Abstract
Trichomes are specialised epidermal structures on plant surfaces of plant aerial organs. They are integral to plant defence and adaptation. However, their elemental composition in Solanum species remains understudied. This research investigates the elemental composition of trichomes from five Solanum species, including representatives of the crops tomato and potato — Solanum berthaultii, Solanum galapagense, Solanum lycopersicum (tomato), Solanum pennellii, and Solanum tuberosum (potato) — using herbarium samples from the Royal Botanic Gardens, Kew. Samples were carbon coated and observed under the Field Emission Gun Scanning Electron Microscopy (FEG SEM). Energy Dispersive X-ray Spectroscopy (EDX/EDS) data were collected using an EDX detector. Results revealed significant variation in elemental content among the five Solanum species. The trichomes of S. berthaultii showed high levels of potassium (K), especially at the tips, suggesting a role in osmoregulation and defence. S. galapagense exhibited notable chlorine (Cl) and calcium (Ca) presence, possibly for ionic balance and pest deterrence. S. lycopersicum and S. pennellii had higher silicon (Si) concentrations in the mid-sections and tips of the trichomes, enhancing structural integrity and herbivory resistance. S. tuberosum displayed significant variability in calcium (Ca), particularly at the base, in which could be of importance for maintaining cellular structure and for rapid damage response. This study provides insights into the elemental composition of the trichomes of Solanum species, contributing to our understanding of their ecological and physiological roles. Our findings underscore the importance of elemental composition in studies of plant adaptation and evolution, offering a foundation for future research in plant defence mechanisms and environmental interactions.
Similar content being viewed by others
Introduction
Trichomes, specialised epidermal structures found on plant leaves and stem surfaces, have garnered considerable interest due to their diverse roles in plant physiology, ecology, ecological interactions, defence mechanisms, and adaptation to environmental stresses1,2. They are known to contribute to plant protection against herbivores, pathogens, and environmental stresses, as well as participate in various physiological processes such as water regulation and UV protection3,4,5. Trichomes are also reservoirs of specialised metabolites, including alkaloids, flavonoids, and terpenoids, which are involved in plant chemical defence5. However, the economy around these epidermal structures is often overlooked and undervalued; many ignore their pervasive presence in everyday life. For instance, cotton textiles, beer flavour, culinary herbs, and some drugs, such as the antimalarial artemisinin from Artemisia annua L6,7. and the antidepressant cannabidiol from Cannabis sativa L8,9, are derived from plant trichomes.
Trichomes perform several ecological functions by conferring tolerance to biotic and abiotic stresses, such as protection against the attack of insects and microorganisms, excess ultraviolet light, heat, drought, and heavy metals2,10. Glandular trichomes produce and accumulate (secondary) specialised compounds11. In the tomato clade, there are species with notable pest resistance due to the presence of type-IV trichomes at a high density, such as Solanum pennellii Correll and S. galapagenseS.C. Darwin & Peralta12,14. Among the sampled species, S. lycopersicum L. (tomato) and S. tuberosum L. (potato) are of particular agricultural importance. At the same time, Solanum berthaultii, Solanum galapagense, and Solanum pennellii are wild species with unique ecological niches and genetic diversity.
The genus Solanum within the Solanaceae family, with 1,290 accepted species distributed worldwide (POWO, 2024), comprises a diverse group of plant species with a wide range of trichome morphologies and ecological adaptations15,16. Trichomes in Solanum species vary in size, shape, density, and glandular structures. Solanum lycopersicum (tomato) is well-known for its glandular trichomes, which secrete various specialised metabolites in chemical defence against herbivores and pathogens12. Similarly, Solanum tuberosum (potato) possesses non-glandular trichomes that contribute to physical defence by reducing herbivore feeding damage17,18. The wild relatives, Solanum berthaultii, S. galapagense, and S. pennellii, exhibit unique trichome morphologies adapted to their respective ecological niches19.
