Abstract
This study aims to monitor and identify adverse events (AEs) associated with cetuximab, a drug used to treat various late-stage (metastatic) tumors, to improve patient safety and guide drug use. This study retrospectively analyzed the cases reported in the FDA adverse event reporting system (FAERS) related to the application of cetuximab from 2013 Q1 to 2022 Q4. Disproportionality analyses, including the reporting odds ratio (ROR), the proportional reporting ratio (PRR), the Bayesian confidence propagation neural network (BCPNN), and the empirical Bayesian geometric mean (EBGM) algorithms, were employed to quantify the signals of cetuximab-associated AEs. A total of 8364225 reports were contained in the FAERS database, of which 5186 reports of cetuximab were identified as ‘primary suspected (PS)’ AEs. The application of cetuximab resulted in AEs in 22 system organ classes (SOCs), which preserved 176 significant disproportionality preferred terms (PTs) through the computation of four algorithms. The main SOCs (Skin and subcutaneous tissue disorders, investigations, metabolism and nutrition disorders, and blood and lymphatic system disorders) accounted for 58.63%. Some AEs were not on the drug label: speech disorder, intervertebral discitis, glomerulonephritis rapidly progressive and disseminated intravascular coagulation. This study identified new signals of adverse drug reactions (ADRs) other than those mentioned in the specification associated with cetuximab, providing valuable insights into the relationship between ADRs and cetuximab use. The findings highlight the importance of continuous surveillance to detect and manage AEs effectively, ultimately improving patient safety during treatment with cetuximab.
Similar content being viewed by others
Introduction
Cetuximab is a monoclonal antibody to epidermal growth factor receptors, which can directly act on the extracellular region or soluble ligand of EGFR, prevent the formation of receptor dimers, and inhibit EGFR signaling1. cetuximab plays a significant role in the treatment of various tumors, including metastatic colorectal cancer (mCRC), where it is particularly effective for patients with KRAS wild-type tumors and has become a key partner in personalized targeted therapy2. It has also been approved by the FDA for the treatment of head and neck squamous cell carcinoma (HNSCC) and as the first-line treatment for recurrent/metastatic HNSCC (R/M HNSCC)3. Although its application in non-small cell lung cancer (NSCLC) has been explored, studies like the SWOG S0819 have not shown additional benefits when combined with chemotherapy4. Research on the use of cetuximab in pancreatic cancer is ongoing, investigating its potential as a standalone or combined treatment5. The importance of cetuximab is underscored by its ability to target specific molecular subgroups within these cancers, offering innovative treatment options and hope for patients facing resistance to conventional therapies.
The FAERS database was maintained by the U.S. Food and Drug Administration (FDA) to collect AEs and medication misinformation6 related to using FDA-regulated medical products. FAERS allows us to monitor and analyze drug-related adverse drug reactions (ADRs)7. FDA uses the information contained in the FAERS database to monitor the safety of medical products and identify potential safety signals that could prompt further investigation or regulatory action. In addition, some studies have found that using database from FAERS to study drugs can uncover new signals of ADRs. It is important to note that the information in FAERS does not necessarily indicate a causal relationship between a medical product and an AE, as reporting systems rely on voluntary submissions. However, it provides valuable information for the FDA when evaluating the safety and benefits of medical products. In cancer patients receiving immunotherapy, antibiotic therapy is associated with immune-related adverse events (irAEs), and in cancer patients receiving anti-PD1/PD-L1, antibiotic use should be carefully assessed to avoid a potential increased risk of irAEs8. In patients with non-small cell lung cancer treated with nivolumab, a higher proportion of interstitial pneumonitis (IP) treated with nivolumab in combination with EGFR-TKI was reported compared with either drug alone9. Analysis of reports of pericardial toxicity associated with the treatment of acute myeloid leukemia showed that while cytarabine has been routinely used and/or preferred for induction chemotherapy for acute myeloid leukemia, alternatives to cladribine may have a higher safety profile in terms of pericardial toxicity10. It can be seen that through extensive data analysis of FAERS, analysts can always get some tips for safe medication.
