Abstract
Prior research has indicated that adopting strict glycemic control measures might elevate the risk of hypoglycemia and result in higher mortality rates among critically ill patients. However, there is a lack of studies investigating the incidence of hypoglycemia and its consequential outcomes in real-world clinical settings. This retrospective cohort study was conducted at Taichung Veterans General Hospital, utilizing critical care databases covering the period from 2015 to 2020. The objective was to examine the outcomes and identify risk factors associated with hypoglycemia in critically ill patients. Out of the total of 16,699 patients admitted to the intensive care units (ICUs), 2,115(12.7%) experienced at least one episode of hypoglycemia were included in the analysis. Critically ill patients who developed hypoglycemia had a significantly higher hospital mortality rate compared to those who did not experience hypoglycemia. (48.9% vs. 15.9%, p < 0.001). Moreover, patients with more severe hypoglycemia exhibited a higher mortality rate (moderate hypoglycemia: hazard ratio [HR] 1.477, 95% confidence interval [C.I.] 1.351–1.614, p < 0.001; severe hypoglycemia: HR 1.847, 95% C.I. 1.607–2.123, p < 0.001) compared to those without hypoglycemia. Additionally, patients with greater frequency of hypoglycemic episodes also showed a higher mortality rate (one episode HR 1.504, 95% C.I. 1.366–1.657, p < 0.001; multiple episodes HR 1.613, 95% C.I. 1.444–1.801, p < 0.001) compared to those without hypoglycemia. Patient who experienced spontaneous hypoglycemia (53.9% vs. 42.4%, p < 0.001) and those without a prior diagnosis of diabetes (60.2% vs. 37.0%, p < 0.001) had higher mortality rate. Hypoglycemia frequently occurs and serves as an independent risk factor for mortality among critically ill individuals, particularly in cases of severe and recurrent episodes. Patients experiencing spontaneous hypoglycemia, as well as those lacking a diabetes diagnosis, demonstrated elevated mortality rates.
Similar content being viewed by others
Background
Maintaining glycemic control is a crucial concern in critically ill patients. Hyperglycemia is frequently observed in this population and has been associated with unfavorable clinical outcomes across various clinical scenarios1,2,3,4,5,6. Proposed mechanisms contributing to glycemic homeostasis include excessive counterregulatory hormones (such as corticosteroids, glucagon, growth hormone, and catecholamines) as well as the release of cytokines like tumor necrosis factor (TNF)-alpha and interleukin (IL)−1. These factors can induce temporary insulin resistance, which in turn can suppress gluconeogenesis and reduce glucose uptake by skeletal muscle7,8,9. Both pharmacological (e.g. steroid, catecholamines) and non-pharmacological (e.g. nutritional intake, intravenous dextrose infusion) interventions can also impact the glycemic status of critically ill patients.
Previous studies10,11,12,13examining glycemic control have yielded conflicting results, as tight glycemic control has been associated with an increased risk of hypoglycemia14, which can ultimately lead to higher mortality rates. Consequently, intensive insulin therapy aiming to maintain blood glucose level between 80 and 110 mg/dL is no longer recommended for critically ill patients. Current guideline suggest initiating insulin therapy when blood glucose level reach or exceed 180 mg/dL, with a target range of between 144 and 180 mg/dL in patients with sepsis or septic shock, in order to mitigate the risk of hypoglycemia15,16.
In critically ill patients, blood glucose level may be influenced by various factors, including dietary intake, medications, adrenal function, or even spontaneously without identifiable factors. To better understand the incidence and outcomes associated with hypoglycemia, we conducted a retrospective study in real-life clinical practice.
Method
Subject enrollment and data collection
We conducted a retrospective cohort study at Taichung Veterans General Hospital (TCVGH), a tertiary referral teaching hospital in central Taiwan with approximately 1500 beds. The study included consecutive adult patients admitted to the Intensive Care Units (ICUs) from January 1st, 2015, to December 31st, 2020.
