXPLORER trial. Nat. Med. 27, 2183–2191 (2021).
Padilla, B. et al. Psychometric evaluation of the indolent systemic mastocytosis Symptom Assessment Form (ISM-SAF) in a phase 2 clinical study. Orphanet J. Rare Dis. 16, 434 (2021).
AYVAKIT Prescribing information. (2024). Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/212608s020lbl.pdf
Gotlib, J. et al. Efficacy and safety of avapritinib in advanced systemic mastocytosis: interim analysis of the phase 2 PATHFINDER trial. Nat. Med. 27, 2192–2199 (2021).
Jones, R. L. et al. Avapritinib in unresectable or metastatic PDGFRA D842V-mutant gastrointestinal stromal tumours: long-term efficacy and safety data from the NAVIGATOR phase I trial. Eur. J. Cancer. 145, 132–142 (2021).
Rong, L., Xie, M., Jiang, M., Qiu, H. & Kong, L. A post-marketing pharmacovigilance study of avapritinib: adverse event data mining and analysis based on the United States Food and Drug Administration adverse event reporting System database. Br. J. Clin. Pharmacol. https://doi.org/10.1111/bcp.15673 (2023).
Barbieri, M. A. et al. Neuropsychiatric adverse drug reactions with tyrosine kinase inhibitors in gastrointestinal stromal tumors: an analysis from the European spontaneous adverse event reporting system. Cancers (Basel). 15, 1851 (2023).
Rodriguez, E. M., Staffa, J. A. & Graham, D. J. The role of databases in drug postmarketing surveillance. Pharmacoepidemiol Drug Saf. 10, 407–410 (2001).
FDA Adverse Event Reporting System. (2024). Available from: https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-latest-quarterly-data-files
Zhou, C. et al. Psychiatric disorders associated with immune checkpoint inhibitors: a pharmacovigilance analysis of the FDA adverse event reporting System (FAERS) database. EClinicalMedicine 59, 101967 (2023).
Nomura, K. et al. Effect of database profile variation on drug safety assessment: an analysis of spontaneous adverse event reports of Japanese cases. Drug Des. Devel Ther. 9, 3031–3041 (2015).
Cui, Z. et al. A pharmacovigilance study of etoposide in the FDA adverse event reporting system (FAERS) database, what does the real world say? Front. Pharmacol. 14, 1259908 (2023).
van Elst, J. M. et al. Taste, smell and mouthfeel disturbances in patients with gastrointestinal stromal tumors treated with tyrosine-kinase inhibitors. Support Care Cancer. 30, 2307–2315 (2022).
Buttiron Webber, T., Briata, I. M., DeCensi, A., Cevasco, I. & Paleari, L. Taste and Smell disorders in Cancer Treatment: results from an Integrative Rapid systematic review. Int. J. Mol. Sci. 24, 2538 (2023).
Alles, S. R. A. & Smith, P. A. Etiology and pharmacology of Neuropathic Pain. Pharmacol. Rev. 70, 315–347 (2018).
Jensen, R. K., Kongsted, A., Kjaer, P. & Koes, B. Diagnosis and treatment of sciatica. BMJ 367, l6273 (2019).
Roskoski, R. Properties of FDA-approved small molecule protein kinase inhibitors: a 2021 update. Pharmacol. Res. 165, 105463 (2021).
Klug, L. R., Kent, J. D. & Heinrich, M. C. Structural and clinical consequences of activation loop mutations in class III receptor tyrosine kinases. Pharmacol. Ther. 191, 123–134 (2018).
Zuccotto, F., Ardini, E., Casale, E. & Angiolini, M. Through the ‘gatekeeper door’: exploiting the active kinase conformation. J. Med. Chem. 53, 2681–2694 (2010).
Serrano, C. & George, S. Gastrointestinal stromal tumor: challenges and opportunities for a New Decade. Clin. Cancer Res. 26, 5078–5085 (2020).
Joseph, C. P. et al. Optimal Avapritinib treatment strategies for patients with metastatic or unresectable gastrointestinal stromal tumors. Oncologist 26, e622–e631 (2021).
R, P., Gd, E. A. & Systematic Review Meta-analysis of the risk of Dementia Associated with Benzodiazepine Use, after Controlling for Protopathic Bias. CNS Drugs. 32, 485–497 (2018).
Lai, Y. R., Yang, Y. S., Tsai, M. L., Huang, C. N. & Chiou, J. Y. Benzodiazepine & nonbenzodiazepine prescriptions for Taiwanese elderly with type 2 diabetes contributes to cognitive dysfunction. Int. Psychogeriatr. 26, 1719–1727 (2014).
Yu, J., Wang, Y. & Ragueneau-Majlessi, I. Pharmacokinetic drug-drug interactions with drugs approved by the US Food and Drug Administration in 2020: mechanistic understanding and clinical recommendations. Drug Metab. Dispos. 50, 1–7 (2022).
Nevado-Holgado, A. J., Kim, C. H., Winchester, L., Gallacher, J. & Lovestone, S. Commonly prescribed drugs associate with cognitive function: a cross-sectional study in UK Biobank. BMJ Open. 6, e012177 (2016).
Mehta, R. S. et al. Association of Proton Pump inhibitor Use With Incident Dementia and Cognitive decline in older adults: a prospective cohort study. Gastroenterology 165, 564–572e1 (2023).
Barus, R., Béné, J., Deguil, J., Gautier, S. & Bordet, R. Drug interactions with dementia-related pathophysiological pathways worsen or prevent dementia. Br. J. Pharmacol. 176, 3413–3434 (2019).
Gao, Y. et al. Effects of centrally acting ACE inhibitors on the rate of cognitive decline in dementia. BMJ Open. 3, e002881 (2013).
Rouch, L. et al. Antihypertensive drugs, prevention of cognitive decline and dementia: a systematic review of observational studies, randomized controlled trials and meta-analyses, with discussion of potential mechanisms. CNS Drugs. 29, 113–130 (2015).
Yasar, S., Zhou, J., Varadhan, R. & Carlson, M. C. The use of angiotensin-converting enzyme inhibitors and diuretics is associated with a reduced incidence of impairment on cognition in elderly women. Clin. Pharmacol. Ther. 84, 119–126 (2008).
Lilly, S. M., Mortensen, E. M., Frei, C. R., Pugh, M. J. & Mansi, I. A. Comparison of the risk of psychological and cognitive disorders between persistent and nonpersistent statin users. Am. J. Cardiol. 114, 1035–1039 (2014).
Wharton, W. et al. Modulation of renin-angiotensin System May slow Conversion from mild cognitive impairment to Alzheimer’s Disease. J. Am. Geriatr. Soc. 63, 1749–1756 (2015).
Golomb, B. A., Kane, T. & Dimsdale, J. E. Severe irritability associated with statin cholesterol-lowering drugs. QJM 97, 229–235 (2004).
Acknowledgements
The authors greatly appreciate FDA for providing access to FAERS database for this study.
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
Conception or design of the work: LZ; Acquisition, analysis, or interpretation of data: MW and JJ; Management and checking of all data: WM, JJ, and YX; Drafting the article: MW and JJ. All authors critically reviewed the manuscript and interpreted the results. The final manuscript was read, checked, and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mao, W., Jiang, J., Xia, Y. et al. Analysis of postmarketing neuropsychiatric adverse events of avapritinib based on the FDA adverse event reporting system. Sci Rep 15, 3108 (2025). https://doi.org/10.1038/s41598-025-86959-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-86959-z