To date, several particle-based analysis algorithms, such as reported odds ratio (ROR)11, proportional reporting ratio (PRR)12, Bayesian confidence propagation neural network (BCPNN)13, and empirical Bayesian geometric mean (EBGM)14, have been used to assess the risk of AEs and detect the signal intensity of AEs associated with cetuximab using FAERS data sources. Cetuximab has been used for R/M HNSCC and mCRC for more than a decade15, and to further improve patient safety and guide drug use, we must pay close attention to the AEs that may occur after cetuximab treatment and data analysis help us further understand its wide clinical application and controllable toxicity. This study used the ROR and PRR algorithms to calculate the association between cetuximab and AEs. We then used the BCPNN algorithm to construct a joint probabilistic model between cetuximab and AE identified by ROR and PRR. Finally, we use the EBGM algorithm to convert the risk association into a corresponding risk index and screen the significant high-risk combinations of cetuximab and AE identified by the above three algorithms. This study combined these four algorithms to detect and identify new AE signals not listed in the cetuximab label.
Materials and methods
Data source and preprocess
The FAERS is a database (https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard) developed and maintained by FDA, collect information on a variety of adverse events and side effects associated with the use of medical products, including prescription and over-the-counter drugs, biologics, medical devices, and dietary supplements.
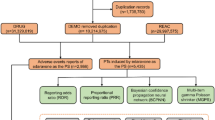
This study analyzed AE data using the relevant FAERS database by generic cetuximab name “cetuximab” and trade name “ERBITUX.” The data acquisition and preprocessing process use SAS and Navcat for MySQL software. It is then cleaned, standardized, and mapped to RxNorm and MedDRA v23.0 concepts to eliminate duplicate case records and identify ADRs, which are statistically identified16. The study collected AEs associated with cetuximab use over ten years and calculated and counted significant ADRs. Then it mapped these ADR terms to PTs and SOCs representing different MedDRA levels17. The study identified cetuximab as the primary suspect drug type for detecting ADR in FAERS and categorized serious clinical outcomes as death, disability, hospitalization, or life-threatening events. Other indicators analyzed included gender, age, and reporter’s country.
Statistical analysis
To identify cetuximab-AEs pairs that are reported more frequently than expected, we use multiple algorithms to measure disproportionately reported. All AEs are calculated by four algorithms: ROR, PRR, BCPNN, and EBGM18, and significant ADRs (must meet the criteria of all four algorithms) is detected. Before calculating the values of these four algorithms., values a, b, c, and d need to be obtained (where ‘a’ represents the number of reports of target adverse events caused by cetuximab, ‘b’ represents the number of reports of non-target adverse events caused by cetuximab, ‘c’ represents the number of reports of target adverse events caused by non-cetuximab, ‘d’ represents the number of reports of non-target adverse events caused by non-cetuximab. N = a + b + c + d, Table 1). The specific formulas of the four algorithms are as follows:
-
(1)
ROR algorithm
$$\:ROR=\left(ad\right)/\left(bc\right)$$$$\:95\%\:CI={e}^{\text{l}\text{n}\left(ROR\right)\pm\:1.96\sqrt{\frac{1}{a}+\frac{1}{b}+\frac{1}{c}+\frac{1}{d}}}$$The criteria of positive safety signal detection: the lower limit of 95% CI > 1, N ≥ 3;
-
(2)
PRR algorithm
$$\:PRR=\left[a\left(c+d\right)\right]/\left[c\left(a+b\right)\right]$$$$\:{\upchi\:}{^2}=\frac{\left(a+b+c+d\right){(ad-bc)}^{2}}{(a+b)(c+d)(a+c)(b+d)}$$The criteria of positive safety signal detection: PRR ≥ 2, χ2 ≥ 4, N ≥ 3;
-
(3)
BPCNN algorithm
$$\:IC={\text{log}}_{2}\frac{a\left(a+b+c+d\right)}{\left(a+b\right)\left(a+c\right)}$$$$\:95\%\:CI=E\left(IC\right)\pm\:2\times\:\sqrt{V\left(IC\right)}$$The criteria of positive safety signal detection: IC025 > 0 (IC025: the lower bound of 95% CI);
-
(4)
EBGM algorithm
$$\:EBGM=\left(aN\right)/\left[\left(a+b\right)\left(a+c\right)\right]$$$$\:95\%\:CI={e}^{\text{l}\text{n}\left(EGBM\right)\pm\:1.96\sqrt{\frac{1}{a}+\frac{1}{b}+\frac{1}{c}+\frac{1}{d}}}$$The criteria of positive safety signal detection: EBGM05 > 2 (EBGM05: the lower bound of 95% CI).
