Abstract
Accurate detection and quantification of biomarkers at ultra-low levels is critical for disease diagnosis and effective treatment. Traditional detection technologies often lack the sensitivity, specificity, throughput, or multiplexing capacity required for comprehensive diagnostics, providing only a subset of these requirements. Here, we introduce AVAC, an automated optical technology for rapid and accurate biomarker detection with ultra-high sensitivity that significantly outperforms standard clinical assays. The core of this technology is the digital counting of plasmonic nanoparticles used as optical labels, enabling multiplexed, high-throughput detection of biomarkers. Validation studies demonstrate AVAC’s high accuracy, with 98.2% specificity and detection limits as low as 26 fg/mL for HIV p24 protein and a quantification range of 160 fg/mL to 850 pg/mL for interleukin-6 (IL-6). The technology supports multiplexed assays without compromising sensitivity, as demonstrated by the simultaneous detection of three key biomarkers associated with cardiovascular disease. A counting range spanning more than four orders of magnitude ensures robust detection from ultra-low to high biomarker concentrations, and its ability to analyze up to 1,000 samples per hour provides high throughput suitable for large laboratories. With its unique combination of capabilities, this versatile platform has significant potential to advance biomarker-based diagnostics in clinical and research settings.
Similar content being viewed by others
Introduction
The early detection and accurate quantification of specific biomarkers are critical to the diagnosis and treatment of a wide range of diseases, including infectious diseases such as AIDS, inflammatory disorders and cardiovascular disease1,2. Biomarkers in body fluids such as blood, saliva or urine provide a non-invasive window into an individual’s physiological state, enabling clinicians to effectively monitor disease progression and therapeutic response3,4.
However, the detection of these biomarkers in the early stages of disease poses significant challenges due to their extremely low concentrations and the complex milieu of biological fluids5,6.
Highly sensitive and specific diagnostic methods are essential to identify these low-abundance molecules while minimizing false positives and negatives7. In addition, many diseases involve complex biological processes characterized by multiple biomarkers that vary significantly in concentration at different stages and under different conditions. This complexity requires detection technologies that are not only highly sensitive and specific, but also capable of multiplexed detection over a wide dynamic range, quantifying targets over several orders of magnitude8.
In practice, most commercial detection technologies rely on label-based methods to overcome the limitations of direct detection. Techniques such as enzyme-linked immunosorbent assays (ELISA), chemiluminescent immunoassays (CLIA), and polymerase chain reaction (PCR) have become standard tools in clinical diagnostics9,10. In these methods, the target biomarker is tagged with a detectable label such as fluorescent dyes, enzymes, or chemiluminescent molecules to enhance the signal and enable quantification.
However, each of these methods has inherent advantages and disadvantages that affect their effectiveness in comprehensive diagnostics. While ELISA and CLIA have been mainstays in clinical laboratories, recent reviews indicate that their limited sensitivity, dynamic range, and multiplexing capabilities have prompted the search for next-generation platforms that can more efficiently address these diagnostic gaps9: ELISA is widely used for protein detection, but often lacks the sensitivity required to detect low-abundance biomarkers and has limited multiplexing capabilities. CLIA improves sensitivity through signal amplification using chemiluminescent labels, but requires specialized equipment and can be costly, and multiplexing is technically challenging9. PCR is highly sensitive for nucleic acid detection but is not directly applicable to protein biomarkers and involves complex procedures that limit its practicality for routine diagnostics11. More recent technologies based on PCR, such as proximity extension assays (PEA), have demonstrated considerable promise. However, their complex liquid-handling process with multiple manual steps and a time-to-results of several hours make their integration into clinical routine difficult12,13.
Given these challenges, there is an urgent need for innovative diagnostic platforms that combine ultrasensitive detection with high specificity, broad dynamic range, and robust multiplexing capabilities. Recent studies have emphasized that these features are critical for improving patient outcomes and facilitating earlier, more accurate diagnostics in clinical settings7. Such platforms should integrate seamlessly into existing laboratory workflows, be cost-effective, and offer scalability from single-sample to high-throughput analyses.
Here we present AVAC, a novel automated optical platform designed to meet these requirements. Using a proprietary technology based on reflective dark-field microscopy and digital counting of plasmonic gold nanoparticles, AVAC enhances detection sensitivity and specificity without the need for complex amplification steps. By using gold nanoparticles (GNPs) as optical labels, we achieve ultra-sensitive detection limits and precise quantification over a wide dynamic range. In addition, AVAC’s ability to distinguish different nanoparticle types based on their optical properties enables true multiplexed detection of multiple biomarkers in a single assay.
We are validating the performance of AVAC through three model applications: ultra-sensitive detection of HIV p24 protein, accurate quantification of IL-6, and simultaneous multiplex detection of IL-6, cardiac troponin I (cTnI), and B-type natriuretic peptide (BNP).
Results
AVAC platform technology
Overview of the AVAC platform
We introduce AVAC, a fully automated optical platform designed for rapid detection and digital counting of plasmonic nanoparticles used as highly specific biomarker labels. The platform leverages reflective dark-field microscopy and features a flexible optical system that allows customization of key components such as objectives, light sources, and cameras. This technology is patented in Europe, the United States, and other major jurisdictions worldwide14,15. The interaction between metallic nanoparticles and incident light is governed by the localized surface plasmon resonance (LSPR), a phenomenon caused by conduction band electrons oscillating in response to the incident light. This resonance affects light scattering and absorption at specific wavelengths, influenced by nanoparticle size, shape, material and surrounding refractive index. In particular, gold nanoparticles exhibit a spectral response in the green and a red-shift as size increases, enabling their differentiation through dark-field microscopy. The standard nanoparticles utilized are spherical gold nanoparticles (GNPs) with a diameter of 100 nm. However, the platform is adaptable to particles made from other materials and of varying sizes, shapes, and structures16,17. The substrates employed are dielectric materials with high flatness and ultra-low roughness, such as glass, silicon, or polymers, which facilitate the plasmonic response of the nanoparticles and ensure compatibility with dark-field microscopy18,19. Moreover, additional surface layers can be applied to significantly enhance the plasmonic signal20,21. The AVAC platform is capable of scanning substrates of arbitrary shapes up to a size of 80 mm × 120 mm. To ensure compatibility with existing laboratory equipment, standard sizes are adhered to, facilitating seamless integration with conventional laboratory equipment, such as that used for 96-well ELISA plates22.
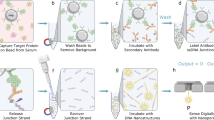
The assay workflow is analogous to an ELISA sandwich structure. A high-density monolayer of oriented capture antibodies is immobilized on the substrates through a series of chemical steps, and blocking agents are used to reduce non-specific binding. During the assay, a removable 96-well structure is attached to the substrate, forming individual wells that contain the samples (Fig. 1A).