Herbarium specimens offer a valuable resource for studying the elemental compositions of different plant parts, including trichomes, across diverse plant species within a genus20. By analysing plant specimens collected from various geographic locations and ecological conditions, we can elucidate the spatial and temporal variation in trichome elemental content and its implications for plant adaptation and evolution.
Energy-Dispersive X-ray Spectroscopy (EDX) is an extremely useful instrument in plant science, with its potential still largely untapped. Analyzing X-ray spectra from plant samples reveals the elemental composition and essential nutrients distributed at the cellular level. This information is crucial for understanding plant behavior, including stress avoidance, disease resistance, and metabolic processes vital for plant development. Consequently, EDX is valuable for breeding programs aimed at developing resilient plants, enhancing agricultural sustainability, and improving global food production21.
The elemental composition of trichomes, encompassing a wide range of essential and trace elements, is particularly interesting due to its potential implications for plant physiology, biochemistry, and ecological interactions22. Recent studies have investigated the presence of essential and trace elements in trichomes in species of Rosaceae and Brassicaceae, including calcium (Ca), potassium (K), magnesium (Mg), iron (Fe), zinc (Zn), and manganese (Mn)27,28. These elements are essential for various physiological processes, such as cell wall structure, enzyme activation, and osmotic regulation.
Despite their ecological and agronomic significance, the elemental composition of trichomes in Solanaceae, particularly in the genus Solanum, remains to be investigated. Determining the elemental profiles of trichomes in these species has great potential for providing unique insights into their physiological functions, ecological roles, nutrient uptake, metal accumulation, and metabolic pathways associated with specialised metabolite biosynthesis. This knowledge can potentially be applied in plant breeding and crop improvement of two important Solanaceae crops, tomato and potato, along with others, such as peppers, eggplant, tobacco, and groundcherry.
Furthermore, understanding the elemental composition of trichomes in Solanum species may lead to a better understanding of their adaptive strategies to biotic and abiotic stresses in their natural habitats, hence having significant ecological and physiological implications for Solanumspecies. For instance, calcium (Ca) and potassium (K) play essential roles in plant defence against herbivores and pathogens by regulating specialised metabolite production and signal transduction pathways23,24. Iron (Fe) and zinc (Zn) are involved in photosynthesis and antioxidant defence mechanisms, which are critical for plant growth and stress tolerance25,26.
In this study, we aim to determine the elemental composition in the trichomes of five Solanum species (Solanum berthaultii, S. galapagense, S. lycopersicum, S. pennellii, and S. tuberosum) from herbarium specimens. By employing scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDS) analysis, we investigated the distribution and concentration of elements within the trichomes of these species.
Materials and methods
Sample collections
Leaf samples of Solanum berthaulitii, Solanum galapagense, Solanum lycopersicum, Solanum pennellii, and Solanum tuberosum were obtained from herbarium specimens housed at the Royal Botanic Gardens, Kew (K Herbarium).
Sample preparation
Trichome areas were carefully dissected from the surfaces of the leaf samples using fine forceps and mounted on 15 mm aluminium stubs with double-sided carbon tape and carbon coated using quorum-Q150Tes, using pulse rod evaporation recipe to enhance conductivity and minimise charging effects during imaging. Special care was taken to avoid contamination and damage to the trichomes during and after the preparation process.
Scanning electron microscopy and elemental analysis (SEM-EDS)
Samples were imaged under a Hitachi Regulus 8230 FEG-SEM with a 10 Kv Elemental compositions of trichomes were determined using Energy-Dispersive X-ray Spectroscopy (EDS) with an Oxford instruments Ultim Extreme detector and point-and-ID technique. The EDS system was calibrated using standard reference materials to ensure accurate quantification of elemental peaks. Elemental spectra were analysed to identify and quantify the presence of elements within the trichome samples using an Oxford EDS detector together with in-lens SE and BSE detector to obtain elemental composition information at the Bioimaging Laboratory, Royal Botanic Gardens, Kew. EDS spectra were acquired from selected areas of interest in the sample, specifically the base, mid-section, and tip of the trichomes. For each region, analyses were done in five replicates, as shown in the representative sample in Fig. 1. Subsequently, the acquired SEM images and EDS spectra were processed and analysed using AZtech software.