Results
Characteristics in real world population
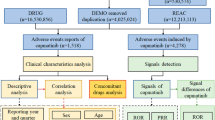
In this study, 8,364,225 cases reported were collected from the FAERS database during the study period (from 2013 Q1 to 2022 Q4). There were 5186 case reports analyzed after duplicates were excluded, and identification was conducted using four algorithms. Among these cases, 176 adverse events (AEs) were found to be adverse drug reactions (ADRs) related to cetuximab as the primary suspect drug. The specific clinical characteristics of events with cetuximab are described in Table 2.
Regarding the case reports documenting all adverse events (AEs), Males accounted for more than half of the total points (57.81%). Among the cases where the patient’s age was recorded, individuals aged 60 to 69 were more likely to experience AEs than other age groups, accounting for 19.59% (case = 1016) of cases. The top five countries with the highest reported number of cetuximab uses were the United States (2630, 50.71%), Germany (1789, 34.50%), Japan (321, 6.19%), China (78, 1.50%), and France (53, 1.02%). Among the recorded AEs, the most commonly reported severe outcome was hospitalization (1158, 22.33%), followed by death (653, 12.59%), life-threatening events (285, 5.5%), and disability (48, 0.93%).
Signal detects at system organ class level
The top five ADRs that occurred and ranked by case numbers at the SOC level in Table 3 were shown in the Fig. 1.
Further analysis of the strength of safety signals reveals that Skin and subcutaneous tissue disorders and Investigations rank first and second, respectively, in meeting the four calculation methods. All these Terms are marked on the label of cetuximab. In addition, not all SOC terms are mentioned on the drug label, such as Gastrointestinal disorders, Infections and infestations, Nervous system disorders, and Vascular disorders complications, which are not marked on the label of cetuximab.
Signal detections ranked by the EBGM at prefer terms level
In this study, four algorithms were used to analyze ADRs and assess their compliance with various screening criteria. The results of the most rigorous algorithm EBGM can be found in Table 4 and Supplementary Table S1.
At the PTs level, there were 176 ADR terms associated with 22 SOCs. The top five strongest signal ADRs, ranked by the EBGM algorithm at the PTs level, are shown in the Fig. 2.
These ADRs were associated with Renal and urinary disorders (case numbers:3, ROR (95% CI) = 10.73 (3.45–33.4), PRR (95% CI) = 10.72 (3.45–33.36), χ2 = 26.27, IC (IC025) = 3.41 (0.49), EBGM (EBGM05) = 10.66 (3.42)) and identified as potential new ADRs not included in the cetuximab label. Moreover, several newly detected ADRs were found to be associated with other SOCs, such as speech disorder (Case number: 80, ROR (95% CI) = 7.67 (6.15–9.57), PRR (95% CI) = 7.57 (6.09–9.41), IC (IC025) = 2.91 (2.19), EBGM: 2.91 (2.19) ), disseminated intravascular coagulation (Case number: 13, ROR (95% CI) = 5.97 (3.46–10.3), PRR (95% CI) = 5.96 (3.46–10.27), IC (IC025) = 2.57 (0.9), EBGM: 5.94 (3.44)), intervertebral discitis (Case number: 4, ROR (95% CI) = 13.85 (5.17–37.06), PRR (95% CI) = 13.84 (5.17–37.01), IC (IC025) = 3.78 (1.11), EBGM: 13.73 (5.13) ), among others. Please refer to the supplementary Table S1 for the EBGM rankings of other PTs.
