Abstract
BRCC3 isopeptidase complex (BRISC) is a JAMM subfamily deubiquitinase that has been revealed to be required for optional activation of NLRP3 inflammasome and TLR4/NF-κB signaling pathway. BRISC plays an important role in lipopolysaccharide (LPS)/D-galactosamine-induced acute liver failure, while its functional contribution to alcoholic liver disease (ALD) is still unclear. In this study, we found that the expression of BRISC components was increased in liver tissues of alcoholic hepatitis (AH) animal models and patients with AH. Mice lacking either the scaffold subunit ABRO1 or the catalytic subunit BRCC3 showed attenuated liver steatosis, inflammation, and liver injury compared to control mice after chronic plus binge ethanol feeding. Moreover, pharmacological inhibition of BRISC activity by a BRISC inhibitor thiolutin potently protected mice from ALD development. Preliminary mechanistical studies showed that BRISC deficiency did not directly affect alcohol-induced hepatocyte injury or the translocation of LPS through the damaged gut mucosa after ethanol feeding, but prevented alcohol-induced NLRP3 inflammasome activation in liver. Collectively, our work revealed a previously unknown role of BRISC in ALD and suggested that BRISC may serve as a promising therapeutic target for ALD treatment.
Similar content being viewed by others
Introduction
Alcoholic liver disease (ALD), including alcoholic fatty liver, alcoholic steatohepatitis (ASH) with or without fibrosis, cirrhosis, and hepatocellular cancer, is becoming a primary cause of chronic liver disease worldwide1,2. Alcohol consumption not only destroys fatty acid metabolism in hepatocytes and adipose tissue, but also damages the intestinal barrier and gut microbiome, leading to hepatic steatosis, and further develops into more severe forms of alcoholic liver injury3,4,5. Unfortunately, to date, the exact knowledge of the pathogenesis of ALD is very limited, and there is no definitive treatment available for any stages of the ALD6.
Accumulation of evidence showed that immune cells and inflammatory signaling pathways are involved in the progression of ALD5,7. Excessive alcohol consumption causes the release of various types of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), triggering hepatic immune cells activation and production of proinflammatory cytokines to drive ALD progression5. The hepatic resident macrophages, Kupffer cells (KCs), are the major cells that initially respond to PAMPs/DAMPs and release large amounts of inflammatory mediators, leading to hepatocyte death and recruitment of circulating inflammatory cells to the liver7,8. Moreover, KCs are involved in the production of reactive oxygen species (ROS) and induce oxidative stress in the liver8. KCs also activate hepatic stellate cells (HSCs) and promote liver fibrosis during ALD5,8. The deletion of KCs prevented ethanol-induced liver damage, highlighting the importance of KCs in ALD9. Many inflammatory mediators are reported to promote the pathological process of ALD5. Among them, the NLRP3 inflammasome has a key role in the cellular response to ethanol10,11. The expression of NLRP3, ASC, and IL-1β in liver were transcriptionally upregulated in both ALD patient and ASH mice models, resulting in elevated levels of circulating IL-1β12,13. The excessive production of ROS, translocation of gut-microbial content (lipopolysaccharide, LPS), and increase of cellular debris (such as ATP and uric acid) induced by alcohol act as key drivers of NLRP3 inflammasome activation10. Deletion of NLRP3, caspase 1, ASC, or IL-1R1 reduced hepatic steatosis and inflammation and attenuated liver injury in ASH mice model13,14, emphasizing that NLRP3 inflammasome plays an important role in ASH pathogenesis and may serve as an attractive therapeutic target for treating ALD.
Previous works have demonstrated that BRISC (BRCC3 isopeptidase complex), a lysine 63 (K63)-specific deubiquitinating enzyme (DUB) that consists of four subunits including BRCC3, ABRO1, BRE, and MERIT40, is required for NLRP3 deubiquitination and activation15,16,17,18. Deficiency of either the catalytic subunit BRCC3 or the scaffold subunit ABRO1 blocks NLRP3 activation in both mouse and human macrophages16. Thus, BRISC deletion significantly attenuated the syndrome severity of NLRP3-associated inflammatory diseases in mice, including LPS-induced sepsis and monosodium urate crystals (MSU)- or aluminum-induced peritonitis16. Moreover, pharmacological inhibition of BRISC alleviated NLRP3-related diseases in mouse models including methionine- and choline-deficient (MCD) diet-induced nonalcoholic fatty liver disease (NAFLD)19. These data indicate that BRISC has a critical role in promoting NLRP3 inflammasome activation and is closely associated with the development of NLRP3-associated diseases. In addition, BRISC has been shown to promote Type 1 interferon signaling20. Recently, we reported that BRISC functions as a positive regulator of Toll-like receptor 4 (TLR4)-mediated nuclear factor kappa B (NF-κB) signaling pathway in KCs. Loss of BRISC significantly prevented LPS/D-galactosamine (D-GalN)-induced acute liver injury by the inhibition of proinflammatory cytokines production of KCs21. These data indicate that BRISC may contribute to the regulation of inflammatory response in liver diseases. Given the fact that the LPS/TLR4 pathway and NLRP3 inflammasome in KCs are critical for ALD development5,8, we speculate that BRISC may contribute to alcohol-induced liver pathology. However, whether BRISC is involved in ASH pathogenesis has yet to be evaluated.