AVAC assay workflow and scanning procedure. (A) A removable 96-well structure is mounted on the substrate with immobilized capture antibodies. (B) Samples and nanoparticles are assayed using conventional laboratory equipment. (C) During incubation, antigens are captured and labeled with nanoparticles (GNPs). (D) The well structure is removed, and the substrate is washed and dried. (E) The substrate is inserted into the AVAC scanner. The scanner automatically approaches all 96 wells or a user-defined selection of samples. (F) Multiple images are taken in each well. (G) Each AVAC image covers approximately 200 μm × 300 μm, allowing imaging of nanoparticles with an apparent size ≤ 0.7 μm. To visualize the well positions in (D) and (F), a substrate with GNP densities orders of magnitude higher than in real assays was used. All photographs and the reader illustration are courtesy of the authors. Illustration (C) was created entirely with Microsoft 365 PowerPoint version 2411 by the authors.
Each sample is incubated with functionalized nanoparticles coated with detection antibodies and blocked to minimize non-specific binding. After washing, the well structure is removed, the surface is dried, and the sample is inserted into the AVAC scanner for analysis (Fig. 1B–E). Details of the materials and protocols used are described in the Methods section. The AVAC platform employs a motorized XY stage to scan the sample(s), capturing several high-resolution dark-field images in each well using a high-speed, low-noise CMOS camera (Fig. 1F and G). Illumination is provided by a high-power warm-white LED, and an active autofocus ensures precise focusing14. Each AVAC image covers approximately 200 μm × 300 μm, and the diffraction-limited resolution allows imaging of nanoparticles with an apparent size of < 0.7 μm.
Simultaneously with scanning, the images are processed by proprietary analysis software (Fig. 2).
AVAC digital counting technology. (A) Image analysis identifies all potential particles, characterizes them in terms of scattering intensity and spectrum (in the examples, color measures are indicated), and classifies and counts them based on these parameters. (B) Classification is typically based on two particle characteristics: particle brightness (horizontal axis) and a relative color component (vertical axis). Nanoparticles can be distinguished from other particles based on color, while different agglomeration states are mainly distinguished based on brightness. (C) From the images of each well, mean particle counts and coefficients of variation (intra-well CV) are determined, resulting in digital counts per well and particle class.
The algorithm executes the following steps15: (i) identification, detect all potential particles based on size and shape criteria; (ii) characterization, analyze particles in terms of scattering intensity and spectrum, and optionally additional parameters; (iii) classification, categorize particles based on characterization results; and (iv) counting, provide a digital count of particles of each class in each image.
From all images of each well, statistical values such as mean and standard deviation are computed. A calibration curve is then employed to convert the mean particle counts into biomarker concentrations. The optimized analysis software allows for synchronous image processing during scanning, minimizing latency. With the capability of scanning and analyzing up to 20,000 images per hour, AVAC can process a 96-well assay in 5–15 minutes, making it suitable for high-throughput settings like central laboratories and blood banks.
Digital single particle counting
A cornerstone of AVAC’s functionality is its ability to identify, classify, and count individual nanoparticles. The synergy of plasmonic nanoparticles and a high-resolution optical system operating in reflective dark-field mode enables robust identification of nanoparticles amidst potential contaminants. Utilizing white-light illumination and a color camera, the system identifies particles based on size and shape and analyzes both the scattering intensity and the spectral properties of each particle. Classification is typically based on a combination of brightness and color measures, which facilitates the differentiation between nanoparticles and contaminants such as dust or chemical residues. This capability ensures resilience against external influences or handling errors, enhancing its robustness.
Moreover, AVAC can distinguish between nanoparticle monomers, dimers, trimers, and higher-order aggregates. By focusing exclusively on the most specific counts—typically monomers—the platform enhances the accuracy of biomarker quantification.
Digital counting advantage
In traditional signal integration methods20,23, detecting the minimal light increase caused by a single nanoparticle is challenging due to inherent image noise and the presence of parasitic objects. The same difficulty is encountered when trying to distinguish two images with N and N + 1 nanoparticles, with N ≫ 1. This limitation stems from the reliance on the total integrated signal, which must exceed noise levels to be reliably detected.
In contrast, AVAC’s approach of identifying and counting particles as individual objects—termed digital counting—circumvents this limitation. This method allows for the clear detection of even a single nanoparticle, as well as to distinguish between images with 1,000 and 1,001 particles.
Two additional advantages are that counts of certain particle classes can be used for quality control purposes, such as identifying and rejecting images with excessive parasitic particles, and that AVAC, unlike integration techniques, adapts seamlessly to variations in nanoparticle characteristics, eliminating the need for recalibration when using different nanoparticle batches or substrates, and also compensates for variations between measurements, such as changes in illumination intensity or spectrum.
Given that AVAC’s ability to accurately classify and count particles is its most distinguishing feature, a rigorous validation is essential. Although AVAC achieves diffraction-limited optical resolution, the 0.7 μm resolution is insufficient to resolve nanoparticles directly, which are typically around 100 nm in diameter. Size and shape alone are therefore insufficient to distinguish nanoparticles from other particles, or to discriminate different agglomeration states of nanoparticles. Instead, AVAC relies on their spectral fingerprints—primarily brightness and color—to achieve reliable classification, as illustrated in Fig. 2B. To verify that this classification is accurate, substrates were imaged using both AVAC and a scanning electron microscope (SEM).
In this validation, particles identified and classified by AVAC were mapped onto both the AVAC images and the corresponding SEM images (Fig. 3). The SEM, with its spatial resolution below 5 nm, provided a clear distinction between monomers, dimers, trimers, and larger aggregates. The overlay of AVAC’s classification results on the SEM images confirmed the accuracy of AVAC’s particle identification and classification. Details on the methodology and analysis are provided in the Methods section.
Validation of AVAC classification using SEM images. Identical substrate areas were imaged with AVAC (left) and SEM (right). The classification of particles obtained with AVAC is mapped onto both images. The images show four monomers, two dimers, one trimer, and two clusters, consistent with observations in the SEM image.
Multiplexing capability
AVAC’s ability to differentiate and classify distinct particle types based on their optical characteristics enables multiplex detection, allowing for the simultaneous measurement of multiple biomarkers within the same sample. One straightforward approach is to use spherical GNPs of different diameters. As the diameter increases, GNPs exhibit higher brightness and a shift of their scattering spectrum towards larger wavelengths (red-shift).
The practical diameter range for GNPs is approximately 50 nm to 150 nm. Smaller GNPs down to 40 nm have been successfully measured, but they produce weaker scattering signals, increasing noise levels and potentially limiting precise identification. Larger GNPs (> 150 nm) have scattering spectra that extend into the near-infrared region, beyond the sensitivity range of standard optics and RGB cameras, and have therefore not been used. The use of a warm-white illumination source in the AVAC reader optimizes the spectral overlap with the scattering spectra of these GNPs, thereby enhancing the contrast between the (specific) nanoparticles and other (non-target) particles, and, in addition, partially offsetting the reduced sensitivity of the CMOS detector in the red region. Within the practical diameter range, larger size differences between GNPs facilitate easier discrimination but require a broader dynamic range in image acquisition. Conversely, smaller size differences simplify imaging with a single camera setting but complicate accurate classification. For a triplex assay, we selected GNP diameters of 80 nm, 100 nm, and 150 nm.