Discussion
Head and neck cancer is the sixth tumor threatening human health globally, and its large number of multi-dimensional information is published in the TCGA and GEO databases; based on these data, it is very promising to find more effective therapeutic targets19. At present, the successfully transformed and marketed drugs are supervised by the FAERS system to ensure safe medication use. Since the EXTREME study20 and CHANGE-2 study21 have established the efficacy of cetuximab in SCCHN patients in the Occident and China. Cetuximab has been approved as a first-line targeted drug for the treatment of R/M SCCHN, and the frequency of use will inevitably be increased in the future, so research of AEs is much more significant.
We use four measures of dis-proportionality22, based on the classic four-grid table (Table 1), to explore the ADRs by combining the calculation methods of ROR, PRR, BCPNN, and EBGM with higher sensitivity and specificity (Tables 3 and 4). Most of the AEs obtained by the study analysis are mentioned in the medicine instructions, and the rash is the most significant AE of cetuximab. However, the rash rarely goes beyond grade 3, and symptom is improved to apply dermatitis pine, hydrocortisone ointment, or erythromycin ointment to the affected area. Our analysis found that the most affected SOC was skin and subcutaneous tissue disorders, with 1495 reports, of which 570 were reported by rash, 152 by acneiform dermatitis, 143 by dry skin, and 58 by skin toxicity. In addition to the surface of tumor cells, EGFR is also expressed in the basal layer of the epidermis and the outer layer of hair follicles, and inhibition of EGFR will cause different degrees of skin toxicity23. It has been reported that the severity of rash is positively correlated with its treatment effect24, and patients must quit smoking before receiving treatment and pay attention to avoid ultraviolet irradiation.
The analysis found that Injury, poisoning, and procedural complications ranked highly, among which radiation mucositis and radiation skin injury were the top 2 signal strength of AEs of cetuximab at preferred terms PTs ranked by EBGM. Radiotherapy is essential in causing the above AEs, and radiotherapy can also cause pharyngeal inflammation, mucosal infection, osteoradionecrosis and other AEs25. The AEs of blood and lymphatic system disorders are mainly bone marrow suppression, mainly reflected in investigations, including leukopenia (case = 29), neutropenia (case = 144), thrombocytopenia (case = 66), decreased hemoglobin (case = 11), anemia (case = 96), etc. If leukocytes and neutrophils decline, chemotherapy should be withheld, and patients should be treated with elevated leukocyte therapy, such as recombinant human granulocyte colony-stimulating factor26. But when blood alkaline phosphatase increased (case = 20), blood lactate dehydrogenase increased (case = 13), and aspartate aminotransferase increased (case = 32), it often means impaired liver function. Increased blood urea (case = 12), accompanied by increased serum creatinine, often predicted renal failure. However, patients with compensable impairment of liver and kidney function have no clinically significant effect on the pharmacokinetics of cetuximab, and the drug can continue to be used when the disease is controllable. In Gastrointestinal disorders, in addition to nausea, vomiting, and diarrhea, adverse reactions include dysphagia (case = 130), stomatitis (case = 100), and dry mouth (case = 130). The most common immune system disorders are infusion-related reactions (case = 207); more than 90% of hypersensitivity occurs at the first infusion of the drug, only a little occur during the infusion, and male smoking patients are a high-risk factor27. Because EGFR is also expressed in alveolar parietal cell type2, we have seen a large number of reports of pneumonia and pulmonary toxicity in Respiratory, thoracic and mediastinal disorders; pulmonary toxicity is mainly manifested as pneumonia and interstitial lung disease, often causing severe tachypnoea or even asphyxia. In this case, it is necessary to immediately stop the drug and inject steroids until the condition of patient is stable28. During treating of cetuximab in combination with FOLFIRI, pulmonary embolism was reported in 4.4% of patients, compared with only 3.4% of patients treated with FOLFIRI alone. Metabolism and nutrition disorders remind us that for patients with loss of appetite and malnutrition, it is important to pay attention to the imbalance of potassium, sodium, magnesium, and calcium in water and electrolytes. Among them, hypomagnesemia (case = 79) was the most reported because EGFR was also more expressed in the distal tubules and collecting ducts of the kidney, and a small amount acted on the proximal tubules, glomerular capillaries, mesangium, bottom epithelial cells, peritubular capillaries, and arterioles. Inhibition of EGFR by cetuximab results in reduced reabsorption of magnesium ions in the thick segment of the ascending branch of the renal loop of the renal medullary cord29.