Data on demographic characteristics of the patients, such as age, sex, Charlson Comorbidity Index (CCI)17, reasons for ICU admission, Acute Physiology and Chronic Health Evaluation (APACHE) II score18, Sequential Organ Failure Assessment (SOFA) scores19, laboratory data, and blood sugar level records during ICU admission, were obtained from the TCVGH critical care clinical data warehouse. Patients who died within 24 h after ICU admission were excluded to ensure complete data for the enrolled subjects. For patients with more than two ICU admissions, only the data of the first ICU admission was used for analysis. The diagnosis of diabetes mellitus included patients with a discharge diagnosis containing any of the ICD-10 code E08-E11, E13 or a measured serum glycated hemoglobin (HbA1c) level greater than or equal to 6.5%20 within 3 months before or during ICU admission.
Glucose control protocol and management
Patients included in the analysis underwent blood glucose measurements, which included both serum glucose readings from laboratory analyzers and those from point-of-care devices (specifically, the Contour Plus One Blood Glucose Monitoring System Glucometer) during their ICU admission. Hypoglycemia was defined based on the clinical practice guideline21and a previous study14, as a blood glucose level below 70 mg/dl. Severe hypoglycemia was defined as a glucose level of 40 mg/dl or less. The glycemic target for critically ill patients was set between 140 and 180 mg/dL. Fingerstick glucose measurements were used in the ICU for convenience and quick response to altering glycemic states. The frequency of glucose testing was at least twice per day and may be increased by the patient’s glycemic status. Continuous insulin infusion was initiated to achieve glycemic control within the target range will when blood glucose level greater than 180 mg/dL. Glucose level was checked every 2 h using the point-of-care device and the infusion rate was adjusted accordingly. Besides, to ensure measurement accuracy, the fingerstick glucose measurement devices were calibrated every day.
The duration of hypoglycemia was calculated from the time of recorded hypoglycemia to the point when the glucose level exceeded 70 mg/dL after intervention. Episodes of hypoglycemia were recorded on a daily basis. Patients who received insulin treatment within 24 h before experiencing hypoglycemia were categorized as having insulin-associated hypoglycemia, while those who experienced hypoglycemia without recent insulin administration were classified as having spontaneous hypoglycemia. The primary outcome of interest was the mortality rate during hospitalization following admission to the ICU.
Statistical analysis
The data were presented as frequency (percentages) for categorical variables and as mean ± standard deviation or median (interquartile range [IQR]) for continuous variables. To compared differences between groups (alive vs. dead, hypoglycemia with or without diabetes, and spontaneous vs. insulin-related), Student’s t-test and the Mann-Whitney U test were used for continuous variables, while the Chi-squared test was used for categorical variables. Kaplan-Meier analysis was performed to access hospital mortality in different hypoglycemia severities, the count of hypoglycemia episodes, hypoglycemia with or without diabetes, and spontaneous or insulin-related hypoglycemia. The log-rank test was used to compare mortality among the three groups. A multivariate COX regression model was constructed to identify independent factors associated with hospital mortality. The model adjusted for potential confounders such as age, BMI, CCI, APACHE II, reasons for ICU admission, episodes of hypoglycemia, severity of hypoglycemia, and other relevant factors. Hazard ratios (HR) for mortality and 95% confidence intervals (C.I.) were calculated. Subgroup analysis was also performed to examine the relationship between hypoglycemia and hospital mortality. Statistical significance was set at a two-sided p-value of < 0.05. The analysis was conducted using R software version 4.3.1.
Ethics approval funding support
The study was adhered to the principles of the Declaration of Helsinki. Approval of the study was obtained from the Institutional Review Board of Taichung Veterans General Hospital (IRB number: SE20249B). The Institutional Review Board of Taichung Veterans General Hospital waived informed consent due to minimal risk to the subjects. All methods were carried out in accordance with the IRB’s guidelines and regulations.
Results
Patient enrollment basic characteristics
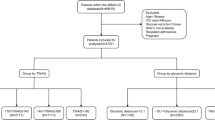
Figure 1 illustrates the patient enrollment process. A total of 29,084 patients were included in this study from 2015 to 2020. Patients with ICU stays of less than 24 h, lacking sugar data records, less than 18 years old, and subsequent records with repeated ICU admission were excluded for analysis. Finally, a total of 16,699 patients were deemed eligible for inclusion in the analysis (Fig. 1). Among these, 13,347 (79.9%) patients survived, while 3,352 (20.1%) died. The characteristics of both survivors and non-survivors are summarized in Table 1. The cohort predominantly consisted of male patients (63.8%), with a median age of 64.4 ± 15.9 years. Furthermore, 35.9% of the patients were diagnosed with diabetes. The mean scores for APACHE II and SOFA were 22.6 ± 7.8 and 7.6 ± 4.0, respectively, on the first day of ICU admission.