Figure 4A presents a two-dimensional histogram (relative green color vs. brightness) from a triplex assay, where the distributions corresponding to the three GNP sizes are distinctly visible. The inset (Fig. 4B) demonstrates the expected increase in brightness and red-shift with increasing particle diameter. Figure 4C shows a magnified area of a dark-field image where this histogram information has been applied to classify particle types.
Multiplexing capability of the AVAC platform. (A) Two-dimensional histogram of relative green color component versus brightness from a triplex assay using GNPs of 80 nm, 100 nm, and 150 nm. (B) The inset displays the relative red contribution and brightness for the three particle types. (C) Zoomed-in AVAC image (~ 3.5% of full image), with particles marked by colored circles corresponding to the five particle classes defined in the 2D histogram.
Dynamic range of the platform
Accurately detecting and quantifying nanoparticles over a wide range of concentrations is crucial for robust biomarker detection, as biomarker levels can vary significantly across different physiological states and disease conditions. Understanding how AVAC’s counting capacity responds to varying nanoparticle densities is essential for optimizing performance. Here, we provide an illustrative explanation of how AVAC’s counting capability adapts from extremely low to very high nanoparticle densities.
Additionally, we compare AVAC’s digital counting method with the analog integration method that sums up the scattering signal from all GNPs. This comparison highlights the advantages and limitations of each method across different GNP concentration regimes, aiding in selecting the most appropriate strategy to maximize AVAC’s dynamic range.
In the low concentration regime, the digital method outperforms the analog method because the latter is susceptible to variations in background signal due to image noise and contaminants, leading to imprecise estimates of nanoparticle numbers, see Fig. 5.
Dynamic range illustration for digital counting (blue curve) and analog signal integration (yellow curve). From the lower counting limit (10–100 GNPs) up to approximately 10⁴ GNPs per well (equivalent to 10 GNPs per AVAC image), digital counting is more precise due to large uncertainties in the analog method caused by background noise and parasitic particles, indicated by the light-yellow area. Between 10⁴ and 107 GNPs per well, both methods perform well. Above 107 GNPs per well, digital counting becomes challenging due to optical overlap, while the analog method remains accurate up to nearly 10⁹ GNPs per well.
Digital counting is unaffected by these issues since it identifies and counts particles individually. Nevertheless, it must be considered that the digital approach also has a lower limit. While AVAC’s ability to discriminate GNPs from other particles is excellent, see Fig. 2B, a very small number of these parasitic particles could still fall into the classification region of the GNPs, resulting in an overestimation of monomer counts. To quantify this effect, we performed experiments in which extremely small numbers of GNPs were incubated on the substrate. The experiment established a lower limit of 8 counts per well, or 8 × 10−3 counts per image, indicated as “LoD” in Fig. 5.
At this point a second fundamental limitation is encountered: the demonstrated counting LoD of approximately ten GNPs implies an intrinsic uncertainty of around 30%, dictated by counting statistics for a Poisson process (Coefficient of Variation, CV =\(\:1/\sqrt{10}\) = 31.6%). To maintain the CV from pure counting statistics at or below 10%, a reasonable minimum lower count is thus 100 GNPs, consistent with the findings of Woolley et al.24, and indicated as “\(\:\sqrt{N}\)” in Fig. 5. It should be emphasized that this limit is roughly one order of magnitude higher than the demonstrated LoD, confirming that AVAC’s accuracy at low counts is constrained by statistical variations rather than instrumental limitations.
Above the lower counting limit, digital counting remains effective over approximately five to six orders of magnitude (from 10 to 100 to about 107 nanoparticles per well), as shown by the blue line in Fig. 5. This extensive range is possible because the sensor substrate provides an expansive capturing area for nanoparticles, unlike detection schemes based on microwell arrays25,26, which are limited by the number of capturing sites.
Each AVAC image, covering approximately 200 μm × 300 μm, can distinguish up to 15,000 particles, given the optical resolution below 0.7 μm and each particle occupying about 4 μm2 in the image. With approximately 1,000 images covering the sensitive area of one well (about 60 mm2), AVAC’s counting range extends to around 1.5 × 107 particles per well.
While it is possible to scan an entire well with 1000 images, this is impractical due to time constraints. In practice, scanning 70 images per well is reasonable, yielding a practical counting range of 1.5 to 15,000 nanoparticles per image, or 100 to 10⁶ nanoparticles per well, still covering four orders of magnitude as indicated in Fig. 5.
At higher particle densities, nanoparticles increasingly overlap optically, making individual counting challenging. However, the total scattering signal remains proportional to the number of nanoparticles. In this high-concentration regime, the analog method—integrating the brightness of the image—allows for accurate estimation of nanoparticle numbers because the signal from nanoparticles completely dominates over background noise. This method extends the upper limit of quantification to nearly 5 × 10⁸ nanoparticles per well (see Methods for calculations).
Biological applications demonstrating AVAC’s capabilities
Ultra-sensitive detection of HIV p24 protein
Human Immunodeficiency Virus (HIV) is the etiologic agent of the Acquired Immunodeficiency Syndrome (AIDS). The p24 protein (~ 24 kDa) is a structural component of the HIV capsid, with approximately 2,000 p24 molecules per virion27. During acute HIV infection, viral replication leads to detectable levels of p24 in the blood28. We developed a highly sensitive p24 immunoassay using AVAC to detect p24 in human serum samples. Here, we evaluate the performance of the AVAC p24 assay as an in vitro diagnostic test for the qualitative determination of p24 in human serum.
Sensitivity (LoD): The limit of detection (LoD) was determined by defining the noise (N) as the mean blank counts plus 2.5 times the blank’s standard deviation (SD). Setting N in this manner ensured that when S/N > 1 is used to establish the LoD, the lowest analyte concentration at which the signal exceeds this stringent noise threshold corresponds to a 98.8% confidence interval (i.e., to 0.6% false positives). The analytical sensitivity or limit of detection (LoD) was determined as the first concentration with S/N > 1. A negative serum pool was spiked with ten levels of p24 and diluted 1:8 in assay diluent, yielding expected concentrations ranging from 0.3 fg/mL to 2.6 ng/mL. The samples were run in duplicate. The LoD was established at 26 fg/mL (Fig. 6A).
Intra- and inter-assay precision: To assess precision, a serum pool was spiked with p24 at three levels (0.208 pg/mL, 2.08 pg/mL, and 20.8 pg/mL) and six replicates of each sample were run alongside twelve replicates of a blank (non-spiked) sample. All samples were diluted 1:8 in assay diluent, corresponding to measured concentrations of 0.026 pg/mL, 0.206 pg/mL, and 2.6 pg/mL, respectively. The experiment was conducted over two days, with one plate per day (n = 12). Results showed intra-assay CVs of 7.5% and inter-assay CVs of 4.2% (Fig. 6A).