Further, we found many ADRs that were not on the label, such as speech disorder (case = 80), disseminated intravascular coagulation (case = 13), intervertebral discitis (case = 4), glomerulonephritis rapidly progressive (case = 3), etc. It is worth paying more attention to clinical use in the future. Speech disorders may also be caused by the pharmacological effects of psychotropic drugs (such as antiepileptic drugs, central nervous system stimulants, and antidepressants) or toxic reactions due to drug accumulation in the body30. Intervertebral discitis may also be triggered in drug adverse reaction events by the following factors: the direct toxic effects of long-term use of drugs (such as nonsteroidal anti-inflammatory drugs and glucocorticoids), and the effect of biologics for autoimmune diseases (such as anti-TNF-a drugs) in altering the body’s immune status31.Rapidly Progressive Glomerulonephritis (RPGN) may also be caused by the following factors: certain drugs (such as hydralazine, propylthiouracil, allopurinol, sulfadiazine, minocycline, penicillamine, rifampicin, aminoguanidine, and sofosbuvir) can induce ANCA-associated vasculitis. Immune checkpoint inhibitors used for cancer treatment (such as PD-1, PD-L1, and CTLA-4 inhibitors) may trigger glomerulonephritis. Antibiotics and nonsteroidal anti-inflammatory drugs (NSAIDs) can alter the function of the immune system, leading to the formation and deposition of immune complexes, thus causing nephritis. Antibiotics and antiviral drugs may directly damage the glomerular filtration membrane, resulting in glomerulonephritis. Patients with autoimmune diseases (such as systemic lupus erythematosus) or chronic kidney disease may be more susceptible to glomerulonephritis when using certain drugs32. Disseminated Intravascular Coagulation (DIC) may also be triggered by the following factors: Antipyretic analgesics (such as aspirin, ibuprofen, etc.) may activate the coagulation system by damaging vascular endothelial cells. Certain antibiotics (such as penicillin and cephalosporin) may trigger allergic reactions or directly damage vascular endothelial cells. Immune checkpoint inhibitors (PD-1 and PD-L1 inhibitors) may activate the immune system. Certain enzyme preparations (fibrinolytic inhibitors) and the inhibition of the fibrinolytic system (e.g., tranexamic acid, aminocaproic acid) may impact the fibrinolytic system. Long-term, high-dose use of glucocorticoids may suppress the function of the mononuclear phagocyte system, leading to a decreased ability to clear procoagulant substances, thereby inducing DIC33. This has great reference value when the above AEs occur in clinical practice.
In Nervous system disorders, besides pharyngeal paraesthesia (case = 3) and neuropathy peripheral (case = 54), speech disorder (case = 80) that was not on the label was detected. Speech and swallowing are directly related to the quality of life34, so speech disorder after taking the medication in advanced patients needs to be paid great attention to. Vascular disorders are most commonly involved in the anaphylactic reaction (case = 87), without mentioning disseminated intravascular coagulation, and the literature reports of arteriovenous thrombosis associated with cetuximab-based chemoradiotherapy35, which reminds us to observe of patients’ vascular endothelial cells, Anticoagulant monitoring, and fibrinolytic activity test results, if necessary, complete the Color Doppler test. In Infections and infestations, discitis is not mentioned on the label, which may be related to the patient’s low immunity, bed rest and symptomatic anti-inflammatory therapy can effectively relieve the worsening of symptoms. Glomerulonephritis rapidly progressive were detected in renal and urinary disorders and not on drug label, and only one report on an unusual case of diffuse proliferative and crescentic glomerulonephritis in a 67- year-old man in close temporal association with cetuximab treatment for recurrent oral squamous cell carcinoma36. After stopping therapy of cetuximab, the patient was treated with short-term cyclophosphamide and steroids, and the renal function improved significantly. In conjunction with reports, we suspect that cetuximab treatment may trigger or exacerbate IgA-mediated glomerular injury and that close monitoring of renal function in patients treated with this EGFR inhibitor is required.