Here, we investigated the effects of genetic deficiency and pharmacological inhibition of BRISC on alcohol-induced liver injury using a mouse model of chronic plus single binge ethanol gavage22. Our results showed that inactivation of BRISC improved symptoms of ALD and suggested that targeting BRISC may be a therapeutic strategy for the treatment of ALD.
Results
BRISC is upregulated in the liver of patients with alcoholic hepatitis and ALD mouse model
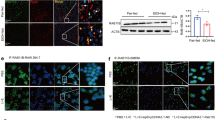
To determine the relevance of BRISC in ALD, we first assessed the correlation of BRISC expression level with alcoholic hepatitis (AH) by analyzing clinical data from the Gene Expression Omnibus (GEO) database, the results showed that both ABRO1 and BRCC3 mRNA, the two most important components of BRISC, were significantly upregulated in liver tissues of patients with AH compared to normal livers (GEO data sets GSE28619) (Fig. 1A). Further, we examined liver BRISC expression in a mouse model of chronic plus binge ethanol feeding, a well-established mouse model of ALD22. Mice received chronic ethanol feeding for 2 weeks plus a single binge ethanol feeding showed mild liver injury, while after 4 or 6 weeks of chronic ethanol feeding, hepatic steatosis, inflammation, and hepatocyte ballooning were significantly increased (Fig. 1B-C). During this process, both ABRO1 and BRCC3 protein expression was markedly up-regulated (Fig. 1D). Consistently, after 10 days of chronic ethanol feeding plus a single binge, the mRNA and protein expression of BRISC components in mouse liver was increased compared to pair-fed counterparts (Fig. 1E-F). These data suggest that BRISC may be functionally involved in ASH.
BRISC is upregulated in liver of ASH patient and mouse model. (A) ABRO1 and BRCC3 expression levels in the liver samples of patients with alcoholic hepatitis from published transcriptome dataset (GSE28619). (B-D) WT mice were subjected to chronic-plus-binge ethanol feeding for 2, 4, 6 weeks (N = 3–6). Representative liver H&E staining images were shown; Scale bar, 100 μm (B). Steatosis were quantified by measuring liver triglyceride content (C). Western blot analysis of ABRO1 and BRCC3 levels in total liver lysates (D). (E, F) WT mice were subjected to chronic-plus-binge ethanol feeding for 10 days (N = 3). Expression of BRISC components was detected by western blot (E) and real-time PCR (F). Data are presented as mean ± SEM; expression scores are shown as box plots, with horizontal lines representing the median; the bottoms and tops of the boxes represent the 25th and 75th percentiles, respectively, and the vertical bars represent the range of data; *P < 0.05, **P < 0.01, ***P < 0.001; two-tailed unpaired t-test.
ABRO1 deficiency ameliorates chronic plus binge ethanol-induced mice liver injury
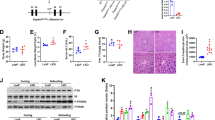
To investigate whether BRISC affects the pathogenesis of ASH in mice, we adopted the 10-day chronic plus binge ethanol feeding model described by the Gao group to create a moderate ALD in mice22. The ethanol food consumption was not different between WT and Abro1−/− mice (Supplementary Fig. 1A). After ethanol feeding, both genotypes of mice showed a similar body weight reduction (Supplementary Fig. 1B). Chronic ethanol feeding increased mice liver/body weight ratio, but there was no difference in Abro1−/− and WT mice (Supplementary Fig. 1C). WT mice fed with ethanol showed obvious hepatic steatosis and liver injury as determined by H&E staining, Oil Red O (ORO) staining, and the measurement of serum alanine aminotransferase (ALT), while these symptoms were alleviated in Abro1−/− mice (Fig. 2A-B). The reduction of hepatic lipid accumulation in Abro1−/− mice fed with ethanol was further confirmed by quantifying liver triglyceride (Fig. 2C). Moreover, ethanol feeding induced significant increase of hepatic TNF-α, IL-6, CXCL1, and CXCL2 mRNA expression, but the increase was prevented by ABRO1 deficiency (Fig. 2D). By immunohistochemical (IHC) analysis, F4/80+ macrophages were decreased in the livers of ethanol-fed mice, but the hepatic F4/80+ positive cells were not different in WT and Abro1−/− mice either before or after ethanol feeding (Fig. 2E). In contrast, Ly6G immunostaining showed that neutrophil infiltration in the livers of ethanol-fed mice was potently increased, but this increase was inhibited by ABRO1 ablation (Fig. 2F). Hepatic 4-Hydroxynonenal (HNE) positive cells were decreased in ethanol-fed Abro1−/− mice, indicating that deficiency of ABRO1 reduced ethanol-induced oxidative stress (Fig. 2G). These data demonstrated ABRO1 deletion attenuates hepatic steatosis, inflammation, and liver injury in mice with ASH.