Specificity: For the specificity test, a screening of HIV-negative patient samples was performed to determine the occurrence of false positives, i.e., positive test results when the individual does not have the disease. False-positive reactions might occur because of sample contamination or non-specific binding and reduce the diagnostic specificity of the assay. 55 individual HIV-negative human serum samples were diluted 1:8 in assay diluent and tested in duplicate on two plates. A signal-to-noise ratio (S/N) was calculated by dividing the observed nanoparticle counts by the counts corresponding to the blank plus 2.5 × blank SD (where the blank is assay diluent with zero p24). Samples with S/N < 1 were considered negative; those with S/N ≥ 1 were considered positive. One false positive was observed among the 55 samples, yielding a specificity of 98.2% (Fig. 6B).
Evaluation of a commercial p24 panel: We analyzed human plasma samples from a commercial HIV seroconversion panel (Zeptometrix, Donor No. 65790, #HIV9016) to further evaluate the AVAC p24 assay. Samples from the seroconversion panel came from a single donor, who was untreated and asymptomatic through the collection period in the detailed days after the first draw. Samples were diluted 1:8 in assay diluent and classified as positive with S/N > 1 (using blank plus 2.5 × blank SD). The AVAC p24 assay identified HIV infection one draw earlier than both the Zeptometrix HIV p24 antigen assay and the Abbott p24 HIV-1 antigen assay, demonstrating improved early detection capabilities over established commercial tests (Fig. 6C).
Summary
The AVAC p24 assay demonstrated a sensitivity (LoD) of 26 fg/mL, a specificity of 98.2%, and intra- and inter-assay precisions of 7.5% and 4.2%, respectively. It was capable of detecting HIV infection earlier than other protein-based detection techniques.
Precise Quantification of Interleukin-6 (IL-6)
Interleukin-6 (IL-6) is a key pro-inflammatory cytokine implicated in conditions such as sepsis, rheumatoid arthritis, and cancer29,30,31. Its accurate quantification supports patient management and disease monitoring. We developed a highly sensitive AVAC immunoassay for IL-6 in human serum and evaluated its performance as an in vitro diagnostic test. The assay’s performance was assessed as an in vitro diagnostic test for quantitative IL-6 determination.
Limit of Blank (LoB) and Limit of Detection (LoD): Using the IL-6 International Standard (NIBSC code 21/308), the assay was performed with 20 replicates of diluent (blank) and 20 replicates of IL-6 spiked at 1 pg/mL. The calculation of LoB and LoD followed the procedure “Parametric option”, see Eqs. (2), (3), (5) and (6) on pages 15–17 of the guideline used32. Cp is the factor with which the corresponding standard deviation (SD) needs to be multiplied to obtain the desired percentile of a normal distribution having this uncertainty SD. In the standard case of the 95th percentile used here, one has \(c_p=1.645⁄((1-1/((4(B-K)) )) )\), with B = total number of blank results in the dataset, and K = number of blank samples; in the case of the LoD, B and K refer to the low-level instead of the blank samples. LoB was established at 0.00 pg/mL, LoD at 0.12 pg/mL.
Lower Limit of Quantification (LLoQ): Determined using a 12-member panel of serum samples spiked with IL-6 and diluted 1:8 (expected concentrations from 0.095 pg/mL to 0.82 pg/mL). The LLoQ was established at 0.16 pg/mL, based on 72 determinations with precision (CV) within 20% and accuracy within 20% of the nominal concentration (Fig. 7A).
Upper Limit of Quantification (ULoQ): Estimated using an 8-member panel of serum samples spiked with IL-6 and diluted 1:8 (expected concentrations from 200 pg/mL to 900 pg/mL). The ULoQ was established at 850 pg/mL, based on 40 determinations with precision (CV) within 15% and accuracy within 15% of nominal concentration (Fig. 7A).
Dilution linearity: A high-concentration IL-6 sample (248.2 pg/mL) was serially diluted up to 1:100 in assay diluent. The assay demonstrated a dilution linearity of 107.7% (average measured concentration of 267.4 pg/mL across n = 9 dilutions), indicating reliable results across dilution levels (Fig. 7A).
Intra- and inter-assay precision: Intra-assay precision was assessed with 21 replicates at low (< 5 pg/mL), medium (< 40 pg/mL), and high (< 200 pg/mL) IL-6 concentrations (dilution 1:8) on a single plate. Inter-assay precision was evaluated over five days, with three replicates per sample, two plates per day, and a total of 30 replicates. Mean intra- and inter-assay CVs were 7.2% and 6.1%, respectively (Fig. 7A).
Method comparison: When benchmarked against the Quanterix Simoa IL-6 Advantage kit (Cat. #101622) on the SR-X® platform, the AVAC IL-6 assay showed an excellent correlation (R2 = 0.99) using 20 individual human serum samples, thereby confirming that its analytical performance aligns closely with a leading ultra-sensitive immunoassay standard (Fig. 7B).
Summary
The AVAC IL-6 assay demonstrated a quantification range of 0.16 pg/mL to 850 pg/mL, dilution linearity of 107.7%, and high precision (intra-assay CV of 7.2% and inter-assay CV of 6.1%). The assay showed excellent agreement with an established ultra-sensitive method.
Multiplexed detection of IL-6, cTnI, and BNP
Leveraging AVAC’s multiplexing capability, we simultaneously detected three biomarkers—interleukin-6 (IL-6), cardiac troponin I (cTnI), and B-type natriuretic peptide (BNP)—using GNPs of distinct sizes (80 nm, 100 nm, and 150 nm). Since the three biomarkers represent key stages of coronary artery disease (CAD), their simultaneous measurement provides a comprehensive view of cardiovascular disease progression33: IL-6 is involved in inflammation and can trigger atheroma plaque rupture, leading to myocardial infarction34; cTnI is a critical marker of cardiac cell death and is recommended for diagnosing myocardial infarction35; BNP is a reliable biomarker for diagnosing heart failure associated with myocardial necrosis and cardiac dysfunction36.
GNPs were used in a ratio of 10 : 3.5 : 1 for 80 nm, 100 nm, and 150 nm particles, respectively, based on sedimentation velocity results and surface area competition observed in previous optimization studies.
For biomarker capture in the triplex configuration, capture antibodies were spatially multiplexed on silicon wafers, meaning each well contained specific capture areas for each biomarker. This approach allowed simultaneous incubation with all three GNP types while minimizing cross-reaction.
In the biological assay, a serum pool was spiked with the three biomarkers at seven concentrations (0.2 pg/mL to 156 pg/mL) and diluted 1:8. Assays were measured with AVAC, and the analysis software classified particles according to Fig. 4, ensuring that only the corresponding GNP type was counted for each biomarker. The lower limits of quantification (LLoQ) were determined for each biomarker, as shown in Fig. 8A. A control experiment using monoplex assays (one capture antibody and one GNP type per well) was conducted for direct comparison. Considering associated uncertainties, the quantification limits in the triplex configuration were identical to those in monoplex assays. This indicates that AVAC maintained its exceptionally low quantification limits even when multiplexing.