Our research is relatively scientific, and we try to overcome the data limitations and serve patients’ health by analyzing FAERS information. Indications of cetuximab cover a variety of cancer types (mainly CRC and HNSCC), while chemoradiotherapy and other combination factors may interfere with monitoring AEs signals. Second, due to the different competencies of the rapporteurs who uploaded the data (physicians vs. patients), the quality of the data may reflect something other than the proper use of the drug. The data provided by the FAERS database is not very comprehensive, and the conclusion is not verified in other databases, such as VigiBase37, Yellow Card Scheme38, EudraVigilance39, MedWatch40 which could not represent the true incidence of ADRs. Therefore, it is necessary to pay special attention to drug interactions in the analysis and try to identify which ADRs are more likely to be associated with a particular drug. Different indications may affect the dose, frequency of administration, and patient population, all of which may influence the discovery of AEs. When mining AEs, it is necessary to consider and use the information of patient’s previous treatment history as much as possible to improve the accuracy of signal detection. Each of these factors may have an impact on the identification and analysis of adverse events, so when using the FAERS database for drug safety monitoring and signal mining, the results should be interpreted with caution and account for these potential confounding factors.
Conclusion
This study identified four potential new AEs signals: speech disorder, disseminated intravascular coagulation, intervertebral discitis, and glomerulonephritis rapidly progressive which may assist in clinical monitoring and risk identification of cetuximab. The use of cetuximab in SCCHN patients is a milestone event, and there is still a long way to go from mitigating chemotherapy toxicity to forming chemo-free regimens (in combination with immunotherapy) in the future. During this period, using FAERS and other databases to obtain cetuximab-related medication feedback can help identify AEs earlier in the clinic and intervene early to ensure the drug safety of patients while monitoring ADRs of drug combination is still very important in future research. However, it should be clear that automated analysis methods for database mining still cannot replace experienced drug safety experts for a clinical case review and interpretation.
Data availability
The data included in this study are available on the request from the corresponding author or the first author.
Abbreviations
- FAERS:
-
FDA adverse event reporting system
- AEs:
-
Adverse events
- ADRs:
-
Adverse drug events
- PTs:
-
Preferred terms
- SOC:
-
System organ class
- ROR:
-
Reporting odds ratio
- PRR:
-
Proportional reporting ratio
- BCPNN:
-
Bayesian confidence propagation neural network
- EBGM:
-
Empirical bayesian geometric mean
- 95% CI:
-
95% Confidence interval
- χ2 :
-
Chi-squared
- IC:
-
Information component
- IC025:
-
The lower limit of 95% CI of the IC
- E(IC):
-
The IC expectations
- V(IC):
-
The variance of IC
- EBGM05:
-
The lower limit of 95% CI of EBGM
- ROR_95:
-
95% Confidence interval of ROR algorithm
- PRR_95:
-
95% Confidence interval of PRR algorithm
References
Oliveira-Silva, R. J., Carolina de Carvalho, A., de Souza Viana, L., Carvalho, A. L. & Reis, R. M. Anti-EGFR therapy: Strategies in head and neck squamous cell carcinoma. Recent. Pat. Anticancer Drug Discov. 11, 170–183 (2016).
Morris, V. K. et al. Treatment of metastatic colorectal cancer: ASCO guideline. J. Clin. Oncol. 41, 678–700 (2023).
Chung, C. H. et al. Phase II Multi-institutional Clinical Trial Result of Concurrent Cetuximab and Nivolumab in Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma. Clin Cancer Res 28, 2329-2338 (2022).
Herbst, R. S. et al. Cetuximab plus carboplatin and paclitaxel with or without bevacizumab versus carboplatin and paclitaxel with or without bevacizumab in advanced NSCLC (SWOG S0819): A randomised, phase 3 study. Lancet Oncol. 19, 101–114 (2018).
