Abstract
Hand, foot and mouth disease (HFMD) is a major public health issue in Hubei Province; however, research on the direct and indirect effects of factors affecting HFMD is limited. This study employed structural equation modeling (SEM) and geographically weighted regression (GWR) to investigate the various impacts and spatial variations in the factors influencing the HFMD epidemic in Hubei Province from 2016 to 2018. The results indicated that (1) with respect to the direct effects, the number of primary school students had the greatest positive direct effect on the number of HFMD cases, with a coefficient of 0.542. Socioeconomic factors strongly influence HFMD cases more directly than natural factors. (2) Concerning indirect effects, the minimum temperature, combined with the per capita disposable income of urban residents, had the greatest positive indirect effect on HFMD cases, with a coefficient of 0.022. Both natural and social factors had more substantial direct impacts on the HFMD epidemic than indirect impacts. (3) Regarding total effects, the number of primary school students, through various natural and social factors, had a total effect coefficient of 0.503 on HFMD incidence. (4) The number of primary school students, per capita GDP, and the number of hospital beds per thousand people had the most significant spatial impacts on HFMD cases. In underdeveloped regions, the HFMD epidemic is more heavily influenced by economic factors.
Similar content being viewed by others
Introduction
Hand, foot, and mouth disease (HFMD) is a common infectious disease in children caused by various enteroviruses, and is transmitted primarily through the fecal‒oral route and close contact. It predominantly affects children under five years of age. The main symptoms include fever, a maculopapular or vesicular rash on the hands and feet, and ulcers in the oral cavity. In rare cases, severe complications such as myocarditis, pulmonary edema, and aseptic meningitis can occur, which can be fatal1,2. HFMD is not a newly emerging infectious disease. Since it was first reported in New Zealand in 1957, it has been widely distributed worldwide, especially in the Asia-Pacific region3. The first reported case of HFMD in China was in Shanghai in 1981. In March 2008, there was an outbreak of HFMD in Fuyang, Anhui Province, which quickly spread to other provinces. In May 2008, HFMD was classified as a notifiable Class C infectious disease in China4,5,6. In recent years, the incidence rate of HFMD has consistently ranked high among the Class C infectious diseases in China. HFMD remains a significant threat to the health and lives of children in China, making its prevention and control crucial for managing Class C infectious diseases.
There is abundant research on the epidemiology and spatiotemporal characteristics of HFMD. Enterovirus A71 (EV71) and coxsackievirus A16 (Cox A16) are the primary enteroviruses that cause HFMD, and predominantly affect children under five years of age, with a higher incidence rate in males than in females7,8. In terms of temporal distribution, HFMD exhibits a biennial high incidence pattern with distinct seasonality. The seasonal characteristics vary across regions, showing either a single peak or double peaks9,10. Many studies have indicated that HFMD is more prevalent in economically developed areas, along rivers and transportation routes, in regions with high population mobility, and at the borders of multiple cities or counties11,12,13,14. Research on the influencing factors of HFMD outbreaks is extensive both domestically and internationally and can generally be categorized into natural and human factors. Among the natural factors, meteorological conditions play crucial roles in the propagation and prevalence of enteroviruses15. The peak period of HFMD outbreaks showed significant seasonal variation, which was consistent to some extent with the seasonal characteristics of the meteorological factors. Temperature, humidity, precipitation, wind speed, and sunlight all influence the spread of HFMD16,17,18,19,20. HFMD transmission routes include droplet transmission, fecal‒oral transmission, and contact transmission, and individuals of all age groups can be infected by the disease. Among human factors, socioeconomic conditions significantly affect HFMD outbreaks through transmission routes and susceptible populations. The key factors influencing the prevalence and spread of HFMD include population density, population mobility, per capita GDP, industrial structure, the urbanization rate, and healthcare resources21,22,23.
Overall, extensive research has focused primarily on the epidemiological analysis and spatiotemporal patterns of HFMD. Studies on the mechanisms influencing HFMD often emphasize meteorological factors, while comprehensive analyses combining natural and socioeconomic factors remain limited, and the exploration of these mechanisms is not in depth. Do various environmental factors have direct and indirect effects on HFMD prevalence? What are the direct and indirect ways in which different factors affect the HFMD epidemic?
In this study, structural equation modeling (SEM) was employed to explore the natural and social environmental factors affecting HFMD prevalence in Hubei. This study identified the direct, indirect, and total effects of geographical environmental factors on HFMD prevalence, and analyzed the regional differences in the influence of various factors. The findings provide geographic perspectives and recommendations for HFMD prevention and control in Hubei Province and other regions. This research enriches health geography studies of HFMD prevalence, offering both practical and theoretical significance.
Materials and methods
Study area
Hubei Province is located in central China, with latitude and longitude coordinates of 29° 01′ − 33° 6′ N, 108° 21° 42′ − 116° 07′ E. The administrative divisions of Hubei include 12 prefecture-level cities (Wuhan, Huanggang, Ezhou, Huangshi, Xianning, Yichang, Xiangyang, Jingzhou, Jingmen, Suizhou, Xiaogan, Shiyan), 1 autonomous prefecture (Enshi), 3 directly administered cities (Tianmen, Qianjiang, Xiantao), and 1 forest district (Shennongjia), totaling 103 counties. As of the end of 2019, the resident population of Hubei Province was 59.27 million, with 30.10 million males and 29.18 million females, accounting for 50.78% and 49.22% of the total population, respectively, resulting in a male‒female ratio of 1.03. The urban population was 36.15 million, while the rural population was 23.12 million, with an urbanization rate of 61%. The economic development of Hubei Province is uneven, exhibiting a gradient difference from east to west. The central region (areas between the three central cities of Wuhan, Xiangyang, and Yichang) has a relatively high economic level, while the eastern and western sides have relatively low economic levels.