To verify the accuracy of the generated triplex assay, four calibrated human serum samples (mix 1 to 4) were spiked with different concentrations of recombinant IL-6, cTnI and BNP and assayed. The GNP counts obtained from the spiked samples were interpolated in the calibration curves of the monoplex and triplex versions of each assay to obtain the percentage of recovery. The analytical acceptability range for recovery was 70–130%. This range is widely accepted and does not compromise the reliability or reproducibility of immunoassays targeting a broad dynamic range37,38. The recovery percentages obtained demonstrate the platform’s ability to multiplex without loss of accuracy, see Fig. 8B. The values highlighted in red indicate a recovery percentage greater than 30%, this percentage was always obtained in a condition very close to the LLoQ of the assays, 0.17 pg/mL for the IL-6 assay and 0.18 pg/mL for the cTnI assay.
Summary
The AVAC triplex assay for IL-6, cTnI, and BNP achieved lower limits of quantification comparable to monoplex assays, demonstrating the platform’s capability to multiplex without sacrificing precision.
Discussion
This study introduces AVAC, an innovative automated optical platform that significantly advances biomarker detection technology by leveraging plasmonic gold nanoparticles (GNPs) as optical labels and reflective dark-field microscopy to achieve ultra-sensitive, high-throughput, and multiplexed detection capabilities. The platform’s ability to digitally count individual nanoparticles enhances sensitivity and specificity by identifying and excluding parasitic objects, thus maintaining precision even at extremely low concentrations. Moreover, AVAC exhibits a broad counting dynamic range spanning more than four orders of magnitude, capable of accurately quantifying nanoparticles from approximately 100 to 107 per well, addressing the need for robust detection across varying biomarker concentrations.
Unlike traditional methods such as ELISA, CLIA, and PCR, which often face limitations in sensitivity, dynamic range, and multiplexing, we have demonstrated that AVAC performs exceptionally across multiple biomarkers, showcasing the full potential of this disruptive technology. For instance, the platform achieved an ultra-low detection limit of 26 fg/mL for HIV p24 antigen, surpassing conventional assays and underscoring AVAC’s potential for early disease detection where biomarkers are present at extremely low concentrations. Additionally, AVAC demonstrated very high sensitivity and the ability to quantify over an extensive dynamic range for IL-6, spanning from 0.16 pg/mL to 850 pg/mL. This wide quantification range is critical for accurately monitoring biomarkers that can vary widely in concentration depending on disease state and progression. Furthermore, the platform’s multiplexing capability was exemplified by the simultaneous detection of IL-6, cTnI, and BNP without sacrificing the lower limit of quantification (LLoQ). This ability to perform true multiplexed assays is fundamental for understanding the profiles of complex pathologies, such as cardiovascular diseases, where multiple biomarkers need to be measured concurrently to provide a comprehensive diagnostic picture.
The results presented here make AVAC a promising platform. Integration of AVAC into existing clinical workflows will require optimization of cost effectiveness and obtaining regulatory approvals, but its compatibility with current laboratory infrastructure and high-throughput operation already position it well for widespread clinical adoption. In addition, the integration of the AVAC scanner with a sample processing module is being considered for the future to further streamline the overall workflow.
In conclusion, AVAC represents a substantial leap forward in biomarker detection, offering a powerful tool for early diagnosis, disease monitoring, and personalized medicine. By overcoming critical limitations of existing technologies—such as limited sensitivity, narrow dynamic range, and challenges with multiplexing—AVAC paves the way for more effective healthcare delivery through improved diagnostic accuracy and comprehensive biomarker analysis.
Methods
Matching of AVAC and SEM images
The optical resolution obtained with AVAC, or any other instrument based on light microscopy, does not allow for resolving individual nanoparticles with a diameter of approximately 100 nm. With a diffraction-limited resolution of about 0.7 μm, see the following section for details, even an agglomeration consisting of roughly a hundred nanoparticles (close to a 5 × 5 × 5 cube) would appear only slightly larger than a single nanoparticle. Only larger agglomerations and other particles of size ≥ 1 μm can be distinguished based on their deviation from the characteristic size and shape of the diffraction-limited pattern. However, smaller aggregates and individual nanoparticles appear similarly sized, making size- or shape-based discrimination insufficient. For this reason, AVAC does not rely primarily on size or shape to discriminate nanoparticles from other particles. Instead, the system uses distinct spectral fingerprints, such as brightness and color. As shown in Fig. 9D, the brightness of the nanoparticles (horizontal axis) increases with their agglomeration state (monomers, dimers, trimers, clusters), as well as it does with the size of other (non-nanoparticle) particles, which can cover the entire brightness range. The key differentiating factor is the color: while nanoparticles consistently exhibit color values above 0.35, “other” particles maintain color values below this threshold, creating a clear gap between the two groups that is preserved across the entire brightness range. Only at brightness values close to 1 (saturation of the image sensor) would these groups potentially overlap, a regime of no practical relevance since large aggregates do not provide a useful assay response. Counting relies on monomers, not on large aggregates, thereby maintaining the specificity and reliability of the assay results.
The accuracy of this particle classification based on brightness and color was validated by matching AVAC images 1:1 with SEM images.
This 1:1 comparison between AVAC and SEM images was achieved as follows, see Fig. 9 for an illustration: (i) An entire well of the sensor substrate was completely covered with AVAC images, and a spatially precise stitching was generated from the corresponding 37 × 61 images (Fig. 9A). (ii) A series of slightly overlapping high-resolution SEM images were taken in the central region of the well. Additional lower resolution SEM images were used to approximate the location of the strip of SEM images in the AVAC stitching (Fig. 9B). (iii) Using this approximate location as a reference point, the individual SEM images could then be precisely registered to the corresponding AVAC image (Fig. 9C). (iv) This allowed us to identify the corresponding area in the AVAC image for each SEM image, resulting in a number of 1:1 matches between AVAC and SEM images of identical substrate locations (Fig. 9E).
The 1:1 matches between the AVAC and SEM images were then used to validate the accurate classification of the AVAC platform. To this end, the particles identified in the AVAC images were classified according to the methodology shown in Fig. 9D, and the resulting particle classes were indicated in both the AVAC and the corresponding SEM images (Fig. 9E). The SEM images allowed for spatial resolution of the nanoparticles, allowing for a clear distinction between different agglomeration states, including monomers, dimers, trimers, etc., while the overlay of the AVAC classification results allowed for verification of its accuracy.
The study showed that AVAC’s estimation of the number of monomers was slightly below the actual value, while its estimation of the number of dimers and trimers was above the actual value. Extensive analysis of the images revealed that the primary factor contributing to this discrepancy is the diffraction limit of the optical images. This phenomenon causes, for example, two closely spaced monomers to be perceived as a single dimer. This effect explains the discrepancies observed in Fig. 9F: The 23 missing monomers correspond to exactly four additional dimers and five trimers (23 = 4 × 2 + 5 × 3).