ABRO1 deficiency ameliorates chronic-plus-binge ethanol-induced mice liver injury. WT and Abro1−/− mice were subjected to chronic-plus-binge ethanol feeding for 10 days (N = 4–9). (A) Liver samples were stained with H&E or ORO, and ORO-positive areas were quantified. Scale bar, 100 μm. (B, C) Liver injury and steatosis were quantified by measuring serum ALT and hepatic triglyceride content. (D) Relative mRNA levels of hepatic pro-inflammatory cytokines. Immunohistochemistry (IHC) staining analysis of F4/80+ cells (E), Ly6G+ cells (F), and4-HNE expression (G) in the liver. Representative IHC images and quantification of positive areas were shown. Scale bar, 50 μm. Data are presented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001; two-tailed unpaired t-test.
BRCC3 deficiency alleviates alcoholic liver steatosis and injury
To further determine the importance of BRISC in alcohol-induced liver injury, we subjected Brcc3−/− mice to the chronic plus binge ethanol feeding model. Like ABRO1 deletion mice, Brcc3−/− mice fed with ethanol showed similar food consumption, body weight reduction, and liver/body weight ratio increase compared to WT controls (Supplementary Fig. 1D-F). BRCC3 deficiency attenuated alcohol-induced liver damage and steatosis, as evidenced by H&E and ORO staining and serum ALT measurement (Fig. 3A-B). Moreover, alcohol-induced upregulation of TNF-α, IL-6, CXCL1, and CXCL2 mRNA expression was inhibited by BRCC3 ablation (Fig. 3C). Taken together, our data suggest that BRISC activity may contribute to the development and progression of ASH.
BRCC3 deficiency alleviates alcoholic liver steatosis and injury. WT and Brcc3−/− mice were subjected to chronic-plus-binge ethanol feeding for 10 days (N = 3–9). (A) Liver samples were stained with H&E or ORO, and ORO-positive areas were quantified. Scale bar, 100 μm. (B) Liver injury was quantified by measuring serum ALT. (C) Relative mRNA levels of hepatic pro-inflammatory cytokines. Data are presented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001; two-tailed unpaired t-test.
Pharmacological inhibition of BRISC attenuates alcoholic liver steatosis and injury
We further evaluated whether pharmacological inhibition of BRISC activity has a protective effect against alcoholic liver injury. WT mice received chronic ethanol feeding for 4 weeks plus a single binge were treated every second day with 1 mg/kg thiolutin (THL), an inhibitor of BRISC19. The liver/body weight ratios of mice fed with alcohol diet or with control diet were not affected by THL injection (Supplementary Fig. 1G). As expected, THL admiration markedly alleviated alcohol-induced liver steatosis and damage, as indicated by H&E and ORO staining, serum ALT and hepatic TG quantification (Fig. 4A-C). The alcohol-induced hepatic TNF-α mRNA expression was impaired in THL-treated mice compared with the control mice (Fig. 4D). Moreover, the increase of Ly6G positive cells in the livers of alcohol-fed mice was attenuated by THL treatment (Fig. 4E). We also observed that the distribution of some Ly6G+ cells in control mice livers appeared to be in a cluster, which represented infiltrating and activated neutrophils; however, Ly6G+ cells were more evenly distributed in THL-treated mice (Fig. 4E). These data demonstrated that pharmacological inhibition of BRISC could achieve the extent of protection from ASH observed in Abro1−/− and Brcc3−/− mice.