Data
The generation and reproduction processes of the HFMD pathogens are affected primarily by meteorological factors19. Eight key meteorological indicators, including maximum temperature, minimum temperature, mean temperature, precipitation, average relative humidity, minimum relative humidity, average relative humidity, wind speed, and sunshine duration, were analyzed. HFMD is transmitted mainly through contact, and its spread is closely related to population density and mobility11,12,13,14,15. Consequently, four demographic indicators were chosen for analysis: urban population, population density, rural population, and the number of primary school students.
The susceptibility of the population to HFMD is influenced by socioeconomic conditions and healthcare levels. Socioeconomic status reflects a region’s population’s ability to resist infectious diseases14. To account for this, several economic indicators, including GDP, per capita GDP, the proportion of tertiary industry, per capita disposable income of urban residents, and per capita disposable income of rural residents, were analyzed. Transportation factors serve as important auxiliary indicators to measure the level of economic development and population mobility. As such, we included railway density, national highway density, provincial highway density, and county road density in the analysis. Healthcare capacity, reflecting the availability of regional medical resources and the ability to prevent and control infectious diseases, was measured via the number of health institutions per 1,000 people, the number of health technicians per 1,000 people, and the number of hospital beds per 1,000 people. Some studies have also indicated that enteroviruses can survive in sewage24, and that land use coverage can affect the spread of infectious diseases such as malaria25. In this study, the impact of water quality and land use coverage on HFMD is explored. Therefore, we incorporated seven water quality indicators—water quality category, pH, dissolved oxygen, permanganate index, ammonia nitrogen, total phosphorus, and total nitrogen, and six land use types—proportion of farmland, forestland, grassland, construction land, water area, and unused land into our analysis.
The selection of influencing factors in this study was based on two considerations: existing literature and data availability. For example, data on the proportion of children under five years old were difficult to obtain, so we used the number of primary school students as a proxy indicator. This indicator is readily available in county-level statistical yearbooks, providing high data accessibility. The study period was set between 2016 and 2018, aligning with the years for which research data were available. The spatial unit of analysis is at the county level, with a sample size of 309 for each indicator. The data sources are listed in detail in Table 1.
Correlation analysis
Current studies have shown that different environmental factors (such as precipitation and humidity) have stronger nonlinear relationships with HFMD incidence rates26. A preliminary exploratory analysis of the correlation between the incidence rate and all natural and socioeconomic factors will be conducted. Factors that pass the significance test of correlation can be further used for confirmatory factor analysis (CFA) in structural equation modeling to cross-verify potential correlations. This cross-verification ensures the scientific validity and reliability of the research results.
Structural equation modeling
Structural equation modeling (SEM) is based on covariance matrices to analyze relationships between variables and is often used to explore and analyze complex multivariable research data27. As a method for establishing, estimating, and testing causal models, SEM can incorporate both observable variables and latent variables, which cannot be directly measured. SEM consists of two components: the measurement model and the structural model. The former describes the relationship between observed variables and latent variables, whereas the latter addresses the relationships between latent variables28,29. The measurement model is generally treated as a confirmatory factor analysis, whereas the structural model aligns with traditional path analysis. The analysis process for both the measurement and structural models in this section follows these steps: hypothesis formulation, data preprocessing, reliability and validity analysis, model fitting and modification, and hypothesis testing28. The detailed steps are provided in Supplementary File 1.
SEM involves numerous fit indices, and achieving perfect a fit for each index, especially with non-scale data, is often unrealistic. The goodness-of-fit of the model is evaluated primarily via various statistical indices, with particular attention given to the following three absolute fit indices: chi-square/df (x²/df, ≤ 3 indicating excellent fit, < 5 acceptable fit); GFI (goodness-of-fit index, > 0.90 indicating excellent fit, > 0.80 indicating good fit); and RMSEA (root mean square error of approximation, < 0.08 indicating excellent fit, 0.08–0.1 acceptable). Some argue that an RMSEA below 0.1 indicates a good fit, an RMSEA below 0.05 indicates a very good fit, and an RMSEA below 0.01 indicates an outstanding fit. However, such results are rarely encountered in practice30. Given the diverse nature of the data in this study, the RMSEA criteria are as follows: values below 0.1 indicate acceptable fit, values below 0.08 suggest good fit, and values below 0.05 reflect excellent fit. The paths in the SEM diagram illustrate the direct, indirect, and total effects between variables. The values shown are standardized regression coefficients, which can be used to compare the magnitude of the effects between different variables. Each effect represents the direction and strength of the relationships between the variables. The SEM in this study was implemented via AMOS 23 software.
Geographically weighted regression model
Geographically weighted regression (GWR) is a local regression model. Compared with traditional ordinary least squares (OLS) linear regression methods, the GWR model incorporates spatial weights, extending the ordinary global regression model to effectively evaluate spatial heterogeneity31. This study aims to utilize the GWR model to analyze the influence and regional differences of various environmental factors on the prevalence of HFMD. The selection of the spatial weight function and the optimization of the function bandwidth are critical in the model. This study chooses the Gaussian kernel function and the Akaike information criterion (AIC) method to determine the optimal bandwidth32. Owing to the relatively weak diagnostic performance of the GWR model, it is advisable to first analyze the research data via the OLS model and then proceed with the GWR model to improve the model’s accuracy33. The GWR and OLS regression expressions are shown in Eqs. (1) and (2):
In Eqs. (1) and (2), \(\:{x}_{i}\) and \(\:{y}_{i}\:\)represent the independent and dependent variables, respectively; (ui, vi)refers to the spatial coordinates of the i-th sample; β0 and β0(ui, vi)denote the global and i-th sample intercept terms, respectively; xij indicates the j-th parameter value of the i-th sample; βj(ui, vi)represents the regression coefficient of the j-th parameter for the i-th sample, reflecting the spatial variation in the influence of different parameters on the sample. The sign of the coefficient indicates whether there is a positive or negative association at a specific location, and their magnitude indicates the strength of the correlation. εi represents the random error term, and k indicates the number of independent variables.