Spatial matching of AVAC and SEM images. (A) An entire well of the sensor substrate was completely covered with AVAC images; the figure shows a stitching of these 37 × 61 images. (B) A series of slightly overlapping high-resolution SEM images were acquired in the central region of the well. Additional lower resolution SEM images were then used to locate the strip of SEM images in the AVAC stitching. (C) Using the approximate location of the strip, the individual SEM images could be spatially registered to the corresponding AVAC image; the example shows AVAC image 1311 with the 18 corresponding SEM images 20…37. E) For each SEM image (here image 26), the corresponding area in the AVAC image can be retrieved. The classification of the particles obtained with AVAC, based on the 2D histogram, D), is mapped onto both AVAC and SEM images (E), with each of the seven classes marked with a circle of a different color. In this example, eight monomers, one dimer and one trimer are identified, consistent with what is observed in the SEM image. (F) Comparison of counts in SEM and AVAC for monomers, dimers and trimers of nanoparticles.
Spatial resolution of nanoparticles in TEM and AVAC images
Imaging of nanoparticles with TEM and AVAC is illustrated in Fig. 10. Graphic A is a TEM image of a 2 × 2 µm2 area showing a monomer, a dimer and a trimer of GNPs with a nominal diameter of 100 nm. Graphic B shows part of an AVAC image, also 2 × 2 µm2, with a single nanoparticle. The scattering pattern shows a donut shape with an orange tint, while the central part appears greenish; this pattern is expected for GNPs of this size on a silicon substrate.
GNPs with a nominal diameter of 100 nm imaged by TEM and AVAC. A: TEM image of an area of 2 × 2 µm2, showing a monomer, a dimer and a trimer of GNPs. B: A GNP imaged with AVAC, also showing an area of 2 × 2 µm2. C: Cross sections through the images of an individual GNP. For the AVAC image, the individual color channels (red, green, and blue) are shown together with their sum (black). For the TEM image, the curve (gray) corresponds to the monomer in the left image.
Graphic C shows cross sections through the images of a single GNP. For the AVAC image, the individual color channels (red, green, and blue) are shown together with their sum (black). As noted for the original image (B), the donut pattern is visible in the red channel and absent in the green one. Evaluation of the cross section reveals a full width at half maximum of 0.66 μm for the AVAC image, while that of the TEM image corresponds to the expected 100 nm.
Upper limit of particle number estimation using an analog approach
As a theoretical upper limit of the analog approach, we can estimate 4 × 300 μm × 200 μm / (0.7 μm)2 = 5∙105 particles per image or 5∙108 per well, assuming that at least four particles can completely overlap optically without blowing out the image sensor; this can be ensured by using shorter exposure times, which may allow factors even higher than the factor “4” assumed here. With a lower limit of 100 nanoparticles, this would mean a usable range of 6–7 orders of magnitude. It should be noted, however, that this theoretical upper limit is orders of magnitude higher than the actual counts obtained in typical AVAC assays focused on low biomarker concentrations. In addition, to be able to reach this limit, the total number of nanoparticles per well incubated with the sample would have to be increased accordingly.
Materials
Silicon surfaces were purchased from Si-Mat Germany. (3-aminopropil)trietoxisilane (APTES), phosphate-buffered saline (PBS) pH 7.4, bovine serum albumin (BSA), sodium chloride and rat IgG were all from Merck. DBCO-PEG1-NHS were obtained from Tebubio. HEPES buffer 50 mM pH 7.5 and anhydrous DMSO were purchased from Life Technologies. Carboxyl-terminated gold nanoparticles (80 nm, 100 nm and 150 nm diameter) were all from Nanopartz. 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), sulfo-N-hydroxysulfosuccinimide (sulfo-NHS), 2-(N-morpholino)ethanesulfonic acid (MES), and Tween-20 were purchased from Merck. Adhesive 96-well silicone superstructure was obtained from Grace Bio-Labs. Antibodies and protein standards were all purchased from commercial suppliers. The capture antibodies were modified using the SiteClick™ Antibody Azido Modification Kit from ThermoFisher. The assay diluent was formulated by Mecwins (10 mg/mL of BSA + 100 mM NaCl + 0.1% Tween 20 + 0.125 mg/mL rat IgG in PBST (PBS + 0.05% Tween-20)).
Serum sample collection
The human serum samples used in this study were obtained from reputable commercial suppliers to ensure ethical compliance and sample quality. Specifically, the pooled human serum used to prepare the spiked samples was purchased from SeraCare (LGC Group, Catalog #1830-0003). SeraCare ensures that all serum pools are collected in accordance with ethical guidelines and regulatory standards. Individual human serum samples were purchased from Grifols Bio Supplies Inc. All donor units were collected at FDA-approved donor centers in the United States, ensuring that informed consent was obtained from all donors and that collection procedures were in accordance with relevant ethical guidelines and regulations.
Silicon surface functionalization
To immobilize capture antibodies in the monoplex assays, amino groups were first generated by chemical vapor deposition (CVD) silanization with APTES for 1 h at 100ºC and a pressure of -950 mbar. The substrate was then incubated with 20 mL of a solution of 100 µM DBCO-PEG1-NHS ester in HEPES buffer 50 mM pH 7.5 + 1% anhydrous DMSO for 1 h on a rocker at 37ºC. The substrate was washed with 20 mL of HEPES buffer 50mM pH 7.5 for 5 min (x2) and PBS pH 7.4 for 5 min (x2) on the rocker at room temperature. In parallel, a solution of capture antibody modified with azide groups according to the SiteClick™ Antibody Azido Modification Kit was prepared at 25 µg/mL in PBS pH 7.4. The substrate was then incubated with 20 mL of the antibody solution for 2 h at 37ºC on the rocker. After incubation, the substrate was washed with 20 mL of PBS pH 7.4 for 5 min (3x) on the rocker at room temperature. Meanwhile, a blocking solution of BSA at 10 mg/mL in PBS pH 7.4 was prepared 30 min before use and shaken on a rocker until completely dissolved. After a final wash with PBS pH 7.4 following incubation with the capture antibody, the substrate was incubated with 20 mL of the blocking solution for 1 h at 37ºC. Finally, the substrate was washed with 20 mL of PBS pH 7.4 for 5 min (x2) and Milli-Q water for 5 min (x2) on the rocker at room temperature. The substrate was dried with N2 and stored at 4ºC until use.
Optimizing the biosensor surface involves screening and selecting the most suitable antibody pairs, ensuring their oriented and dense immobilization, implementing effective blocking procedures, and refining washing protocols to minimize non-specific binding and unbound nanoparticle residues. These measures are crucial for maximizing the sensitivity and specificity of AVAC-based assays.