Pharmacological inhibition of BRISC attenuates alcoholic liver steatosis and injury. Mice were subjected to chronic-plus-binge ethanol feeding for 4 weeks. Mice were treated with THL (1 mg/kg by intraperitoneal injection) or vehicle control (DMSO/PBS) (N = 3–7). (A) Liver samples were stained with H&E or ORO, and ORO-positive areas were quantified. Scale bar, 100 μm. (B, C) Liver injury and steatosis were quantified by measuring serum ALT and liver triglyceride content. (D) Hepatic TNF-α expression was evaluated by real-time PCR. (E) IHC staining analysis of Ly6G+ cells in liver. Representative IHC images and quantification of positive areas were shown; Scale bar, 50 μm. Data are presented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001; two-tailed unpaired t-test.
BRISC has limited effect on ethanol metabolism and alcohol-induced cytotoxicity in hepatocytes in vitro
To explore the potential mechanism by which BRISC regulates ASH progression, we first examined the involvement of BRISC in ethanol metabolism by detecting the expression of key ethanol metabolizing enzymes in liver tissues of mice fed with ethanol and pair-fed diet. The results showed that alcohol-induced CYP2E1 protein expression was comparable between the livers of WT mice and ABRO1 or BRCC3-deficient mice (Fig. 5A). Moreover, ABRO1 or BRCC3 deficiency has no effect on the protein expression of alcohol dehydrogenase 1 (ADH1) and aldehyde dehydrogenase 1A1 (ALDH1A1) in liver of mice no matter fed with alcohol or not (Fig. 5A). These data indicate that alcohol metabolism may not be altered in ABRO1 or BRCC3 deficient mice. In addition, the protein levels of endoplasmic reticulum (ER) stress markers CHOP and GRP78 and autophagy marker p62 were not affected by ABRO1 or BRCC3 deficiency under steady-state or alcohol stress conditions (Fig. 5A).
BRISC has limited effect on ethanol metabolism and alcohol-induced cytotoxicity in hepatocytes in vitro. (A) WT and Abro1−/− or Brcc3−/− mice were subjected to chronic-plus-binge ethanol feeding for 10 days. Western blot analysis of the indicated proteins in mice liver. (B) Primary hepatocytes from WT and Abro1−/− were stimulated with 100 mM ethanol for 48 h. The cell viability, LDH, and ROS production were analyzed. (C) The HepG2 cells were transduced with shCtrl or shABRO1 lentivirus and expression of ABRO1 was analyzed by western blot. (D) The HepG2 cells transduced with shCtrl or shABRO1 lentivirus were stimulated with 50 mM ethanol for 48–60 h. The cell viability, LDH and ROS release were analyzed. (E) The HepG2 cells transduced with shCtrl or shABRO1 lentivirus were stimulated with 400 mM ethanol for 6 h. Lipid droplet formation was detected by ORO staining; Scale bar, 50 μm. All experiments were replicated three times independently with cells. Data are presented as mean ± SEM; ***P < 0.001; two-tailed unpaired t-test.
We next investigated whether BRISC deficiency directly protects hepatocytes from alcohol-induced injury. Primary hepatocytes isolated from both Abro1−/− and WT mice showed significant increase of lactate dehydrogenase (LDH) release, reactive oxygen species (ROS) production, and cell death after ethanol treatment, but no differences were observed between these two genotypes (Fig. 5B). Similarly, in human hepatocellular carcinoma HepG2 cells, knockdown of ABRO1 did not affect ethanol-induced LDH release, ROS production, and cell death (Fig. 5C-D). Alcohol-induced lipid accumulation in HepG2 cells with stable ABRO1 knockdown was comparable with that in control cells (Fig. 5E). Together, these data suggest BRISC has a limited effect on alcohol-induced direct toxicity of hepatocytes.