Results
Model fitting results
First, correlation analysis and confirmatory factor analyses were conducted on various indicators of natural factors (meteorological factors, proportion of land use types, and water quality) and socioeconomic factors (economic factors, demographic factors, transportation factors, and healthcare factors). The natural and social factors that have passed the significance test were included in the SEM. The factors, layers, and specific indicators included in the SEM are shown in Table 2.
Owing to the numerous measurement variables for natural and social environmental factors and the intricate internal paths of the model, even when the model fit results meet the test standards, they may not always be within an interpretable framework. Therefore, multiple experiments and corresponding trade-offs are necessary. To investigate the various impacts of the geographical environmental factors on the HFMD prevalence, three initial models were constructed on the basis of prior knowledge. Model 1 is a conventional nonrecursive (reversible) model, Model 2 is a classic second-order model, and Model 3 is a path model (without latent variables, only observed variables). On the basis of the model fitting results and MI correction suggestions, different models were optimized and compared multiple times. In the results of Model 1, the path connections between the latent and observed variables, latent variables and latent variables, and observed variables and observed variables were complex and difficult to understand, so they were discarded. Although the Model 2 results indicated that the economic factors had a stronger relationship with HFMD prevalence than did the natural environmental factors, they obscured the role of specific measurement indicators, leading to their exclusion. Therefore, Model 3 was adopted in this study (Fig. 1). This model clearly delineates the interactive effects between natural and social factors, aiding in understanding the influence of the natural‒social environmental interactions on HFMD prevalence. The choice of HFMD cases over the incidence rate is based on multiple experiments showing that many factors have more pronounced effects on the HFMD cases.
For the fit indicators of the model, χ²/df = 3.915 (< 5, acceptable), p < 0.05, RMSEA = 0.098 (between 0.08 and 0.1, acceptable), SRMR = 0.0638 (< 0.08, acceptable), GFI = 0.889 (between 0.8 and 0.9, acceptable), AGFI = 0.804 (between 0.8 and 0.9, acceptable), and CFI = 0.927 (> 0.9, excellent fit). Overall, the model fit is acceptable.
The Pathway model of the impact of the geographical environment on the HFMD epidemic. Note: The non-standardized coefficients of all the values in the figure are significant at the 0.05 level or above. The values shown in the figure are standardized coefficients, which mainly reflect the degree of influence of the factors.
Effect path analysis of influencing factors
By calculating path coefficients, we can determine the direct and indirect effects of the geographical environmental factors on the HFMD epidemic. From the perspective of direct effects (Fig. 1), the socioeconomic factors had a stronger direct impact on HFMD prevalence than did the natural factors. Among these variables, the number of primary school students had the greatest direct effect on HFMD prevalence, with a coefficient of 0.542. Numerous studies have shown a positive correlation between population density and HFMD prevalence34,35. Compared with population density, the number of primary school students is more closely related to HFMD cases. Among the natural factors, only total phosphorus had a positive direct effect on HFMD prevalence, with a coefficient of 0.078, indicating that water quality may influence the suitable environment for enteroviruses of HFMD. The proportion of construction land had a negative direct effect on HFMD prevalence. Theoretically, due to higher population density, greater population mobility, and more complex environmental factors in areas with constructed land, the risk of HFMD prevalence may increase. However, children, as a high-risk group, often concentrate activities in areas such as residential and public facility lands. An “ecological fallacy” exists between the incidence of HFMD and constructed land categories, thus the positive effect between the two is not significant.
From the perspective of indirect effects (Fig. 1; Table 3), there were a total of 22 pathways through which natural factors and human factors jointly affect the epidemic of HFMD. Among these pathways, 3 pathways started from urban residents’ per capita disposable income, 13 pathways started from the number of primary school students, 5 pathways started from the lowest temperature, and 1 pathway started from the average relative humidity. All pathways ended with the number of HFMD cases. Therefore, these pathways can be divided into four categories, namely Pathway 1, Pathway 2, Pathway 3, and Pathway 4. In Pathway 1, the effect value of urban residents’ per capita disposable income combined with construction land indirectly affecting the number of HFMD cases was the highest, at -0.064. In Pathway 2, there was only one pathway, where the effect value of average relative humidity combined with the number of technical personnel per thousand people indirectly affecting the number of HFMD cases was 0.007. In Pathway 3, the effect value of the minimum temperature combined with urban residents’ per capita disposable income indirectly affecting the number of HFMD cases was the highest, at 0.022. In Pathway 4, the effect value of the number of primary school students combined with urban residents’ per capita disposable income, total phosphorus, and construction land indirectly affecting the number of HFMD cases was the highest, at -0.014. Among the meteorological factors, the positive impact of the minimum temperature on the HFMD epidemic was more obvious, indicating that an increase in the minimum temperature is conducive to the breeding and survival of HFMD enteric pathogens. This is mainly because areas with higher urban residents’ per capita disposable income also have higher population density and frequent economic activities, which facilitate the spread of HFMD among the population. The indirect effect of urban residents’ per capita disposable income on the number of HFMD cases through construction land was negative, which is related to the previously mentioned “ecological fallacy” factor. Comparatively, the direct impacts of the natural environment and social factors on the HFMD epidemic were greater than the indirect impacts. Different geographical environmental factors jointly influence the HFMD epidemic through complex interrelationships.