Functionalization of gold nanoparticles
The surface functionalization of GNPs was performed by carbodiimide coupling through the terminal carboxylic acid groups. A solution of 100 µL of the appropriate detection antibody at 100 µg/mL in PBS and 100 µL of GNPs at 250 µg/mL in Milli-Q water was prepared. The required volume of 10 mM MES pH 3.8 was then mixed with the solution of the detection antibody and the GNPs to give a final volume of 1200 µL. The stock solution of GNPs was sonicated for 30 s and vortexed for 5 s before mixing with the detection antibody. In parallel, a solution of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and sulfo-N-hydroxysulfosuccinimide (sulfo-NHS) was prepared at 1 mg/mL and 1.5 mg/mL, respectively, in 10 mM MES pH 3.8. 100 µL of a 1:1 solution of the EDC and sulfo-NHS solutions was prepared in an Eppendorf tube, vortexed for 5 s, and immediately added to the previously prepared mixture of MES 10 mM pH 3.8, detection antibody, and GNPs. The final solution was incubated on a rotator at room temperature for 1 h. The solution was centrifuged at 17,000 g for 5 min and 4ºC, the supernatant was removed, and the pellet was resuspended in 1 mL PBS + 0.05% Tween-20 (PBST). The solution was sonicated for 15 s, vortexed for 5 s, and this washing step was repeated twice. After the last washing step, the GNPs were resuspended in 1300 µL of blocking solution (PBS + 0.05% Tween-20 + BSA 1 mg/mL) and incubated for 1 h at room temperature on a rotator. The nanoparticles were then centrifuged at 17,000 g for 5 min at 4ºC, the supernatant was removed, and the pellet was resuspended in the blocking solution. The solution was sonicated for 15 s, vortexed for 5 s, and this washing step was repeated twice. Finally, the concentration of functionalized GNPs was calculated by measuring the absorbance at the SPR peak using a NanoDrop 2000 spectrophotometer (ThermoScientific). The functionalized GNPs were stored at 4ºC until use.
Biological assay
First, the adhesive 96-well silicone superstructure was placed on the silicon wafer using a tool developed by Mecwins. Calibrators and samples were prepared at 1:8 (or 1:x) dilution in a 96 deep-well plate. The required volume of diluent was first aliquoted, taking into account a final volume of 200 µL per well and the volume of sample and GNPs to be added. The required volume of calibrator or individual sample was added and gently mixed by shaking the plate in a thermomixer (1 min, 800 rpm at 4ºC).
For each reaction well, the required amount of GNPs was added to obtain a final number of 75·106 nanoparticles per well in the monoplex assays, while in the triplex assay the different GNPs were used in a ratio of 10 : 3.5 : 1 (number of particles per sample for GNPs of 80 nm, 100 nm and 150 nm for a total number of GNPs per well of 225·106). The functionalized GNPs were sonicated for 30 s and vortexed for 5 s prior to addition. This process must be performed as quickly as possible to avoid sedimentation of the GNPs. After adding the GNPs to the reaction, the plate was gently mixed by shaking in a thermomixer (1 min, 800 rpm at 4ºC).
Finally, the mixture of sample and GNPs was transferred with a multichannel pipette (150 µL per well) to the assay silicon substrate and incubated at 37ºC for 60 min without agitation. After incubation, the substrate was washed thoroughly with PBS + 0.05% Tween-20 (PBST), the superstructure was removed under water with tweezers, and finally it was rinsed with Milli-Q water. Finally, the substrate was dried with dry N2 and its surface was scanned with the AVAC platform.
AVAC measurements and analysis
A 50x/0.80 dark-field objective was used for all AVAC measurements. Images were acquired as shown in Fig. 1F, using a rectangular grid of 13 × 13 images per well. This geometry ensures that the images cover the entire well (9 mm × 9 mm), including the areas corresponding to the position of the removable well structure. These latter images were not used to calculate the average number of nanoparticles per image but served as quality control of the scanning procedure to ensure that the images were correctly centered with respect to each well.
After verifying correct centering, all 13 × 13 images of all wells were analyzed, and the central 9 × 9 images of each well were used together to calculate a two-dimensional histogram (blue color vs. brightness) for the entire plate, and to automatically derive the particle classification, as shown in Fig. 9D. Application of the classification criteria resulted in a particle count for each of the seven defined classes for each image in each well. Only the monomer counts were used as a measure of biomarker concentration, to ensure the highest possible specificity.
To calculate the mean number of monomers for each well, again only the central 9 × 9 images were used. Before calculating the mean number, an automatic removal of potential outliers was applied to the counts from the 81 images of each well. Since the well structure can cause systematic spatial dependence within each well, the counts are not necessarily normally distributed, so outliers were identified as being more than 1.5 interquartile ranges above the upper quartile or below the lower quartile. This procedure provided a mean number of nanoparticle monomers per AVAC image for each well as a measure of biomarker concentration within that well.
In cases where replicates were assayed on the same plate, the arithmetic mean of the corresponding counts was then calculated along with the unbiased standard deviation.
To calculate the quantification range of IL-6, a calibration curve (mean monomer counts as a function of IL-6 concentration) was fitted with a 5PL function, using a weighting model based on the calculated standard deviations per concentration. This 5PL curve was used to convert the monomer counts of the serum samples (72 for the LLoQ, 40 for the ULoQ) into IL-6 concentrations. These concentrations were evaluated following the methodology laid out by the Clinical and Laboratory Standards Institute32, as indicated in the text.
To calculate the lower and upper limits of quantification (LLoQ and ULoQ) for the triplex assay and the monoplex used in comparison, the concentration dependencies of the biomarkers were again fitted with 5PL functions. The resulting 5PL curves were employed to ascertain the ranges within which the precision and accuracy of quantification were within the requisite limits, namely 20% for the LLoQ and 15% for the ULoQ.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bodaghi, A., Fattahi, N. & Ramazani, A. Biomarkers promising and valuable tools towards diagnosis, prognosis and treatment of Covid-19 and other diseases. Heliyon, 9(2), (2023).
Johann Jr, D. J. et al. Clinical proteomics and biomarker discovery. Ann. N. Y. Acad. Sci. 1022(1), 295–305 (2004).
Ahmad, A., Imran, M. & Ahsan, H. Biomarkers as biomedical bioindicators: Approaches and techniques for the detection, analysis, and validation of novel biomarkers of diseases. Pharmaceutics 15(6), 1630 (2023).
Rifai, N., Gillette, M. A. & Carr, S. A. Protein biomarker discovery and validation: The long and uncertain path to clinical utility. Nat. Biotechnol. 24(8), 971–983 (2006).
Anderson, N. L. & Anderson, N. G. The human plasma proteome: History, character, and diagnostic prospects. Mol. Cell. Proteom. 1(11), 845–867 (2002).
Petricoin, E. F. & Liotta, L. A. Clinical applications of proteomics. J. Nutr. 133(7), 2476S–2484S (2003).
Nimse, S. B., Sonawane, M. D., Song, K. S. & Kim, T. Biomarker detection technologies and future directions. Analyst 141(3), 740–755 (2016).
Su, M. Multiplexed detection of molecular biomarkers with phase-change nanoparticles. Nanomedicine 8(2), 253–263 (2013).
Khan, M. et al. Enzyme-linked immunosorbent assay versus chemiluminescent immunoassay: a general overview. Global J. Med. Pharm. Biomedical Update, 18 (2023).
Zhu, H. et al. PCR past, present and future. Biotechniques 69(4), 317–325 (2020).