BRISC deficiency suppresses alcohol-induced NLRP3 inflammasome activation in liver
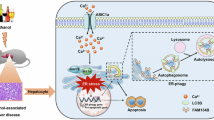
Our previous studies have demonstrated that BRISC is required for optimal activation of the NLRP3 inflammasome in BMDMs16. Considering that NLRP3 inflammasome plays a critical role in ALD progression, we further tested whether BRISC deficiency affects hepatic NLRP3 inflammasome activation induced by alcohol challenge. We first showed that alcohol-induced increase of serum LPS concentration was not affected by ABRO1 or BRCC3 deficiency, indicating that BRISC inactivation did not alter endotoxin translocated through the damaged gut mucosa after ethanol feeding (Fig. 6A). As expected, alcohol exposure significantly elevated NLRP3 and IL-1β mRNA expression in WT livers, but the increase was markedly blocked in Abro1−/− and Brcc3−/− livers (Fig. 6B-C). Accordingly, the protein levels of NLRP3 and pro-IL1β were reduced in the livers of Abro1−/− mice fed with alcohol diet (Fig. 6D-E). ABRO1 deficiency also decreased the levels of cleaved caspase 1, IL-1β, and GSDMD induced by alcohol diet feeding (Fig. 6D-E). These data suggest that BRISC may be involved in NLRP3 inflammasome activation in liver following alcohol exposure. As KCs are the main IL-1β-producing cells in ALD, we thus examined whether BRISC affects DAMPs-driven activation of NLRP3 inflammasome in KCs. Abro1−/− and control KCs were primed with LPS and then stimulated with the conditioned media derived from in vitro cultured primary hepatocytes from WT mice fed with ethanol diet. The results showed that the IL-1β production was significantly lower in Abro1−/− KCs than in control KCs (Fig. 6F). BRISC-mediated K63-linked deubiquitination of NLRP3 is critical for optimal NLRP3 activaiton16. We found that feeding with ethanol diet led to a potent decrease of K63-linked ubiquitination in KCs, while this decrease was blocked by ABRO1 deficiency (Fig. 6G). Collectively, these results suggest that inactivation of BRISC inhibits DAMPs-mediated NLRP3 inflammasome activation in KCs during ALD.
BRISC deficiency suppresses alcohol-induced NLRP3 inflammasome activation in liver. (A-E) WT and Abro1−/− or Brcc3−/− mice were subjected to chronic-plus-binge ethanol feeding for 10 days. Serum LPS levels were analyzed using ELISA (A). Hepatic NLRP3 and IL-1β expression was evaluated by real-time PCR (B, C). Western blot analysis of the indicated proteins in mice liver (D, E). (F) WT and Abro1−/− KCs were primed with 500 ng/ml LPS for 4 h, then stimulated with the conditioned media from in vitro cultured primary hepatocytes of ethanol diet-fed WT mice for 30 min. The IL-1β level in the culture supernatants was measured. (G) K63-linked ubiquitination of NLRP3 in KCs isolated from mice livers fed with ethanol or control diet. The KCs from each group were pooled from three mice. Data are presented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001; two-tailed unpaired t-test.
Discussion
In this study, we demonstrated, for the first time to our knowledge, that global deficiency of ABRO1 and BRCC3 protected mice from alcohol-induced liver inflammation and steatosis. As ABRO1 and BRCC3 are two essential subunits of BRISC, our results thus supported that BRISC has an important role in the pathological process of ALD. Moreover, pharmacological inhibition of BRISC activity had protective effects on ALD. Both the expression of ABRO1 and BRCC3 were increased in liver tissues of AH animal models and patients with AH, suggesting that BRISC is correlated with the pathogenesis and progression of ALD. Based on these findings, we conclude that BRISC may be a potential therapeutic target for ALD treatment.
Accumulating evidence indicates that DUBs are involved in the pathogenesis of multiple liver diseases, especially NAFLD23,24,25. Many DUBs of the Ubiquitin-specific proteases (USPs) family have been reported to play essential roles in the progression of NAFLD. Among them, hepatic USP2, 11, 14, 15, 20, and 33 exacerbate NAFLD, while USP4, 10, 13, 18, and CYLD in hepatocytes have restorative effects23,26,27,28,29. Besides, a JAMM family DUB RPN11 was recently shown to have deteriorative effects on NAFLD30. In contrast, the roles of DUBs in ALD are much less known. Up till now, there are just a few DUBs that have been clearly confirmed to contribute to ALD. For example, USP22 ameliorates chronic ALD by deubiquitinating BRD431. In the present study, we revealed BRISC, a JAMM family DUB, positively regulated alcohol-induced hepatic steatosis and inflammation, thus contributing to ALD progression. Importantly, our results demonstrated that THL, a chemical inhibitor of BRISC enzyme activity, effectively alleviated alcohol-induced liver injury in ALD mice model. Overall, this study extended our understanding of DUBs function in ALD and provided a novel therapeutic strategy for the treatment of ALD. As most of DUBs can be drugged according to their cysteine proteases or metalo proteases, DUBs are considered to be promising drug targets in various diseases32,33,34. It is worth carrying out further studies to broadly explore the roles of DUBs in ALD pathogenesis and progression.