Elemental composition analysis of trichome regions from representative species using EDX-SEM. (A) Tip, (B) Mid-section, and (C) Base of the trichome are shown on the left. Corresponding graphs on the right depict the elemental composition of specific regions highlighted in each respective micrograph.
Data analysis
Elemental data were statistically assessed with an analysis of variance (ANOVA) to assess differences in elemental compositions among Solanum species and trichome types, looking for the differences between data groups, which are statistically significant differences between groups and analysing the levels of variance within each group.
Quality control
All sample preparation and analysis procedures were conducted under controlled laboratory conditions to ensure the reliability and reproducibility of results. Standard reference materials and calibration procedures were used to verify the accuracy of elemental composition.
Results
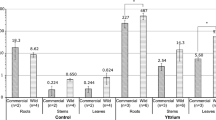
The elemental composition of trichomes in the sampled Solanum species revealed significant variation among species and trichome types. Across the five species studied (Solanum berthaultii, S. galapagense, S. lycopersicum, S. pennellii, and S. tuberosum), differences in elemental content were observed, reflecting their ecological and physiological adaptations (Tables 1, 2 and 3; Figs. 2, 3 and 4; Appendix). Oxygen is consistently the most abundant element in the trichomes of all species and regions (base, mid-section, and tip). However, the proportions of other elements like silicon (Si), potassium (K), iron (Fe), aluminium (Al), magnesium (Mg), calcium (Ca), chlorine (Cl), sodium (Na), phosphorus (P), and sulphur (S) vary markedly. For instance, Solanum tuberosum exhibits a higher percentage of calcium (Ca) and potassium (K) in the trichome tips than other species, suggesting a unique adaptation or functional role.
Elemental Composition Comparison via ANOVA. One-way ANOVA comparing elemental compositions across Solanum species studied and trichome types, revealing significant differences (p < 0.05). See Supplemental Table S1 for detailed statistics.
The results also evidenced a variation in the elemental composition between different sections of the trichomes. Solanum berthaultii shows a notable presence of iron (Fe) and aluminium (Al) at the trichome bases, significantly lower in Solanum galapagense. This could be attributed to ecological and physiological differences among these species. The mid regions of the trichomes in Solanum galapagense are enriched with silicon (Si) and chlorine (Cl), which are relatively lower in Solanum lycopersicum. Such variations could indicate different defensive or structural functions provided by these elements, as silicon (Si), for instance, is known for enhancing plant defence mechanisms13. In respect to the tips of the trichomes, Solanum pennellii stood out for exhibiting a higher percentage of potassium (K) and calcium (Ca) in this section, possibly playing a role in specific defence or metabolic processes. The variability in elemental composition, especially in the tip regions, could reflect diverse ecological pressures and evolutionary adaptations. In essence, these elemental differences among trichome regions and species are evidence of the complexity of plant adaptations and the potential multifunctionality of trichomes in particular in Solanum.
ANOVA analysis (Supplemental table S1, Fig. 2)
The analysis of variance (ANOVA) table reveals significant effects of the group on certain elemental levels. Specifically, the group significantly affects oxygen levels (F (2, 97) = 6.16, p = 0.00303), with the mean Oxygen (O) levels differing substantially between groups, as indicated by the p-value. This suggests that the group has a notable impact on Oxygen (O) levels, further supported by the relatively small residuals’ mean square value compared to the mean square value of the group, indicating that the group explains a significant proportion of the variance.