From the perspective of total effects (Table 4), in Pathway 1, economic factors combined with water quality and land use type had both direct and indirect effects on the number of HFMD cases, with a total effect value of 0.058. In Pathway 2, meteorological factors combined with healthcare factors only had an indirect effect on the number of HFMD cases, with a total effect value of 0.007. In Pathway 3, meteorological factors combined with various factors, such as the economy, land use type, healthcare, and transportation, had an indirect effect on only the HFMD cases, with a total effect value of 0.016. In Pathway 4, population factors combined with meteorological, economic, water quality, land use, healthcare, and transportation factors had both direct and indirect effects on the HFMD cases, with a total effect value of 0.527. The total effects of the four pathways, from largest to smallest, are as follows: Pathway 4 > Pathway 1 > Pathway 3 > Pathway 2. Starting from the number of primary school students and ending with the number of HFMD cases, the four pathways can be merged into one overall pathway, with a total effect value of 0.503. This means that the number of primary school students, through the direct and indirect effects of various natural and social factors, increases the number of HFMD cases by 0.503 standard units. The results revealed the significant roles of population factors, economic factors, meteorological factors, water quality factors, transportation factors, and healthcare factors in the HFMD epidemic in 22 branch pathways, 4 major pathways, and one overall pathway. In particular, the number of primary school students, urban residents’ per capita disposable income, and the minimum temperature were the three observed variables that had a significant impact on the number of HFMD cases.
The three key elements of an infectious disease epidemic are the source of infection, transmission pathways, and susceptible populations. Overall, with primary school students as the susceptible population, meteorological factors represented by the minimum temperature, influence the HFMD epidemic through the source of infection (the breeding and survival of enteroviruses). Social and economic factors, such as per capita GDP, urban residents’ per capita disposable income, county road density, and the number of hospital beds, influence HFMD epidemics through the cumulative impact of multiple transmission pathways and susceptible populations. Many factors have direct, indirect, and total effects on the HFMD epidemic through the three key elements of infectious diseases.
Regional differences in influencing factors
Both the HFMD epidemic and the geographical environmental factors have spatiotemporal attributes, making it necessary to further explore the regional differences in the influence of various environmental factors on the HFMD epidemic. This study provides scientific evidence for the implementation of targeted prevention and control measures in different regions. After performing multicollinearity tests on the aforementioned variables, variables with a variance inflation factor (VIF) greater than 10 and those that did not pass the significance test were excluded. The remaining variables were then included in the GWR model. The goodness of fit (adjusted R2) of the GWR model was 0.55 (p < 0.05) when the number of HFMD cases was the dependent variable, and 0.04 when the HFMD incidence was the dependent variable. Therefore, the results of the GWR model with the dependent variable as the number of cases were used as basis for analysis in this section. Figure 2 shows that three economic factors had significant spatial heterogeneity (p < 0.05, see Supplementary File 2) in their impact on the HFMD epidemic, with their spatial impact decreasing in the following order: the number of primary school students, per capita GDP, and the number of beds per thousand people.
The regression coefficients for the number of primary school students ranged from 0.37 to 1.34, indicating that its spatial impact on the HFMD epidemic was strong. The influence of the number of primary school students on the HFMD epidemic was positive across the entire province, generally showing a gradual decline from the periphery toward the center, with significant spatial heterogeneity within regions. Notably, the influence of number of primary school student on HFMD epidemic was most pronounced in Xiangyang and Suizhou.The positive influence of the number of primary school students on the HFMD epidemic was relatively stable. Population factors, especially child density, are the most fundamental factors affecting the spread and epidemic of HFMD.
The regression coefficients for per capita GDP ranged from − 0.22 to 0.58, indicating that the economy’s impact on HFMD was unstable. The negative impact of per capita GDP on the HFMD epidemic was pronounced in the central Hubei. This indicated that in economically developed areas, better economic conditions could help reduce the prevalence of the disease. The positive impact of per capita GDP on the HFMD epidemic generally showed a spatial pattern of increasing from central-southern Hubei to the surrounding areas. The influence of per capita GDP on the HFMD epidemic was greater in southwestern Hubei, and some counties and cities in eastern Hubei than in the central-southern region (especially Yichang). This indicated that the level of economic development had a more significant impact on the HFMD epidemic in economically underdeveloped areas. In underdeveloped areas where economic activities were frequent but medical resources lagged behind the pace of economic development, HFMD was more likely to spread.
The regression coefficient for the number of hospital beds per thousand people ranged from − 0.36 to 0.15 indicating that this factor’s impact on HFMD was unstable. The coefficient values fluctuated between positive and negative, suggesting variability in its effect on HFMD prevalence across different regions of Hubei. The negative effect of hospital beds per thousand people on HFMD prevalence was mainly concentrated in central and western Hubei. In contrast, the western region of Hubei, particularly western Enshi, showed a primarily positive effect of hospital beds per thousand people on HFMD prevalence. This unstable effect needs to be analyzed in conjunction with the local socioeconomic conditions. The eastern and central regions of Hubei had higher levels of economic development than did western Hubei. Areas with better economic development, such as Wuhan city, also tend to have richer medical resources, including a greater number of hospital beds per capita, which effectively suppresses the prevalence of HFMD. In economically underdeveloped areas such as Enshi, however, the overall level of medical resources, including hospital beds per capita, is lower than that in eastern Hubei. Consequently, the effectiveness of HFMD prevention and control efforts in these regions is not as significant. These findings suggest that increasing investment in healthcare resources in economically underdeveloped regions can effectively enhance local government capabilities in preventing and controlling HFMD. By tailoring strategies to local economic conditions and healthcare resource availability, more targeted and effective measures can be implemented to mitigate the impact of HFMD in different regions of Hubei.
Like the global R2, the local R2 values from the GWR model vary between 0 and 1, quantifying the performance of the local regression models. This metric explains the proportion of the dependent variable variance covered by the local regression model. The regression coefficients for local R2 ranged from 0.48 to 0.70, showing a clear spatial differentiation with an increasing trend from east to west overall, with higher local R2 values observed in northwestern and southwestern Hubei. These findings indicate that in economically underdeveloped regions, economic factors such as per capita GDP, the number of primary school children, and the number of hospital beds per thousand people play stronger roles in explaining the prevalence of HFMD. Different factors exhibit varying spatial differentiations in their impacts on HFMD, with the intensity of the same factor’s effect varying across different regions. In economically disadvantaged areas, economic factors have a greater impact, highlighting the importance of improving local residents’ living standards and economic development as primary measures to enhance the prevention and control of infectious diseases such as HFMD.