BRISC is ubiquitously expressed and functions in various types of cells, including macrophages, hematopoietic stem cells, endothelial cells, vascular smooth muscle cells, and cardiomyocytes16,35,36,37,38. Our recent study has shown that BRISC is expressed in hepatocytes and KCs. However, BRISC deficiency did not affect the responses of hepatocytes to TNF-α21. Here, we also found that BRISC had a very limited effect on ethanol metabolism and alcohol-induced cytotoxicity in hepatocytes. Thus, the physiological role of BRISC in hepatocytes is still unrevealed. BRISC deficiency impaired LPS-induced TLR4/NF-κB pathway activation and inflammatory factors production in KCs21. Moreover, BRISC mediates NLRP3 deubiquitination and promotes the activation of NLRP3 inflammasome in macrophages16. In line with these results, our study showed that lack of BRISC inhibited the expression of NF-κB target genes, including TNF-α, IL-6, CXCL1, CXCL2, NLRP3, and IL-1β, in liver of mice fed with alcohol. During ALD, translocated LPS derived from the gut microflora provides the first signal for TLR4-mediated upregulation of inflammatory mediators5, while we did not detect an obvious difference in circulating LPS concentration between BRISC-deficient and WT mice after alcohol exposure. This excluded the possibility that the decreased expression of hepatic proinflammatory factors is because of global deficiency of BRISC reduced endotoxin translocated through the damaged gut mucosa, thus BRISC deletion may attenuate the response of liver cells to PAMPs/DAMPs. As KCs are the main proinflammatory cytokines-producing cells in ALD8, we further detected the effect of BRISC deficiency on DAMPs-induced KCs activation and found that the production of IL-1β in KCs triggered by DAMPs from alcohol-treated hepatocytes was reduced by BRISC deficiency. Besides, IL-1β cleavage induced by alcohol challenge was prevented in BRISC-deficient liver. These data suggest that BRISC may be involved in the regulation of TLR4-induced inflammatory factors expression and NLRP3 inflammasome activation in KCs following alcohol exposure.
Previous studies have demonstrated that TLR4 signaling and NLRP3 inflammasome play important roles in the initiation and development of ALD. Alcohol consumption results in leakage of gut-derived LPS, leading to TLR4 signaling activation in liver macrophages and the subsequent increase of hepatic inflammatory responses5. TLR4 ablation was shown to protect mice from early alcohol-induced injury39. The NLRP3 inflammasome exerts its pathogenic role involving the maintenance of macrophages in an inflammatory state, upregulation of hepatocyte lipid synthesis, activation of hepatic stellate cells, and sensitivity of hepatocytes to TNF-α-induced cytotoxicity10. Mice lacking NLRP3, caspase 1, ASC, or IL-1R1 displayed attenuated liver injury in ASH mice model13,14. In vivo administration of MCC950, a specific NLRP3 inhibitor, reduced steatohepatitis in a murine model of AH40. Thus, pharmacological inhibition of TLR4 signaling and NLRP3 inflammasome activation may be an attractive therapeutic strategy to treat ALD. Our previous work has reported that BRISC is essential for TLR4 signaling activation in KCs and promotes NLRP3 inflammasome activation in BMDMs16,21. Here, we also showed that BRISC contributed to alcohol-induced hepatic proinflammatory factors expression and NLRP3 inflammasome activation. Thus, BRISC inactivation could inhibit both TLR4 signaling and NLRP3 inflammasome in liver, which can be developed as a novel therapeutic approach for ALD. In fact, the present and our previous work showed that BRISC inhibitor THL effectively alleviated MCD diet-induced NAFLD, LPS/D-GalN-induced acute liver failure, and ALD19,21. Collectively, these data suggested that BRISC may serve as a promising therapeutic target in the treatment of various liver inflammatory diseases.
In conclusion, our present findings reveal that BRISC acts as a positive regulator of the progression of ethanol-induced liver injury. Strategies for pharmacological targeting BRISC are likely to be a relevant therapeutic approach to treat ALD.
Materials and methods
Mice
Wild type C57BL/6 mice were obtained from the Beijing Vital River Laboratory Animal Technology Co., Ltd (Beijing, China). Abro1−/− and Brcc3−/− mice were described previously16. All mice were housed in a controlled environment of specific pathogen-free conditions at the Animal Facility of Laboratory Animal Center of Beijing Institute of Radiation Medicine. Male mice between 8 and 10 weeks of age were used in the study.
Ethics Statement
The animal experiments conducted in this study underwent the review process and received approval from the Institutional Animal Care and Use Committee of Beijing Institute of Radiation Medicine. All methods were performed in accordance with relevant guidelines and regulations. Anesthesia was induced by intraperitoneal injection of sodium pentobarbital (50 mg/kg). The animals were euthanized by cervical dislocation after anesthesia. The study is reported in accordance with ARRIVE guidelines.