A comparative analysis revealed that non-survivors were older (67.2 ± 15.6 years vs. 63.7 ± 15.8 years, p < 0.001), had lower body mass index (BMI) values (23.8 ± 4.9 vs. 24.6 ± 4.7, p < 0.001), and exhibited higher Charlson Comorbidity Index (CCI) scores (2.7 ± 1.6 vs. 1.9 ± 1.5, p < 0.001). The mortality rate was significantly higher in the medical ICU compared to other units (p < 0.001). Acute respiratory failure and sepsis were identified as the primary reasons for ICU admission associated with increased mortality rates (p < 0.001). Furthermore, non-survivors presented with higher APACHE II (28.4 ± 7.4 vs. 20.8 ± 7.0, p < 0.001) and SOFA scores (10.2 ± 4.1 vs. 6.6 ± 3.5, p < 0.001) indicative of greater severity. The average lengths of stay in the ICU and hospital were 8.6 ± 8.1 days, and 17.1 ± 10.1 days, respectively.
Glycemic status
Table 2 provides an overview of glycemic control status. A total of 2,115 patients (12.7%) experienced hypoglycemia during their stay in the ICU. Specifically, 1,742 patients (10.4%) had moderate hypoglycemia, and 373 patients (2.2%) had severe hypoglycemia. Analysis of 1,326 patients revealed that 591 individuals (7.9%) encountered a single episode of hypoglycemia, while 789 (4.7%) had multiple episodes. Among those with hypoglycemia, 926 individuals (44.1%) received an insulin infusion in the 24 h preceding the episodes. The analysis further included the examination of glucose records, encompassing parameters such as minimum, maximum, average, and time-weighted average glucose levels.
Hypoglycemia and risk of mortality
Within this cohort, 2,115 patients (12.7%) experienced hypoglycemia. Among these individuals, there were 1,034 deaths, resulting in a mortality rate of 48.9% which is significantly higher compared to the mortality rate of 15.9% in patients without hypoglycemia (p < 0.001) (Table 3). After adjusting for baseline characteristics, comorbidities, reasons for ICU admission, disease severity, use of mechanical ventilation, renal replacement therapy, and laboratory data, the hazard ratio for death increased significantly with the severity and number of episodes of hypoglycemia (Table 4). Compared to individuals without hypoglycemia, those experiencing moderate hypoglycemia had a hazard ratio for death of 1.477 (95% CI 1.351–1.614, p < 0.001), while severe hypoglycemia was associated with a hazard ratio for death of 1.847 (95% CI 1.607–2.123, p < 0.001). Similarly, compared to individuals without hypoglycemia, those experiencing one episode of hypoglycemia had a hazard ratio for death of 1.504 (95% CI 1.366–1.657, p < 0.001), while multiple episodes of hypoglycemia were associated with a hazard ratio for death of 1.613 (95% CI 1.444–1.801, p < 0.001).
The association between hypoglycemia and mortality varied between patients with and without a diagnosis of diabetes. Patients without a diabetes diagnosis exhibited a significantly higher risk of mortality compared to diabetic patients, particularly concerning the severity (Fig. 2a, p < 0.001) and frequency of hypoglycemic episodes (Fig. 2b, p < 0.001). Patients experiencing spontaneous hypoglycemia exhibited a greater risk of mortality compared to those who had received an insulin infusion 24 h prior to the onset of hypoglycemia (53.9% vs. 42.4%, p < 0.001, Fig. 2c, Table S5).
Hazard Ratios for Hospital Mortality According to Severity and Frequency of Hypoglycemia, Diagnosis of Diabetes, and Type of Hypoglycemia. The relationship of severity (Panel a) or frequency (Panel b) of hypoglycemia and death differs between patients with and without diabetes. Patients experiencing spontaneous hypoglycemia demonstrated a notably elevated risk of mortality compared to those categorized under the insulin-related group (Panel c).