Conclusion
In conclusion, we utilized SEM to investigate the direct, indirect, and total effects of the natural and social factors on the prevalence of HFMD. Additionally, GWR was employed to analyze the regional differences in the influence of various factors on HFMD prevalence. The results indicated that socioeconomic factors had a stronger direct impact on HFMD prevalence than natural factors did. The combination of the minimum temperature and urban residents’ per capita disposable income had the strongest positive indirect effect on HFMD prevalence. Both natural and social factors had greater direct effects on HFMD prevalence than indirect effects did. There were regional disparities in the impact of the geographical environment on HFMD prevalence. In economically underdeveloped regions, socioeconomic factors exert a greater influence. The number of primary school children, per capita GDP, and number of hospital beds per thousand people presented the most pronounced spatial impacts on HFMD prevalence. These findings enhance the understanding of the relationships between various factors and HFMD, providing evidence to support the development of relevant preventive measures and the establishment of early warning systems in different areas.
Discussion
On the basis of the epidemiological characteristics and spatiotemporal distribution of HFMD, numerous studies have preliminarily identified key environmental factors influencing its prevalence from the perspective of the epidemiological triad, including meteorological factors17, demographic factors21, economic factors23, and transportation factors36. This study systematically summarized the impact factors of the natural and socioeconomic environments on HFMD prevalence. In the natural environment, meteorological factors and water quality have emerged as critical influences on HFMD prevalence. They primarily affect the propagation and proliferation of infectious agents such as enteroviruses, thereby influencing the spread of HFMD. Within the socioeconomic environment, demographic factors stand out as the foundational element influencing HFMD prevalence. They primarily affect the accumulation and contact of susceptible populations, thus impacting HFMD transmission dynamics. Economic factors have a complex influence on HFMD prevalence. They operate primarily through diverse pathways such as increased economic activities and regional financial investments in environmental modifications, which can either promote or suppress HFMD transmission. Among the economic factors, the per capita disposable income of urban residents has a particularly significant effect on HFMD prevalence. This income not only reflects the economic level of urban households but also reflects the overall economic strength of the town or city. A region’s economic level is closely linked to population density and mobility, both of which facilitate the spread of HFMD. As a result, the influence of urban residents’ per capita disposable income on the HFMD cases is a positive effect.
Transportation and healthcare factors have relatively weak impacts on HFMD prevalence. The limited availability of data, particularly concerning transportation factors such as road density, may have influenced the study outcomes. Moreover, the uneven distribution of healthcare resources across regions limits the full potential of the healthcare advantages in HFMD prevention and control, especially in underdeveloped areas where healthcare service capabilities urgently need improvement. The number of health technicians per 1,000 people has a positive relationship with HFMD incidence. Economically developed counties tend to have a greater number of health technicians per 1,000 people, which correlates with higher incidence rates. However, this does not imply that a greater number of health technicians necessarily leads to a higher incidence of HFMD. The underlying factors driving this relationship are still largely economic. Furthermore, the results indicate that the suppressive effect of health technicians per 1,000 people on HFMD incidence is not as pronounced as that of hospital beds per 1,000 people. In short, under the comprehensive effects of meteorology, economic factors, demographic factors, transportation factors, and healthcare factors, the epidemic path of HFMD is increasingly complicated.
The spread of HFMD exhibits population density-dependent characteristics, which are particularly influenced by the density of children. Many studies have demonstrated a positive correlation between population density and the prevalence of HFMD20,37. This study further highlights that the number of primary school students is more closely related to HFMD incidence than the overall population density. Deng et al. reported a positive correlation between the proportion of children under the age of five and HFMD incidence35. This finding is closely related to the population distribution characteristics of HFMD, which primarily affects children under the age of five. Compared with the less accessible data on the proportion of children under five, the number of elementary school students is a commonly available statistic in county-level statistical yearbooks, offering greater data accessibility. Therefore, researchers can use the number of primary school students as a proxy for population-related factors in studies on HFMD when data on children are difficult to obtain.
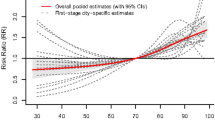
Meteorological factors can create a more favorable environment for infectious agents, and the survival of enteroviruses causing HFMD varies under different climate conditions. In southern China, HFMD often shows a bimodal distribution, whereas in northern China, it presents a single peak11, indicating that climate factors have a stable and predictable impact on enteroviruses. An analysis of the cumulative daily incidence of HFMD in Hubei Province from 2009 to 2019 and its relationship with temperature revealed that when the temperature reached 16 °C, the HFMD incidence increased significantly, and when the temperature exceeded 26 °C, the incidence began to decline (Fig. 3). This suggests that 16–26 °C might be the optimal temperature range for the survival and reproduction of enteroviruses responsible for HFMD.
Moreover, it should be noted that the transmission and spread of HFMD depend on contact and interaction between infected individuals or asymptomatic carriers. The temperature range of 16–26 °C coincides with the average spring and early summer temperatures in Hubei, which also corresponds to the human comfort zone (18–26 °C). During this period, when the weather is neither too cold nor too hot, parents are more likely to take their young children outdoors. Children, who feel more comfortable, are more active and likely to explore a wider range of environments. A weaker immune system, combined with behaviors such as thumb-sucking and a curiosity about their surroundings, increases the risk of coming into contact with contaminated environments or objects, thereby increasing the likelihood of HFMD infection. This highlights the need for caregivers to strengthen preventive measures during the spring and summer months, such as paying attention to children’s hygiene, maintaining clean living environments, and ensuring timely vaccination against HFMD.