Iron (Fe) levels also show a significant effect of the group (F (2, 97) = 5.58, p = 0.00508), with the p-value below the 0.05 threshold. This suggests that group differences have a statistically significant impact on iron (Fe) levels. Similarly, sulphur (S) levels are significantly affected by the group (F (2, 97) = 4.966, p = 0.00884), again indicated by the p-value being below 0.05.
In contrast, several other elements did not show significant effects between groups. Silicon (Si) levels do not significantly differ between groups (F (2, 97) = 0.254, p = 0.776), as the p-value is higher than 0.05, indicating no statistical significance. Potassium (K) levels similarly show no significant group effect (F (2, 97) = 1.1, p = 0.337), with a non-significant p-value. Aluminium (Al) levels (F (2, 97) = 0.708, p = 0.495) and magnesium (Mg) levels (F (2, 97) = 0.096, p = 0.908) also show no significant effects, with their p-values much higher than 0.05. Chlorine (Cl) (F (2, 97) = 0.432, p = 0.651), sodium (Na) (F (2, 97) = 0.295, p = 0.745), and phosphorus (P) (F (2, 97) = 1.366, p = 0.26) levels similarly indicate no significant group differences.
Overall, while oxygen (O), iron (Fe), and sulphur (S) levels were significantly impacted by group differences, the levels of silicon (Si), potassium (K), aluminium (Al), magnesium (Mg), chlorine (Cl), sodium (Na), phosphorus (P), and to a lesser extent calcium (Ca), were not significantly affected.
Variance between groups (Supplemental table S2, Fig. 3)
Base data group
Oxygen (O) had the highest variance (93.6), indicating significant variability in its data. Phosphorus (P) had the lowest variance (0.201), suggesting minimal variability. Other elements, such as Calcium (Ca) and Chlorine (Cl), also showed relatively high variance values (80.3 and 28.9, respectively).
Mid data group
The Oxygen (O) data again showed a high variance (33.7), but lower than that of the Base Data group. Phosphorus (P) and Iron (Fe) had low variances (1.06 and 0.252, respectively), indicating consistent data for these elements. Overall, most elements had lower variances than the Base Data group.
Tip data group
Potassium (K) had a high variance (37.7), indicating a widespread in the data. Sodium (Na) had the lowest variance (0.367), similar to its low variance in other groups. Variance values for elements like Oxygen (O) and Silicon (Si) were between the Base and Mid-section groups.
Correlations of combined data (Supplemental table S3)
The correlation analysis of combined data revealed several significant relationships among the elements (Fig. 4). Oxygen (O) exhibited strong negative correlations with iron (Fe) (−0.525) and calcium (Ca) (−0.600), indicating that these elements are less likely to co-exist in high concentrations with oxygen.
Silicon (Si) showed a strong positive correlation with Aluminium (Al) (0.794) and a moderate positive correlation with iron (Fe) (0.551), suggesting that these elements often occur together. Similarly, iron (Fe) was strongly correlated with silicon (0.551) and aluminium (0.630), reinforcing the notion that these elements co-exist in these structures.
Potassium (K) presented strong negative correlations with sodium (Na) (−0.737) and magnesium (Mg) (−0.609), alongside moderate positive correlations with silicon (0.363) and iron (0.400). These patterns indicate complex interrelationships among these elements.
Aluminium (Al) also showed strong positive correlations with silicon (0.794) and iron (0.630), further indicating a strong association between these elements. In contrast, magnesium (Mg) strongly positively correlated with chlorine (Cl) (0.664) and sodium (0.636) but had strong negative correlations with silicon (−0.522) and potassium (−0.609).
Calcium (Ca) displayed a strong negative correlation with oxygen (−0.600) and a moderate negative correlation with sodium (−0.358), suggesting specific exclusion patterns with these elements. Chlorine (Cl) had a strong positive correlation with sodium (0.643) and a moderate positive correlation with magnesium (0.664), indicating a tendency for these elements to be found together.