Characteristics of patients with hypoglycemia
Table 3 provides a comparison of characteristics between patients with and without hypoglycemia. Patients who experienced hypoglycemia were older (66.7 ± 15.6 years vs. 64.1 ± 15.9 years, p < 0.001), more likely to be male (64.2% vs. 61.7%, p = 0.025), had lower BMI (23.6 ± 4.6 vs. 24.5 ± 4.7, p < 0.001), higher comorbidity as indicated by the Charlson Comorbidity Index (CCI: 2.7 ± 1.6 vs. 2.0 ± 1.5, p < 0.001), and were more likely to have a diagnosis of diabetes (48.9% vs. 34.0%, p < 0.001). The incidence of hypoglycemia was higher among patients in medical ICUs compared to other ICU types (p < 0.001). Patients with acute respiratory failure and sepsis also had a higher risk of hypoglycemia (p < 0.001). Additionally, patients with higher severity scores (APACHE II score: 27.8 ± 7.3 vs. 21.7 ± 7.6, p < 0.001; SOFA score: 10.0 ± 4.1 vs. 7.1 ± 3.8, p < 0.001), those requiring mechanical ventilation (85.5% vs. 68.2%, p < 0.001), and renal replacement therapy (37.1% vs. 10.2%, p < 0.001) had higher risk of hypoglycemia. Furthermore, patients experiencing hypoglycemia had higher hospital mortality (48.9% vs. 15.9%, p < 0.001), longer ICU stays (14.4 ± 10.1 vs. 7.8 ± 7.4 days, p < 0.001), and longer hospital stays (20.6 ± 10.4 vs. 16.6 ± 10.0 days, p < 0.001).
Discussion
The primary finding of this study highlights the common occurrence of hypoglycemia among critically ill patients in real-life ICU settings. Patients who experienced hypoglycemia were associated with a higher mortality rate, particularly those with severe hypoglycemia and multiple episodes. Notably, non-diabetic patients with hypoglycemia demonstrate poorer outcomes and higher mortality rates. Additionally, over half of the patients with hypoglycemia experienced spontaneous hypoglycemia, resulting in higher mortality rates compared to those who received insulin infusion prior to the onset of hypoglycemia.
The focus on hypoglycemia intensified due to its correlation with the use of intensive insulin therapy aimed at achieving meticulous glycemic control. Intensive insulin therapy has been associated with an increased risk of hypoglycemia and consequently, higher mortality rates. Currently, there is a lack of data regarding the prevalence, risk factors, and outcomes of hypoglycemia in critically ill patients in real-world ICU settings. Previous study, such as Krinsley J.S. et al., conducted a retrospective study in a university-affiliated community hospital, revealing that the occurrence of severe hypoglycemia (blood glucose < 40 mg/dL) was 7.8%22. In another study conducted in two hospitals in Australia by Egi M. et al., 22.4% of those patients experienced hypoglycemia (blood glucose < 81 mg/dL) during ICU admission23. In our study, 12.7% of all participants experienced hypoglycemia, with only 2.2% classified as severe. The differences in these rates may be attributed to varying definitions of hypoglycemia, glycemic control goals, and the initiation of continuous intravenous insulin infusion. The main discovery of this study underscores the common occurrence of hypoglycemia among critically ill patients in real-life practice. Moreover, it highlights that patients with hypoglycemia are associated with a higher mortality rate, particularly those with severe hypoglycemia and multiple episodes. Risk factors for hypoglycemia in critically ill patients include age, higher severity scores (such as APACHE II and SOFA scores), higher CCI scores, a diagnosis of diabetes, admission to medical ICUs, and the presence of sepsis and acute respiratory failure. Patients who developed hypoglycemia not only faced increased mortality rates but also utilized more medical resources, resulting in longer stays in both the ICU and hospital. These findings emphasize the critical importance of monitoring glycemic status to prevent the occurrence of hypoglycemia and ensure timely intervention in critically ill patients.