Mouse model of chronic-plus-binge ethanol feeding
The mouse model of chronic plus binge ethanol feeding were established according to a previous study with minor adjustments22. Mice were fed a control Lieber-DeCarli ad libitum diet (Dyets, D710260) for 5 days, then divided into two groups: ethanol (EtOH)-fed groups, which were given free access to the Lieber-DeCarli diet containing 5% (v/v) ethanol for the specified periods; and control groups, which were pair-fed with an isocaloric control diet for the same durations. On the final day, both ethanol-fed and pair-fed mice were gavaged between 7:00 am and 9:00 am with a single dose of either ethanol (5 g/kg body weight) or isocaloric maltose dextrin, respectively. 9 h later, the mice were euthanized. The liquid diet served as the sole source of food and water, eliminating the need for additional diets or drinking water.
BRISC inhibitor treatment
For a period of 4 weeks, wild-type mice were administered the Lieber-DeCarli ad libitum diet and concurrently treated with either a single dose of the small molecule inhibitor of BRISC complex, thiolutin (THL, 1 mg/kg, Cayman, 11350) or a vehicle control (DMSO/PBS) intraperitoneally. Injections of THL or vehicle control commenced on the second day following the 5% (v/v) ethanol feeding and continued every other day for the duration of the 4-week period. On the final day, 2 h after THL or vehicle control treatment, the mice were gavaged with a single dose of either ethanol (5 g/kg body weight) or maltose, and then sacrificed 9 h after the gavage procedure.
Clinical datasets analysis
The datasets were retrieved from the Gene Expression Omnibus (GEO) repository (GSE28619) and processed using oligo package for published clinical datasets analysis. The raw data underwent normalization through the robust multiarray average (RMA) technique41.
Biochemical assays
Serum alanine aminotransferase (ALT) levels were determined in accordance with the primary reference procedures established by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) at the Beijing CIC Clinical Laboratory (Beijing, China). The assessment of liver triglyceride content were carried out utilizing a triglyceride assay kit (Applygen Technologies, E1013). Quantification of total protein in the liver was accomplished with the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, 23227).
Western blotting
Proteins were separated by SDS-PAGE and subsequently transferred from the gel onto the PVDF membranes. The membranes were then incubated with the specified primary antibodies. The immune complexes formed on the membrane were detected using HRP conjugated secondary antibodies. For visualization, the immune complex on PVDF membranes were detected using the SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, A43840) and captured on X-ray films. The list of primary antibodies utilized in this study is provided in Supplementary Table 1.
Assay of NLRP3 ubiquitination
Ten days after chronic-plus-binge ethanol feeding, KCs were isolated from WT and Abro1−/− mice and then homogenized in lysis buffer (0.1%SDS, 150mM NaCl, 100mM pH 8.0 tris-HCl, 2 mM EDTA, and 1% Triton X-100) with 50 µM N-ethymaleimide (Sigma-Aldrich, E3876) and protease inhibitor (Roche, 04693116001). The lysates were sonicated and centrifugation at 12,000 g for 30 min at 4 °C. Supernatants were gently shaken 4 h at 4 °C with 2 µg of anti-NLRP3 antibody followed by protein A/G agarose beads (Santa Cruz Biotechnology, sc-2003) for 6 h. The beads were washed with the lysis buffer 3 times by centrifugation at 300 g at 4 °C and lysed in an SDS samples buffer and boiled for 10 min. The samples were then subjected to western blotting analysis.
Liver histology and immunohistochemical staining
A certain size of liver tissue was excised, preserved in 4% buffered formalin for at least 48 h, and subsequently embedded in paraffin. The tissue sections were used for Hematoxylin and Eosin (H&E) staining and immunohistochemical analysis for Ly6G (Abcam, ab25377) and F4/80 (Abcam, ab111101). To assess the presence of 4-Hydroxynonenal, immunohistochemical staining was performed using the anti-4-Hydroxynonenal antibody (Abclonal, A26085). Fresh liver tissues were rapidly frozen in OCT embedding medium for subsequent Oil Red O (ORO) staining. For each sample, positive cells or areas were analyzed by randomly selecting and examining three fields under either ×200 or ×400 magnification. This process was repeated for at least three random fields per slide to ensure comprehensive analysis. The captured images of the tissue sections were recorded utilizing a Nikon Digital Sight DS-U3 camera, and the subsequent image analysis was facilitated by the Image Pro Plus v6.0 software (Media Cybernetics, Inc.).
Cell isolation and culture
Primary hepatocytes and KCs were extracted following a previously established protocol21. Following a two-step collagenase perfusion procedure, the single-cell suspension was centrifuged at 50 g for 5 min at 4 °C. The pellet was resuspended in 10 ml of 50% Percoll solution (GE, 17089102) and subjected to centrifugation at 50 g for 10 min at 4 °C, without applying the brake. The hepatocyte-containing pellet was then collected, washed twice with PBS, and then cultured in hepatocyte-specific medium (Gibco, 17705021) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S).