Sodium (Na) is strongly negatively correlated with potassium (−0.737) and moderately with silicon (−0.326) and aluminium (−0.373), which suggests a separation between these element concentrations. Lastly, phosphorus (P) shows weaker correlations than other elements but has a moderate positive correlation with magnesium (0.347), indicating some level of association.
Discussion
The research on the elemental composition of trichomes in five Solanum species (S. berthaultii, S. galapagense, S. lycopersicum, S. pennellii, and S. tuberosum) revealed significant interspecies and intraspecies variation. This variation possibly reflects both ecological and physiological adaptations. By examining the elemental composition at the trichome base, mid-section, and tip, distinct patterns emerge that could influence the species’ interaction with their environment, pest resistance, and overall physiology.
The base of trichomes across the species showed a high content of oxygen (O), with notable variations in other elements like silicon (Si), potassium (K), and calcium (Ca). For example, S. berthaultiiexhibited a wide range of potassium (K) content (8.2–18.0%), suggesting a potential role in physiological processes such as turgor regulation and defence mechanisms as described by29. On the other hand, S. galapagense and S. lycopersicum had relatively lower silicon (Si) levels compared to S. tuberosum and S. pennellii, which might relate to their different environmental stress adaptations (Supplemental Table S4). Notably, S. tuberosum trichomes showed significant variability in calcium (Ca) content (2.6–30.2%), indicating potential differences in cellular structure and rigidity.
At the mid-section, oxygen (O) remained predominant, but the distribution of other elements shifted. S. pennellii and S. tuberosum showed higher silicon (Si) content, which could enhance their structural integrity and resistance to herbivory, which has been confirmed in other plants like cucumber, radish, rice, etc30,31,32,33. Potassium (K) levels were also high in S. berthaultii, which aligns with its physiological role in plant stress responses. The presence of magnesium (Mg) and sulphur (S) in varying concentrations suggests their involvement in metabolic and defensive processes, as supported by previous research linking these elements to enzyme activity and specialised metabolite production34,35,36.
The trichome tips displayed the most significant variations in elemental composition. S. berthaultii showed high potassium (K) levels (up to 24.9%), which could enhance the plant’s defensive secretion capability. In contrast, S. galapagense had lower elemental diversity but a significant presence of chlorine (Cl) and calcium (Ca), possibly contributing to ionic balance and pest deterrence provided by calcium chlorides37. The varied elemental content at the trichome tips across species underscores the role of these structures in ecological interactions and defence mechanisms.
The presence, absence, abundance, and correlations of specific elements in trichomes across different regions have several implications. High oxygen (O) levels across all areas indicate its fundamental role in maintaining trichome structure and function. Muravnik38 found leaf glandular trichomes highly oxygenated in all the plant species he studied. Also, the significant presence of potassium (K), particularly in S. berthaultii and S. tuberosum, suggests its importance in osmoregulation and defence, corroborating studies that indicate a critical role of potassium (K) in plant stress tolerance and pathogen resistance39,40,41.
The variability in silicon (Si) content, especially in the mid-section and tips of S. pennellii and S. tuberosum, aligns with research suggesting that silicon (Si) can enhance structural defences and reduce herbivory. The elevated calcium (Ca) levels in the trichome of S. tuberosum point to its role in maintaining cellular integrity and facilitating rapid response to physical damage, consistent with findings that calcium (Ca) signalling is crucial in plant defence42,43 .
Magnesium (Mg) and sulphur’s (S) varying levels suggest their involvement in enzymatic functions and secondary metabolism. Magnesium (Mg) is essential for chlorophyll production and enzyme activation44,45while sulphur (S) is a critical component of amino acids and defensive compounds46,47. Their presence in the trichomes suggests these elements play a role in supporting the metabolic demands of these structures and, in this way, enhancing the plants’ overall resilience to biotic and abiotic adversities.
Conclusion
In summary, the elemental composition of trichomes in the studied Solanum species reveals critical insights into their ecological and physiological strategies. The significant variations among species and trichome regions indicate adaptive mechanisms that likely enhance survival, stress tolerance, and defence. Understanding these elemental distributions helps decipher the complex interactions between plants and their environment, providing a foundation for future research and potential agricultural applications.