Forster, T. et al. Cetuximab in pancreatic cancer therapy: A systematic review and Meta-analysis. Oncology 98, 53–60 (2020).
Filippini, D. M. et al. Bone fracture as a novel immune-related adverse event with immune checkpoint inhibitors: Case series and large-scale pharmacovigilance analysis. Int. J. Cancer 149, 675–683 (2021).
Zhao, B. et al. A real-world disproportionality analysis of everolimus: Data mining of the public version of FDA adverse event reporting system. Front. Pharmacol. 15, 1333662 (2024).
Jing, Y. et al. Association of antibiotic treatment with immune-related adverse events in patients with cancer receiving immunotherapy. J. Immunother Cancer 10 (2022).
Oshima, Y., Tanimoto, T., Yuji, K. & Tojo, A. EGFR-TKI-associated interstitial pneumonitis in nivolumab-treated patients with non-small cell lung cancer. JAMA Oncol. 4, 1112–1115 (2018).
Janus, S. E. et al. Reported pericardial toxicities associated with acute myelogenous leukemia treatments: A pharmacovigilance analysis of the FDA adverse reporting database. Curr. Probl. Cardiol. 47, 101345 (2022).
Rothman, K. J., Lanes, S. & Sacks, S. T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 13, 519–523 (2004).
Evans, S. J., Waller, P. C. & Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 10, 483–486 (2001).
Bate, A. et al. A bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 54, 315–321 (1998).
DuMouchel, W. Bayesian Data Mining in Large Frequency Tables, With an Application to the FDA Spontaneous. (American Statistician, 1999).
Psyrri, A. et al. Durvalumab with or without tremelimumab versus the EXTREME regimen as first-line treatment for recurrent or metastatic squamous cell carcinoma of the head and neck: KESTREL, a randomized, open-label, phase III study. Ann. Oncol. 34, 262–274 (2023).
Liu, S., Ma, W., Moore, R., Ganesan, V. & Nelson, S. RxNorm: Prescription for electronic drug information exchange. IT Prof. 7, 17–23 (2005).
Brown, E. G. Using MedDRA. Drug Saf. 27, 591–602 (2004).
Tada, K., Maruo, K., Isogawa, N., Yamaguchi, Y. & Gosho, M. Borrowing external information to improve bayesian confidence propagation neural network. Eur. J. Clin. Pharmacol. 76, 1311–1319 (2020).
Leemans, C. R., Snijders, P. J. F. & Brakenhoff, R. H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 18, 269–282 (2018).
Guigay, J. et al. Cetuximab, docetaxel, and cisplatin versus platinum, fluorouracil, and cetuximab as first-line treatment in patients with recurrent or metastatic head and neck squamous-cell carcinoma (GORTEC 2014-01 TPExtreme): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 22, 463–475 (2021).
Guo, Y. et al. First-line treatment with chemotherapy plus cetuximab in Chinese patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck: Efficacy and safety results of the randomised, phase III CHANGE-2 trial. Eur. J. Cancer. 156, 35–45 (2021).
Hauben, M. & Zhou, X. Quantitative methods in pharmacovigilance: Focus on signal detection. Drug Saf. 26, 159–186 (2003).
Hsu, W. H., Yang, J. C., Mok, T. S. & Loong, H. H. Overview of current systemic management of EGFR-mutant NSCLC. Ann. Oncol. 29, i3–i9 (2018).
Mitchell, E. P., Perez-Soler, R., Van Cutsem, E. & Lacouture, M. E. Clinical presentation and pathophysiology of EGFRI dermatologic toxicities. Oncol. (Williston Park) 21, 4–9 (2007).
Gutiontov, S. I., Shin, E. J., Lok, B., Lee, N. Y. & Cabanillas, R. Intensity-modulated radiotherapy for head and neck surgeons. Head Neck 38(Suppl 1), E2368–E2373 (2016).
Michel, L. et al. Phase I trial of palbociclib, a selective cyclin dependent kinase 4/6 inhibitor, in combination with cetuximab in patients with recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol. 58, 41–48 (2016).