Dysglycemia, encompassing hyperglycemia, hypoglycemia, and glucose variability, serves as a biomarker of disease severity and is associated with increased mortality in critically ill patients. Diabetic patients are at an elevated risk of experiencing dysglycemia. Within our cohort, diabetes was present in over one-third of patients (35.9%), with 13.8% being diagnosed solely based on an HbA1c level equal to or greater than 6.5%, without any prior medical history of the condition. This indicated that diabetes might be underdiagnosed in critically ill patients. Given that pre-morbid hyperglycemia is linked to an increased risk of hypoglycemia24, routine screening for glycated hemoglobin is recommended for ICU patients. Although diabetic patients were more susceptibility to hypoglycemia, our findings revealed that their mortality rate was lower compared to non-diabetic patients. Previous studies had also indicated that blood glucose level may have different clinical implications between diabetic and non-diabetic patients25,26. Therefore, glycemic control strategies should be tailored differently for diabetic and non-diabetic patients, and the future research is needed to explore optimal approaches.
Hypoglycemia can occur in critically ill patients even without insulin infusion. Patients with significant underlying medical conditions, such as malnutrition, liver cirrhosis, chronic renal failure, adrenal insufficiency, and hypothyroidism, are at an increased risk of developing hypoglycemia27,28,29,30. The proposed biochemical mechanism connecting hypoglycemia to elevated mortality remains unclear. Previous studies indicate that recurrent hypoglycemia can exacerbate ischemic damage to the brain, probably resulting in hormonal dysregulation and harmful systemic effects, or could lead to cell death and causing end-organ damage. The basic mechanism of hypoglycemia, along with its impact on organ damage and clinical outcomes, need additional research and investigation31,32. Our findings revealed that over half of the patients who experienced hypoglycemia encountered it spontaneously, without receiving insulin infusion in the preceding 24 h. These patients exhibited even higher mortality rates compared to those whose hypoglycemia was associated with insulin infusion. The factors contributing to the occurrence of hypoglycemia in critically ill patients remain unclear, and previous studies have shown conflicting outcomes regarding their prognosis23,33,34,35. In our study, more than half hypoglycemic patients did not receive insulin infusion before the episode of hypoglycemia. Previous studies have demonstrated risk factors of the development of spontaneous hypoglycemia including fulminant hepatic failure and/or overt adrenal failure during septic shock, especially in patients with severe comorbidities such as malnutrition, liver cirrhosis, chronic renal failure, adrenal insufficiency and hypothyroidism. Proposed mechanisms responsible for glycemic homeostasis includes excessive counter regulatory hormones and release of cytokines tumor necrosis factor (TNF)-alpha and interleukin (IL)−1. These factors can result in transient insulin resistance that can lead to suppression of gluconeogenesis and decreased glucose uptake by skeletal muscle7,27,28,29,30. However, the risk of development of spontaneous hypoglycemia and its impact on clinical outcome of critically ill patients remains unknown. In our study, individuals experiencing spontaneous hypoglycemia tended to be younger, male, and have lower Charlson Comorbidity Index (CCI), APACHE II, and SOFA scores compared to those with diabetes. Additionally, they exhibited a lower prevalence of diabetes, lower HbA1c levels, but higher serum lactate levels. Due to the potential association of spontaneous hypoglycemia with poorer outcomes and its possible oversight or delayed detection, there is an urgent requirement for research aimed at exploring its underlying causes and clinical manifestations.
Our study has several limitations that should be acknowledged. Firstly, as a retrospective study, it offers insights into real-world clinical practices but is constrained by potential issues of incomplete or biased data, and it lacks the ability to establish causality. Secondly, being a single-center study, the findings may not be generalizable to other hospitals or medical systems, limiting their applicability. However, because of the high accessibility of medical care in Taiwan, this cohort may well reflect the prevalence and clinical features of critically ill patients with hypoglycemia in clinical practice. We thus suggest a prospective multiple-center study to validate these findings and propose principles for glycemic control and monitoring. Thirdly, the collection of glucose data lacked a standardized protocol, which might have led to missed episodes of hypoglycemia. Additionally, the use of both serum glucose and finger-stick samples for blood glucose measurements resulted in non-standardized results, which could affect the reliability of the findings. However, in our study, most of the records of blood sugar were from fingerstick glucose measurements instead of blood sugar level due to consideration of convenience and rapid response to glycemic management. We only checked serum glucose level once at ICU admission. However, fingerstick glucose measurement devices were calibrated every day to minimize measurement bias. Furthermore, there is lack of post-discharge follow-up data of this cohort. The influence of hypoglycemia and effect of clinical outcome need further evaluation for those who transferring out from ICUs. Additional prospective studies or trials to investigate the association between hypoglycemia and mortality in critically ill patients are needed to find out risk factors of developing hypoglycemia in critically ill patients. In addition, we proposed studies for the application of continuous glucose monitor in the intensive care unit. This may help early detection and timely intervention of hypoglycemia and thus improve outcomes of these patients.