This study presents, for the first time, information on the elemental composition of trichomes in family Solanaceae, in particular of the genus Solanum and the crops tomato and potato. While this is a starting point for future studies, advancing knowledge of the elemental composition of these species will have important practical implications for plant breeding and conservation efforts. Breeding programs aimed at developing stress-tolerant cultivars can utilise information on elemental profiles to select traits associated with enhanced nutrient uptake, metal tolerance, and pest resistance. Furthermore, conservation strategies for endangered species can benefit from understanding the elemental strategies employed by wild relatives in diverse ecological niches. Thus, our study has also demonstrated that herbarium specimens may be used successfully to sample elemental components in plant species, particularly with the SEM-EDS technique, and therefore, herbarium collections can be a helpful source of material for the expansion of these studies.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Liu, Y., Jing, S. X., Luo, S. H. & Li, S. H. Non-volatile natural products in plant glandular trichomes: chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 36 (4), 626–665 (2019).
Zhang, Y. et al. The roles of different types of trichomes in tomato resistance to cold, drought, whiteflies, and botrytis. Agronomy 10 (3), 411 (2020).
Glas, J. J. et al. Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int. J. Mol. Sci. 13 (12), 17077–17103 (2012).
Karabourniotis, G., Liakopoulos, G., Nikolopoulos, D. & Bresta, P. Protective and defensive roles of non-glandular trichomes against multiple stresses: structure–function coordination. J. Forestry Res. 31 (1), 1–12 (2020).
Kaur, J. & Kariyat, R. Role of trichomes in plant stress biology. Evolutionary Ecology of plant-herbivore Interaction, pp. 15–35. (2020).
Duke, S. et al. Current and potential exploitation of plant glandular trichome productivity, pp. 121–151. (2000).
Soetaert, S. Trichomes, the key to an increased production of artemisinin in Artemisia annua Ghent University. (2013).
Ghosh, D., Chaudhary, N., Shanker, K., Kumar, B. & Kumar, N. Monoecious Cannabis sativa L. discloses the organ-specific variation in glandular trichomes, cannabinoids content and antioxidant potential. J. Appl. Res. Med. Aromatic Plants. 35, 100–476 (2023).
Xie, Z. et al. Cannabis sativa: origin and history, glandular trichome development, and cannabinoid biosynthesis. Hortic. Res. 10 (9), uhad150 (2023).
Ojo, F. M., Nwokeocha, C. C. & Faluyi, J. O. Foliar Anatomical studies of Andropogon gayanus-Andropogon tectorum Complex in Southwestern Nigeria. Notulae Scientia Biologicae (2021). https://doi.org/10.15835/nsb13411031
Wang, G. Recent progress in secondary metabolism of plant glandular trichomes. Plant. Biotechnol. 31 (5), 353–361 (2014).
Vendemiatti, E. Genetic and Molecular Characterization of type-IV Glandular Trichome Development in Tomato (Solanum lycopersicum Cv. Micro-Tom) and its Participation in Arthropod Resistance (Universidade de São Paulo], 2020).
Reynolds, O. L., Padula, M. P., Zeng, R. & Gurr, G. M. Silicon: potential to promote direct and indirect effects on plant defence against arthropod pests in agriculture. Front. Plant Sci. 7, 744 (2016).
Vendemiatti, E. et al. The genetic complexity of type-IV trichome development reveals the steps towards an insect-resistant tomato. Plants 11 (10), 1309 (2022).
Bar, M. & Shtein, I. Plant trichomes and the biomechanics of defence in various systems, with Solanaceae as a model. Botany 97 (12), 651–660 (2019).
Hassan, R. A. & Hamdy, R. Comparative study on trichomes types of wild species of Solanum L., 1753 (Solanales, Solanaceae) in Egypt and its taxonomic significance. Bulletin of the Iraq Natural History Museum (P-ISSN: 1017–8678, E-ISSN: 2311–9799). 17(3): 349–373. (2023).