Hopps, S. et al. Cetuximab hypersensitivity infusion reactions: Incidence and risk factors. J. Oncol. Pharm. Pract. 19, 222–227 (2013).
Mayfield, J. D., Mercado, C. E., Kaye, F. J. & Mendenhall, W. M. Cetuximab-associated pulmonary toxicity in concurrent chemoradiation for the treatment of a squamous cell carcinoma of the head and neck. Head Neck 41, E55–e8 (2019).
Petrelli, F., Borgonovo, K., Cabiddu, M., Ghilardi, M. & Barni, S. Risk of anti-EGFR monoclonal antibody-related hypomagnesemia: Systematic review and pooled analysis of randomized studies. Expert Opin. Drug Saf. 11(Suppl 1), S9–19 (2012).
Pruett, D. G. et al. Characterizing drug-induced stuttering in electronic health records. J. Commun. Disord. 113, 106475 (2024).
Loft, J. A. et al. The Induced Immune response in patients with infectious spondylodiscitis: A prospective Observational Cohort Study. Front. Immunol. 13, 858934 (2022).
Koirala, A., Sharma, P. D., Jhaveri, K. D., Jain, K. & Geetha, D. Rapidly progressive glomerulonephritis. Adv. Kidney Dis. Health 31, 485–495 (2024).
Gong, F. et al. Disseminated intravascular coagulation: cause, molecular mechanism, diagnosis, and therapy. MedComm 2025(6), e70058 (2020).
Mouw, K. W. et al. Factors associated with long-term speech and swallowing outcomes after chemoradiotherapy for locoregionally advanced head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 136, 1226–1234 (2010).
Gupta, D. et al. Deep vein and artery thrombosis associated with cetuximab-based chemoradiotherapy. Indian J. Pharmacol. 43, 478–480 (2011).
Sasaki, K., Anderson, E., Shankland, S. J. & Nicosia, R. F. Diffuse proliferative glomerulonephritis associated with cetuximab, an epidermal growth factor receptor inhibitor. Am. J. Kidney Dis. 61, 988–991 (2013).
Lindquist, M. VigiBase, the WHO global ICSR database system: Basic facts. Drug Inform. Assoc. 42, 409–419 (2008).
Avery, A. J. et al. Evaluation of patient reporting of adverse drug reactions to the UK ‘Yellow Card Scheme’: Literature review, descriptive and qualitative analyses, and questionnaire surveys. Health Technol Assess. 15, 1–234 (2011).
Postigo, R. et al. EudraVigilance medicines safety database: Publicly accessible data for research and public health protection. Drug Saf. 41, 665–675 (2018).
Kessler, D. A. Introducing MEDWatch. A new approach to reporting medication and device adverse effects and product problems. Jama 269, 2765–2768 (1993).
Acknowledgements
This study was performed using the FDA Adverse Event Reporting System (FAERS) source that was provided by the FDA. The information, results, or interpretation of the current study do not represent any opinion of the FDA. We thank Zhao’s team (Official WeChat Account: SCIPhD) of Sheng Xin Zhu Shou for their suggestions and language editing of this article.
Funding
The study was supported by Jiangsu Province Hospital (the First Affiliated Hospital with Nanjing Medical University) Clinical Capacity Enhancement Project (JSPH-MA-2023-1), Clinical Diagnosis and Treatment Technology Innovation Challenge Project of Jiangsu Province Hospital (JBGS202420), the Natural Science Foundation of Jiangsu Province (Grants No. BK20230739) and Jiuquan City Science and Technology Livelihood Program Project Class A (2023MA3047).
Author information
Authors and Affiliations
Contributions
Shuai Zhao and Yan Wang designed the study; Zhaoyi Lu wrote the manuscript; Xi Chen coordinated the project; Xiaoli Deng performed data analysis; All members participated in discussion. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, S., Wang, Y., Deng, X. et al. Analysis of ADR reports of cetuximab based on the FDA adverse event reporting system database. Sci Rep 15, 4104 (2025). https://doi.org/10.1038/s41598-025-88838-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-88838-z