Conclusion
The major finding of this study is that hypoglycemia common and associated with increased mortality among critically ill patients in real-life practice, particularly in cases with greater severity and frequency of hypoglycemic episodes. Within this cohort, patients without diabetes and those experiencing spontaneous hypoglycemia exhibited elevated mortality rates. These findings emphasize the critical significance of preventing and promptly addressing hypoglycemia in critically ill patients.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to rule of Institutional Review Board but are available from the corresponding author on reasonable request.
Abbreviations
- Term:
-
Definition
- DM:
-
Diabetes mellitus
- BMI:
-
Body Mass Index
- ICU:
-
Intensive care unit
- APCHE II:
-
Acute Physiology and Chronic Health Evaluation II
- SOFA:
-
The sequential organ failure assessment score
- Hb:
-
Hemoglobin
- CCI:
-
Charlson Comorbidity Index
- RRT:
-
Renal replacement therapy
- CRP:
-
C-reactive protein
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- HR:
-
Hazard ratios
- CI:
-
Confidence interval
References
Capes, S. E., Hunt, D., Malmberg, K. & Gerstein, H. C. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 355 (9206), 773–778 (2000).
Laird, A. M., Miller, P. R., Kilgo, P. D., Meredith, J. W. & Chang, M. C. Relationship of early hyperglycemia to mortality in trauma patients. J. Trauma. 56 (5), 1058–1062 (2004).
Elhadd, T. A., Al-Amoudi, A. A. & Alzahrani, A. S. Epidemiology, clinical and complications profile of diabetes in Saudi Arabia: a review. Ann. Saudi Med. 27 (4), 241–250 (2007).
Stöllberger, C., Exner, I., Finsterer, J., Slany, J. & Steger, C. Stroke in diabetic and non-diabetic patients: course and prognostic value of admission serum glucose. Ann. Med. 37 (5), 357–364 (2005).
Jones, K. W. et al. Hyperglycemia predicts mortality after CABG: postoperative hyperglycemia predicts dramatic increases in mortality after coronary artery bypass graft surgery. J. Diabetes Complications. 22 (6), 365–370 (2008).
Pasternak, J. J. et al. Hyperglycemia in patients undergoing cerebral aneurysm surgery: its association with long-term gross neurologic and neuropsychological function. Mayo Clin. Proc. 83 (4), 406–417 (2008).
McCowen, K. C., Malhotra, A. & Bistrian, B. R. Stress-induced hyperglycemia. Crit. Care Clin. 17 (1), 107–124 (2001).
Robinson, L. E. & van Soeren, M. H. Insulin resistance and hyperglycemia in critical illness: role of insulin in glycemic control. AACN Clin. Issues. 15 (1), 45–62 (2004).
Mizock, B. A. Alterations in carbohydrate metabolism during stress: a review of the literature. Am. J. Med. 98 (1), 75–84 (1995).
van den Berghe, G. et al. Intensive insulin therapy in critically ill patients. N Engl. J. Med. 345 (19), 1359–1367 (2001).
Van den Berghe, G. et al. Intensive insulin therapy in the medical ICU. N. Engl. J. Med. 354 (5), 449–461 (2006).
Brunkhorst, F. M. et al. Intensive insulin therapy and pentastarch resuscitation in severe Sepsis. N. Engl. J. Med. 358 (2), 125–139 (2008).
Intensive versus conventional glucose control in critically ill patients. N. Engl. J. Med., 360(13): pp. 1283–1297. (2009).
Hypoglycemia and risk of death in critically ill patients. N. Engl. J. Med., 367(12): pp. 1108–1118. (2012).
Evans, L. et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 47 (11), 1181–1247 (2021).
Evans, L. et al. Surviving Sepsis Campaign: International guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 49 (11), e1063–e1143 (2021).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40 (5), 373–383 (1987).
Knaus, W. A., Zimmerman, J. E., Wagner, D. P., Draper, E. A. & Lawrence, D. E. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit. Care Med. 9 (8), 591–597 (1981).