Cho, K. S. et al. Characterization of trichome morphology and aphid resistance in cultivated and wild species of potato. Hortic. Environ. Biotechnol. 58, 450–457 (2017).
Pelletier, Y., Horgan, F. G. & Pompon, J. Potato resistance against insect herbivores: Resources and opportunities. Insect pests of potato: global perspectives on biology and management, pp. 439–462. (2013).
Dupin, J. G. R. Historical Biogeography and the Evolution of Environmental Niche and Fruit type in Datureae (Solanaceae) (University of Colorado at Boulder, 2017).
Heberling, J. M. Herbaria as big data sources of plant traits. Int. J. Plant Sci. 183 (2), 87–118 (2022).
Adeniyi, A. G. et al. Unlocking the potential of teak seed waste: carbonization for sustainable resource transformation. Biofuels, Bioprod. Biorefin. 18 (1), 226–236 (2024).
Tissier, A. Glandular trichomes: what comes after expressed sequence tags? Plant J. 70 (1), 51–68 (2012).
Mostafa, S., Wang, Y., Zeng, W. & Jin, B. Plant responses to herbivory, wounding, and infection. Int. J. Mol. Sci. 23 (13), 7031 (2022).
Zaynab, M. et al. Role of primary metabolites in plant defence against pathogens. Microb. Pathog. 137, 103–728 (2019).
Chen, Q. et al. Hemin-mediated alleviation of zinc, lead and chromium toxicity is associated with elevated photosynthesis, antioxidative capacity; suppressed metal uptake and oxidative stress in rice seedlings. Plant. Growth Regul. 81, 253–264 (2017).
ul Hassan, Z. et al. Role of zinc in alleviating heavy metal stress. Essential Plant Nutrients: Uptake, Use Efficiency, and Management, pp. 351–366. (2017).
Chwil, M. & Kostryco, M. Histochemical assays of secretory trichomes and the structure and content of mineral nutrients in Rubus idaeus L. leaves. Protoplasma 257 (1), 119–139 (2020).
Hopewell, T., Selvi, F., Ensikat, H. J. & Weigend, M. Trichome biomineralization and soil chemistry in Brassicaceae from Mediterranean ultramafic and calcareous soils. Plants 10 (2), 377 (2021).
Altaf, M. A. et al. Tolerance and adaptation mechanism of Solanaceous crops under salinity stress. Funct. Plant Biol. 51, FP22158 (2022).
Fan, N. et al. Silicon quantum dots promote radish resistance to root herbivores without impairing rhizosphere microenvironment health. Environ. Science: Nano. 10 (9), 2232–2244 (2023).
Islam, T., Moore, B. D. & Johnson, S. N. Novel evidence for systemic induction of silicon defences in cucumber following attack by a global insect herbivore. Ecol. Entomol. 45 (6), 1373–1381 (2020).
Meharg, C. & Meharg, A. A. Silicon, the silver bullet for mitigating biotic and abiotic stress, and improving grain quality, in rice? Environ. Exp. Bot. 120, 8–17 (2015).
Singh, A., Kumar, A., Hartley, S. & Singh, I. K. Silicon: its ameliorative effect on plant defence against herbivory. J. Exp. Bot. 71 (21), 6730–6743 (2020).
Abdalla, M. A. et al. Comparative metabolite profile, biological activity and overall quality of three lettuce (Lactuca sativa L., Asteraceae) cultivars in response to sulphur nutrition. Pharmaceutics 13 (5), 713 (2021).
Isah, T. Stress and defence responses in plant specialised metabolites production. Biological research, pp (2019). 52. Epub 28-Ago-2019.
Zenda, T., Liu, S., Dong, A. & Duan, H. Revisiting sulphur—the once neglected nutrient: it’s roles in plant growth, metabolism, stress tolerance and crop production. Agriculture 11 (7), 626 (2021).