Chakraborty, S. Democratizing nucleic acid-based molecular diagnostic tests for infectious diseases at resource-limited settings–from point of care to extreme point of care. Sens. Diagnostics. 3 (4), 536–561 (2024).
Álvez, M. B. et al. Next generation pan-cancer blood proteome profiling using proximity extension assay. Nat. Commun. 14, 4308 (2023).
Feng, W. et al. NULISA: a proteomic liquid biopsy platform with attomolar sensitivity and high multiplexing. Nat. Commun. 14, 7238 (2023).
Pini, V. et al. Biosensor Platform and Method for the Simultaneous, Multiplexed, ultra-sensitive and high Throughput Optical Detection of Biomarkers (U.S. Patent No. 11,519,843) (United States Patent and Trademark Office, 2022).
Thon, A. et al. Method for Optically Detecting Biomarkers (U.S. Patent No. 11,519,856) (United States Patent and Trademark Office, 2022).
Calvo, R. et al. Size characterization of plasmonic nanoparticles with dark-field single particle spectrophotometry. Sci. Rep. 12(1), 17231 (2022).
Calvo, R. et al. Novel characterization techniques for multifunctional plasmonic–magnetic nanoparticles in biomedical applications. Nanomaterials 13(22), 2929 (2023).
Olson, J. et al. Optical characterization of single plasmonic nanoparticles. Chem. Soc. Rev. 44(1), 40–57 (2015).
Mayer, K. M. & Hafner, J. H. Localized surface plasmon resonance sensors. Chem. Rev. 111(6), 3828–3857 (2011).
Kosaka, P. M. et al. Detection of cancer biomarkers in serum using a hybrid mechanical and optoplasmonic nanosensor. Nat. Nanotechnol. 9(12), 1047–1053 (2014).
Schmidt, M. A., Lei, D. Y., Wondraczek, L., Nazabal, V. & Maier, S. A. Hybrid nanoparticle–microcavity-based plasmonic nanosensors with improved detection resolution and extended remote-sensing ability. Nat. Commun. 3(1), 1108 (2012).
Aydin, S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 72, 4–15 (2015).
Kosaka, P. M., Pini, V., Calleja, M. & Tamayo, J. Ultrasensitive detection of HIV-1 p24 antigen by a hybrid nanomechanical-optoplasmonic platform with potential for detecting HIV-1 at first week after infection. PLoS One. 12(2), e0171899 (2017).
Woolley, C. F., Hayes, M. A., Mahanti, P., Douglass Gilman, S. & Taylor, T. Theoretical limitations of quantification for noncompetitive sandwich immunoassays. Anal. Bioanal. Chem. 407, 8605–8615 (2015).
Rissin, D. M. et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 28(6), 595–599 (2010).
Wilson, D. H. et al. The Simoa HD-1 analyzer: a novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J. Lab. Autom. 21(4), 533–547 (2016).
Chang, L. et al. Simple diffusion-constrained immunoassay for p24 protein with the sensitivity of nucleic acid amplification for detecting acute HIV infection. J. Virol. Methods. 188(1–2), 153–160 (2013).
Cohen, M. S., Gay, C. L., Busch, M. P. & Hecht, F. M. The detection of acute HIV infection. J. Infect. Dis. 202(Supplement_2), S270–S277 (2010).
Akdis, M. et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 138(4), 984–1010 (2016).
Sánchez-Zauco, N. et al. Circulating blood levels of IL-6, IFN-γ, and IL-10 as potential diagnostic biomarkers in gastric cancer: a controlled study. BMC cancer. 17, 1–10 (2017).
Kim, M. H. & Choi, J. H. An update on sepsis biomarkers. Infect. Chemother. 52(1), 1 (2020).
Pierson-Perry, J. F. et al. Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures; Approved Guideline—Second Edition. CLSI document EP17-A2 (ISBN 1-56238-795-2) (Clinical and Laboratory Standards Institute, 2012).
Lee, J. K. et al. Pre-clinical tests of an integrated CMOS biomolecular sensor for cardiac diseases diagnosis. Sensors 17(12), 2733 (2017).
Wainstein, M. V. et al. Elevated serum interleukin-6 is predictive of coronary artery disease in intermediate risk overweight patients referred for coronary angiography. Diabetol. Metab. Syndr. 9, 1–7 (2017).
Byrne, R. A. et al. 2023 ESC guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart Journal: Acute Cardiovasc. Care. 13(1), 55–161 (2024).
Gaggin, H. K. & Januzzi, J. L. Jr Biomarkers and diagnostics in heart failure. Biochim. et Biophys. Acta (BBA)-molecular Basis Disease. 1832(12), 2442–2450 (2013).
U.S. Food and Drug Administration. Bioanalytical Method Validation Guidance for Industry (2018).
European Medicines Agency. ICH Guideline M10 on Bioanalytical Method Validation and Study Sample Analysis (2022).
Acknowledgements
The authors would like to thank their retired colleague Carlos García for his invaluable contributions to the mechanical setup of the first AVAC prototypes. They also wish to express their gratitude to their colleague Teresa Ramón for her extensive work on the biochemistry used for the assay work presented here.Valerio Pini and Andreas Thon thank the Spanish State Research Agency for the Torres Quevedo grants, PTQ-16-08540 & PTQ-15-07565.
Author information
Authors and Affiliations
Contributions
V.C., V.P., and A.T. contributed equally to the work presented in this manuscript, their individual focus being the biological applications of the AVAC platform (V.C.), and its multiple instrumental (V.P.) and software aspects (A.T.). O.A. supervised the work, both regarding the instrument and its applications. V.P., A.S.M., and A.T. conceived and designed the instrumental part of the platform and developed the hardware and the control SW of the AVAC platform, including its integration; V.P. supervised these tasks. A.T. developed the analysis software run by the platform, and additional software used to analyze the data. V.C. conceived, directed and supervised all aspects of the biological application work. N.M. and C.R. designed the work on p24 and IL-6 (N.M.) and the multiplex assay (C.R.), respectively, carried out the corresponding experiments, and analyzed the results. All authors contributed to the collection, analysis, or interpretation of data. O.A., V.C., V.P., and A.T. conceived, drafted, and substantially revised the manuscript with contributions from the other authors.
Corresponding author
Ethics declarations
Competing interests
All authors are employed by Mecwins S.A., Madrid, Spain, the company which has developed the AVAC platform. Several authors (A.S.M., A.T., O.A., V.C., V.P.) are inventors of patents which cover the AVAC platform.This work was supported by a grant from the Spanish Ministry of Science and Innovation, Retos “Spectrasens”, RTC-2017-6311-1-1 (authors involved in the work related to this grant: A.S.M., A.T., O.A., V.C., V.P.). Other authors have no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cebrián, V., Pini, V., Thon, A. et al. Introducing AVAC as an ultra-sensitive platform with broad dynamical range for high-throughput multiplexed biomarker detection using digital counting of plasmonic nanoparticles. Sci Rep 15, 5390 (2025). https://doi.org/10.1038/s41598-025-88992-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-88992-4