HFMD spreads through multiple transmission routes. Humans are the only known hosts for human enteroviruses, and close contact is the primary mode of transmission. During the acute phase, patients can shed the virus from the throat; secretions from ruptured blisters or conjunctival discharge from hemorrhagic conjunctivitis are also infectious. Additionally, patients can continue to shed the virus through feces for several weeks after recovery. The period of highest infectivity is within the first week after symptom onset, but contagiousness can persist for several weeks after symptoms and signs have subsided. In summary, HFMD can be transmitted via several routes, including the digestive tract (fecal‒oral), respiratory tract (droplets from coughing or sneezing), and through direct contact. Water sources may also facilitate the transmission of enteroviruses if they are not properly treated. Children frequently spend time in places such as kindergartens, daycare centers, elementary schools, residential areas, shopping malls, and parks. Additionally, economic factors, transportation conditions, water quality, and land use types can have both direct and indirect impacts on the transmission of HFMD.
Kindergartens and elementary schools are often located near children’s homes, as most parents prefer to enroll their children in nearby institutions for convenience. The surrounding environments of these locations, such as schools, residential communities, shopping centers, and parks, often involve convenient transportation and active economic activities. These conditions increase the likelihood of close contact between children, which facilitates the spread of HFMD. If economic activities are frequent, and there is a high flow of people, inadequate public hygiene may harbor enterovirus on shared objects, increasing the chances of infection when children come into contact with contaminated items. In addition, the communal environments of kindergartens and elementary schools contribute to cluster infections of HFMD. Items such as towels, utensils, toys, and learning tools frequently used by children can become contaminated and serve as transmission vectors, spreading the virus to other children in the same environment. This explains one of the reasons why children often become sick after starting kindergarten. Moreover, if water sources are contaminated, children may contract enteroviruses through contact with untreated water.
Since kindergartens and elementary schools are communal living environments with a high density of children, the accumulation of susceptible individuals occurs quickly. Once a child is infected, close contact among them increases the risk of transmission, increasing the likelihood of cluster outbreaks. Therefore, public hygiene in such environments must be given special attention. Daily health checks in kindergartens must be strictly enforced to ensure a clean and safe environment for children’s learning and living. Additionally, if an HFMD case occurs in childcare facilities, there is a risk that children could bring enteroviruses home, potentially infecting family members regardless of the living environment. This risk is especially high for other children in the household, such as siblings. As a result, caregivers need to increase their awareness of HFMD prevention and closely monitor the daily health of children.
Infectious disease outbreaks are often characterized by the epidemiological triad of infectious agents, transmission routes, and susceptible populations, whereas natural and social environments are recognized as two crucial factors influencing disease spread38. Research on dengue fever has indicated that the “triad” facilitates its outbreaks, whereas the “two factors” determine the conditions under which outbreaks can occur39,40. This study suggested that the “triad” is essential for localized outbreaks of diseases, while the “two factors” provide the breeding ground for widespread and geographically expansive outbreaks. For example, the occurrence of HFMD outbreaks in Hubei is attributed to interactions among infectious agents (affected individuals and asymptomatic carriers), transmission routes (fecal-oral and contact transmission), and susceptible populations (generally susceptible with children being the most vulnerable). The geographical spread of HFMD is influenced primarily by the combined effects of socioeconomic factors (economic development level, population dynamics, transportation density, healthcare standards) and natural environmental factors (meteorological conditions, proportion of land use types, and water quality).
Different factors exhibit spatial heterogeneity in their impacts on the prevalence of HFMD. The spatial distribution of HFMD outbreaks was associated with uneven regional economic development, with higher transmission rates observed in urban areas with higher per capita GDP21. In peri-urban areas characterized by high population density and significant population mobility, HFMD incidence rates are also elevated41. Furthermore, research has indicated that in regions with higher per capita GDP, temperature significantly influences the incidence of HFMD23. This study demonstrated that socioeconomic factors play a crucial role in influencing the spatial disparities in HFMD prevalence, particularly factors such as per capita GDP, the number of school-aged children, and the number of hospital beds per thousand people. The influence of economic factors on the HFMD epidemic tends to be more pronounced in regions with lower economic development levels. Therefore, enhancing the prevention and control of infectious diseases such as HFMD in economically disadvantaged areas remains contingent upon poverty alleviation efforts and improving the living standards of local residents.
On the basis of our previous analysis of the spatial distribution characteristics of HFMD in Hubei, prevention and control strategies should differ between economically developed areas and underdeveloped areas. In economically developed regions, such as Wuhan and Xiangyang, where HFMD cases are more common, a higher incidence is associated with population density, frequent economic activity, and the higher reporting rates of HFMD. In contrast, the higher HFMD incidence in economically underdeveloped areas may be due to a lack of healthcare resources and insufficient public health awareness. For the former, efforts should focus on densely populated areas with a high concentration of young children, particularly in districts with a high density of kindergartens. For the latter, improving local healthcare resources and raising public awareness of prevention and control measures are key. Additionally, all high-incidence areas should encourage the vaccination of children against HFMD to reduce the risk of outbreaks. The prevention and control of HFMD is not an overnight task; it is a long-term and challenging endeavor. Focusing on the spatial distribution of susceptible populations, monitoring changes in meteorological factors, encouraging vaccination in regions or among populations where feasible, creating clean and livable community environments, and promoting health and hygiene awareness can all contribute to the precise prevention and control of HFMD.