White, P. J. & Broadley, M. R. Chloride in soils and its uptake and movement within the plant: a review. Ann. Botany. 88 (6), 967–988 (2001).
Muravnik, L. The structural peculiarities of the leaf glandular trichomes: A review. Plant Cell and Tissue Differentiation and Specialised metabolites: Fundamentals and Applications, pp. 63–97. (2021).
Kamanga, R., Mbega, E. & Ndakidemi, P. Drought tolerance mechanisms in plants: physiological responses associated with water deficit stress in Solanum lycopersicum. Adv. Crop Sci. Technol. 6, 3 (2018).
Rajagopal, D., Sopory, S. K. & Mathew, M. Dealing with environmental fluctuations: diversity of Potassium Uptake systems across the three domains of life. J. Plant Growth Regul. 42 (10), 6104–6136 (2023).
Sofy, M. R., Elhawat, N. & Alshaal, T. Glycine betaine counters salinity stress by maintaining high K+/Na + ratio and antioxidant defence via limiting na + uptake in common bean (Phaseolus vulgaris L). Ecotoxicol. Environ. Saf. 200, 110732 (2020).
Lamalakshmi Devi, E. et al. R. A. Adaptation strategies and defence mechanisms of plants during environmental stress. Med. Plants Environ. Challenges, pp. 359–413. (2017).
Vega-Muñoz, I. et al. Breaking bad news: dynamic molecular mechanisms of wound response in plants. Front. Plant Sci. 11, 610445 (2020).
Guo, W., Nazim, H., Liang, Z. & Yang, D. Magnesium deficiency in plants: an urgent problem. Crop J. 4 (2), 83–91 (2016).
Peng, Y. Y. et al. Magnesium deficiency triggers SGR–mediated chlorophyll degradation for magnesium remobilization. Plant Physiol. 181 (1), 262–275 (2019).
Capaldi, F. R., Gratão, P. L., Reis, A. R., Lima, L. W. & Azevedo, R. A. Sulfur metabolism and stress defense responses in plants. Trop. Plant. Biology. 8, 60–73 (2015).
Francioso, A., Conrado, A. B., Mosca, L. & Fontana, M. Chemistry and biochemistry of sulfur natural compounds: Key intermediates of metabolism and redox biology. Oxidative Medicine and Cellular Longevity. 2020(1): 8294158. (2020).
Acknowledgements
The authors thank TETFUND for postdoctoral sponsorship to FMO under the Academic Staff Training and Development Program (AST&D, Year 2022 Intervention) to the Royal Botanic Gardens, Kew London. We thank Saba Rokni for training at the herbarium. We also thank Dr. Atinuke Bagbe of Mathematics Department (Statistics Unit) at Olusegun Agagu University of Science and Technology, Okitipupa, Ondo State, Nigeria for the statistical analysis in this study.
Author information
Authors and Affiliations
Contributions
All authors reviewed the manuscriptConceptualization: FMO, ARS, VAB, EV, JLJ, MKM, Collection of preliminary data; Methodology; Investigation; Resources- FMO, ARS, VAB, EV, JLJ, MKM, Writing original draft; Reviewing and editing - FMO Collection of samples during survey; funding acquisition - FMO, ARS, Supply of modified method for sample preparation - FMO, ARS, MKM. All authors read and approved the final manuscript FMO – Funmilola Mabel Ojo EV - Eloisa Vendemiatti MKM - Manoj Kumar Mahto JLJ - Jehová Lourenço Júnior VAB – Vagner Augusto Benedito ARS – Ana Rita Giraldes Simões.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ojo, F.M., Vendemiatti, E., Júnior, J.L. et al. Determining trichome elemental composition in Solanum wild and domesticated species using SEM-EDS. Sci Rep 15, 15292 (2025). https://doi.org/10.1038/s41598-025-87643-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87643-y