Vincent, J. L. et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on sepsis-related problems of the European Society of Intensive Care Medicine. Crit. Care Med. 26 (11), 1793–1800 (1998).
ElSayed, N. A. et al. 2. Classification and diagnosis of diabetes: standards of Care in Diabetes—2023. Diabetes Care. 46 (Supplement_1), S19–S40 (2022).
Committee, A. D. A. P. P. 6. Glycemic targets: standards of Medical Care in Diabetes—2022. Diabetes Care. 45 (Supplement_1), S83–S96 (2021).
Krinsley, J. S. & Grover, A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit. Care Med. 35 (10), 2262–2267 (2007).
Egi, M. et al. Hypoglycemia and outcome in critically ill patients. Mayo Clin. Proc. 85 (3), 217–224 (2010).
Egi, M. et al. Pre-morbid glycemic control modifies the interaction between acute hypoglycemia and mortality. Intensive Care Med. 42 (4), 562–571 (2016).
Fong, K. M., Au, S. Y. & Ng, G. W. Y. Glycemic control in critically ill patients with or without diabetes. BMC Anesthesiol. 22 (1), 227 (2022).
Chan, M. C. et al. A minimum blood glucose value less than or equal to 120 mg/dL under glycemic control is associated with increased 14-day mortality in nondiabetic intensive care unit patients with sepsis and stress hyperglycemia. J. Crit. Care. 34, 69–73 (2016).
Nirantharakumar, K. et al. Hypoglycemia in non-diabetic in-patients: clinical or criminal? PLoS One. 7 (7), e40384 (2012).
Sako, A. et al. Hospitalization with hypoglycemia in patients without diabetes mellitus: a retrospective study using a national inpatient database in Japan, 2008–2012. Med. (Baltim). 96 (25), e7271 (2017).
Eom, Y. S., Wilson, J. R. & Bernet, V. J. Links between thyroid disorders and glucose homeostasis. Diabetes Metab. J. 46 (2), 239–256 (2022).
Arky, R. A. Hypoglycemia Associated with liver disease and ethanol. Endocrinol. Metab. Clin. North Am. 18 (1), 75–90 (1989).
Dave, K. R., Pileggi, A. & Raval, A. P. Recurrent hypoglycemia increases oxygen glucose deprivation-induced damage in hippocampal organotypic slices. Neurosci. Lett. 496 (1), 25–29 (2011).
Widlansky, M., Wang, J., Alexannian, A. & Gutterman, D. Abstract 1088: Acute Hypoglycemia induces mitochondria-mediated superoxide production and apoptosis in endothelial cells: inhibition by AMP kinase activation through an eNOS dependent mechanism. Circulation, 120. (2009).
Kosiborod, M. et al. Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. Jama 301 (15), 1556–1564 (2009).
Cichosz, S. L., Redke, F. & Hejlesen, O. K. Spontaneous and iatrogenic hypoglycaemia related to mortality in the ICU. Diabetes Metab. 45 (6), 545–549 (2019).
Saliba, L., Cook, C. H., Dungan, K. M., Porter, K. & Murphy, C. V. Medication-induced and spontaneous hypoglycemia carry the same risk for hospital mortality in critically ill patients. J. Crit. Care. 36, 13–17 (2016).
Acknowledgements
We appreciate Yu Yi Yu and Wei Ting Hsiao, our research assistants, Biostatistics Group, Department of Medical Research, Taichung Veterans General Hospital, and the staff of Artificial Intelligence Studio at Taichung Veterans General Hospital for their cooperation in this study.
Funding
This study was supported by Taiwan National Science and Technology Council (NSTC 112-2321-B-075 A-001). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
HFY was a major contributor in drafting the manuscript. WCC and CLW analyzed the patient data and are also responsible for the acquisition and interpretation of data. MCC was responsible for the conception and the design of the work and involved in study coordination. All authors participated in the revision of the article for important intellectual content and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Given that the research was limited to the secondary use of data previously collected during daily practice and the patients’ identity were completely unidentifiable, the informed consent was hence waived.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yeh, HF., Chao, WC., Wu, CL. et al. Hypoglycemia and hospital mortality in critically ill patients. Sci Rep 15, 2642 (2025). https://doi.org/10.1038/s41598-025-87163-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87163-9