Several factors were not included in the study due to their difficulty in quantification, such as the health awareness of young children or their guardians, the characteristics of thumb-sucking behavior in children, the interventions of vaccination, the sanitation conditions within childcare institutions, and the environmental management levels of the communities where the children reside. Some indicators, such as economic factors, may have potential confusing effects on HFMD epidemic and are difficult to distinguish thoroughly. These factors, however, directly, or indirectly influence whether susceptible populations are exposed to multiple infection pathways for HFMD, as well as the survival of enteroviruses in the surrounding environment. Furthermore, the adjusted R² value of the GWR model in this study is relatively low. On the one hand, this can be attributed to the complexity of the factors involved, making it difficult to achieve a perfect fit with a single model, especially when both the natural and social environmental factors are considered. On the other hand, this suggests the existence of undiscovered explanatory variables, highlighting the complexity of the factors contributing to the spatial variability in HFMD prevalence. This also indicates the existence of unexplored explanatory variables, highlighting the complexity of the factors influencing the spatial variation in HFMD transmission, which warrants further investigation in the future. This research focuses on analyzing the factors influencing HFMD in Hubei Province via data from 2016 to 2018. In the future, we plan to continue collecting relevant data to further explore the factors influencing HFMD transmission in the post-pandemic period and compare them with pre-pandemic conditions.
Data availability
The data that support the findings of this study are available from the National Communicable Disease Surveillance Network System (http://www.chinacdc.cn/), but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Hongying Chen (E-mail: 348166087@qq.com).
References
Ministry of Health of the People’s Republic of China. Guidelines for prevention and control of HFMD (2009). Chin. Practical J. Rural Doctor. 16 (2), 125–127. https://doi.org/10.13558/j.cnki.issn1672-3686.2010.02.039 (2010).
Tyring, S. K. Hand foot and mouth disease: enteroviral load and disease severity. EBioMedicine 62. https://doi.org/10.1016/j.ebiom.2020.103115 (2020).
Ang, L. W. et al. Epidemiology and control of hand, foot and mouth disease in Singapore. Ann. Acad. Med. Singap. 38 (2), 106–112. https://doi.org/10.47102/annals-acadmedsg.V38N2p106 (2009).
Zheng, Z. M. et al. Enterovirus 71 isolated from China is serologically similar to the prototype E71 BrCr strain but differs in the 5′-noncoding region. J. Med. Virol. 47 (2), 161–167. https://doi.org/10.1002/jmv.1890470209 (1995).
Cui, J. Z. et al. Epidemiological characteristics of fatal cases of hand, foot and mouth disease in children under 5 years old in China, 2008–2018. Chin. J. Epidemiol. 41 (07), 1041–1046. https://doi.org/10.3760/cma.j.cn112338-20200114-00031 (2020).
Mao, L. X. et al. Epidemiology of hand, foot, and mouth disease and genotype characterization of Enterovirus 71 in Jiangsu, China. J. Clin. Virol. 49 (2), 100–104. https://doi.org/10.1016/j.jcv.2010.07.009 (2010).
Chen, H. Y. Practical Guidelines for Disease Monitoring and Analysis (Hubei science and technology publishing house, 2018).
Fong, S. Y. et al. A five-year retrospective study on the epidemiology of hand, foot and mouth disease in Sabah. Malaysia Sci. Rep. 11 (1), 17814. https://doi.org/10.1038/s41598-021-96083-3 (2021).
Huang, R. et al. Spatial-temporal mapping and risk factors for hand foot and mouth disease in northwestern inland China. PLoS Negl. Trop. Dis. 15 (3), e9210. https://doi.org/10.1371/journal.pntd.0009210 (2021).
Sun, J. et al. Using geographically weighted regression to study the seasonal influence of potential risk factors on the incidence of HFMD on the Chinese mainland. ISPRS Int. J. Geo-Information. 10 (7), 448. https://doi.org/10.3390/ijgi10070448 (2021).
Wu, Y. et al. Spatiotemporal cluster patterns of hand, foot, and mouth disease at the province level in mainland China, 2011–2018. Plos One. 17 (8), e0270061. https://doi.org/10.1371/journal.pone.0270061 (2022).
Wang, C. et al. Spatiotemporal cluster patterns of hand, foot, and mouth disease at the county level in mainland China, 2008–2012. PloS One. 11 (1), e147532. https://doi.org/10.1371/journal.pone.0147532 (2016).
Truong, P. N. et al. A spatial-temporal statistical analysis of health seasonality: explaining HFMD infections within a children population along the Vietnamese south central coast. BMC Public. Health. 19, 1–10. https://doi.org/10.1186/s12889-019-7281-4 (2019).
Gong, S. S. et al. Geographical characteristics and influencing factors of the prevalence of hand, foot and mouth disease in Hubei Province. Scientia Geogr. Sinica 2020, 40(6):999–1009. https://doi.org/10.13249/j.cnki.sgs.2020.06.016
Zhang, K. J. et al. Spatiotemporal distribution patterns and Predicting modeling researches of Hand, Foot and Mouth Disease in Mainland China. J. Prev. Med. Chin. PLA 2017, 35(06):683–686. https://doi.org/10.13704/j.cnki.jyyx.2017.06.045
Gou, F. et al. Different responses of weather factors on hand, foot and mouth disease in three different climate areas of Gansu, China. BMC Infect. Dis. 18 (1), 15–24. https://doi.org/10.1186/s12879-017-2860-4 (2018).
Yang, L. et al. Non-linear association between daily mean temperature and children’s hand foot and mouth disease in Chongqing, China. Sci. Rep. 13 (1), 20355. https://doi.org/10.1038/s41598-023-47858-3 (2023).
Du, Z. et al. Weather effects on hand, foot, and mouth disease at individual level: a case-crossover study. BMC Infect. Dis. 19 (1), 1029. https://doi.org/10.1186/s12879-019-4645-4 (2019).
Nguyen, H. X. et al. Temporal and spatial analysis of hand, foot, and mouth disease in relation to climate factors: a study in the Mekong Delta region, Vietnam. Sci. Total Environ. 581, 766–772. https://doi.org/10.1016/j.scitotenv.2017.01.006 (2017).
Jixia, H. et al. Identification of health risks of hand, foot and mouth disease in China using the geographical detector technique. Int. J. Environ. Res. Public. Health. 11 (3), 3407–3423. https://doi.org/10.3390/ijerph110303407 (2014).
Xu, C. Spatio-temporal pattern and risk factor analysis of hand, foot and mouth disease associated with under-five morbidity in the Beijing–Tianjin–Hebei region of China. Int. J. Environ. Res. Public Health. 14 (4), 416. https://doi.org/10.3390/ijerph14040416 (2017).
Kua, J. A. & Pang, J. The epidemiological risk factors of hand, foot, mouth disease among children in Singapore: a retrospective case-control study. PLOS ONE. 15 (8), e236711. https://doi.org/10.1371/journal.pone.0236711 (2020).
Liao, J. et al. Spatial-temporal mapping of hand foot and mouth disease and the long-term effects associated with climate and socio-economic variables in Sichuan Province, China from 2009 to 2013. Sci. Total Environ. 563–564. https://doi.org/10.1016/j.scitotenv.2016.03.159 (2016).
Liang, X. et al. Effects of land use /cover change on lake water quality in the semi-arid region of northern China: a case study in Lake Daihai Basin (2000-2018). J. Lake Sci. 33 (3), 727–738. https://doi.org/10.18307/2021 (2021).
Patz, J. A. et al. Climate change: Regional warming and malaria resurgence. Nature 420 (6916), 627 (2002).
Huang, R. et al. Effects of meteorological parameters and PM10 on the incidence of hand, foot, and mouth disease in children in China. Int. J. Environ. Res. Public Health. 13 (5), 481. https://doi.org/10.3390/ijerph13050481 (2016).
Qiu, H. Z. & Lin, B. F. Principles and Applications of Structural Equation Modeling (China Light Industry Press, 2009).
Luan, R. S. et al. Principles and Methods of Epidemiological Research (Second Edition). Chengdu: Sichuan science and technology publishing house, (2014).
Liu, D. et al. The impact of ecosystem services on human well-being and its group differences in the loess hilly and gully region. Geographic Res. 41 (05), 1298–1310. https://doi.org/10.11821/dlyj020210558 (2022).
Hou J T, Wen Z L & Cheng Z J. Structural equation model and its applications. Beijing: Educational Science Publishing house, (2004).
Wang, S. J., Gao, S. & Chen, J. Spatial heterogeneity of driving factors of urban haze pollution in China based on GWR model. Geographic Res. 39 (03), 651–668 (2020). DOI: CNKI:SUN:DLYJ.0.2020-03-012.
Cui, W. et al. Industrial Electricity Consumption and Economic Growth: a spatio-temporal analysis across Prefecture-Level cities in China from 1999 to 2014. Energy, 2021, 222(4):119932. https://doi.org/10.1016/j.energy.2021.119932
Liu, Y. W. et al. Spatial-temporal evolution of ecological land and influence factors in Wuhan urban agglomeration based on geographically weighted regression model. Chin. J. Appl. Ecol. 31 (03), 987–998. https://doi.org/10.13287/j.1001-9332.202003.016 (2020).
Pan, Q. et al. Regional-level risk factors for severe hand-foot-and-mouth disease: an ecological study from mainland China. Environ. Health Prev. Med. 26 (1), 4–13. https://doi.org/10.1186/s12199-020-00927-9 (2021).
Deng, T. et al. Spatial-temporal clusters and risk factors of hand, foot, and mouth disease at the district level in Guangdong Province, China. PloS One. 8 (2), e56943. https://doi.org/10.1371/journal.pone.0056943 (2013).
Song, Y. et al. Spatio-temporal differentiation characteristics and influencing factors of hand, foot, and mouth disease in China. Acta Geogr. Sin. 77 (03), 574–588. https://doi.org/10.11821/dlxb202203006 (2022).
Gao, Y. et al. Spatial and temporal characteristics of hand-foot-and-mouth disease and their influencing factors in Urumqi, China. Int. J. Environ. Res. Public Health. 18 (9), 4919. https://doi.org/10.3390/ijerph18094919 (2021).
Wu, P. C. et al. Higher temperature and urbanization affect the spatial patterns of dengue fever transmission in subtropical Taiwan. Sci. Total Environ. 407 (7), 2224–2233. https://doi.org/10.1016/j.scitotenv.2008.11.034 (2009).
Qi, X. et al. The effects of socioeconomic and environmental factors on the incidence of dengue fever in the Pearl River Delta, China, 2013. PLoS Negl. Trop. Dis. 9 (10), e0004159. https://doi.org/10.1371/journal.pntd.0004159 (2015).
Wang, C. G. A Study on the Epidemiological Characterisitics of Dengue in Guangdong Province and the Impact of Meteorological Factors in Guangzhou (Shandong University, 2014).
Wang, W. et al. Epidemiological characteristics and spatiotemporal patterns of hand, foot, and mouth disease in Hubei, China from 2009 to 2019. Plos One. 18 (6), e0287539. https://doi.org/10.1371/journal.pone.0287539 (2023).
Acknowledgements
This work was supported by the National Social Science Foundation of China (Grant No. 20BZZ104), Hubei Province Postdoctoral Innovation Research Project (Grant No. 30205202353), the Ministry of Education, Humanities and Social Sciences Youth Fund (Grant No. 24YJCZH313), and the China Postdoctoral Science Foundation (Grant No. 2024M751061).
Author information
Authors and Affiliations
Contributions
W.W.W. performed the statistical analysis and initial drafing of the manuscript. C.H.Y. and H.L. collected the data and reviewed the manuscript. G.S.S and D.D.C.participated in study conceptualization, data collection, initial writing of the manuscript, and fnal submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.






Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, W., Deng, D., Gong, S. et al. Influencing factors of hand, foot, and mouth disease based on structural equation modeling in Hubei, China. Sci Rep 15, 3571 (2025). https://doi.org/10.1038/s41598-025-87853-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87853-4