For the isolation of KCs, the supernatant was subjected to high-speed centrifugation at 600 g for 10 min at 4 °C. The precipitated cells were resuspended in 3 ml of 24% OptiPrep (Axis-Shield, AS1114542) and layered over a gradient of OptiPrep solutions (17.6%, 8.4%, and 0%). The solutions were then centrifuged at 1420 g for 20 min at 4 °C without the brake. The KC-rich fraction, located at the interface between the 8.4% and 17.6% OptiPrep layers, was carefully aspirated, washed with ice-cold sorting buffer, and pelleted by centrifugation at 600 g for 10 min at 4 °C. These cells were further refined using MACS technology, targeting the F4/80 surface marker (Miltenyi Biotec, 130-110-443), as per the manufacturer’s guidelines. Finally, the purified KCs were verified via flow cytometry and cultured in DMEM containing 20% heat-inactivated FBS and 20 ng/ml M-CSF (Peprotech, 315-02).
HepG2 cells were maintained in DMEM supplemented with 10% FBS and 1% P/S. Cells were incubated in a humidified chamber containing 5% CO2 at 37 °C for growth. HepG2 cell line with stable knockdown of ABRO1 was established by lentivirus-mediated short hairpin RNA (shRNA) targeting human ABRO1 as described in our previous study16.
Activation of NLRP3 inflammasome in Kupffer cells
Ten days after chronic-plus-binge ethanol feeding, primary hepatocytes were isolated from WT mice and incubated for 48 h in 6-well plates. The culture media were then collected and cell debris was removed by centrifugation at 300 g for 10 min. Primary KCs from WT and Abro1−/− mice were isolated and cultured in 24-well plates. The KCs were primed with 500 ng/ml LPS (InvivoGen, tlrl-pb5lps) for 4 h, followed by washing with PBS. Subsequently, the KCs were treated with 0.2 ml of conditioned medium collected from in vitro cultured primary hepatocytes. After 30 min, the culture media were collected, and the levels of IL-1β in the media were quantified. The IL-1β concentration in the culture supernatants was determined using a Mouse IL-1β ELISA Kit (Abcam, ab197742).
Cell viability, LDH release, and ROS release analysis
Primary hepatocytes and HepG2 cells were seeded in 96-well plates and stimulated with 50 mM or 100 mM ethanol for 48–60 h. Cell viability, lactate dehydrogenase (LDH) release, and reactive oxygen species (ROS) production were measured using the following kits, following the manufacturers’ guidelines: Cell Titer-Glo Luminescent Cell Viability Assay (Promega, G7570), LDH Cytotoxicity Assay Kit (Beyotime, C0017), and DCFDA/H2DCFDA-Cellular ROS Assay Kit (Abcam, ab113851).
Oil Red O staining of HepG2 cells
The HepG2 cells were seeded in 24-well plates and stimulated with 50 mM ethanol for 48 h. Then HepG2 cells were fixed using 4% paraformaldehyde solution and subsequently stained with ORO (Solarbio, G1260) and Cole’s Hematoxylin Solution (Solarbio, G1140).
Real-time PCR
Total RNA was extracted from the liver tissue using the TRIzol reagent (Thermo Fisher Scientific, 15596026). Subsequently, 4 µg of RNA was reverse-transcribed into cDNA employing the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, K1622). Real-time PCR amplification was conducted on the Light Cycler 480 I instrument (Roche) with SYBR Green Master mix (TOYOBO, QPK-212) as the detection agent. The gene-specific primers utilized were designed based on sequences available in Primer Bank and are detailed in Supplementary Table 2.
Statistical analysis
All data were expressed as means ± SEM. The Kolmogorov-Smirnov test was applied to assess the distribution of variables. For single comparisons, a standard two-tailed unpaired Student’s t-test was conducted. In cases where multiple comparisons were necessary, either one-way or two-way analysis of variance (ANOVA) was utilized. Statistics were performed using GraphPad Prism version 9.0. P-value < 0.05 were considered statistically significant.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Seitz, H. K. et al. Alcoholic liver disease. Nat. Rev. Dis. Primers. 4, 16. https://doi.org/10.1038/s41572-018-0014-7 (2018).
Mackowiak, B., Fu, Y., Maccioni, L. & Gao, B. Alcohol-associated liver disease. J. Clin. Invest. 134 https://doi.org/10.1172/jci176345 (2024).
Pohl, K., Moodley, P. & Dhanda, A. D. Alcohol’s Impact on the Gut and Liver. Nutrients 13 https://doi.org/10.3390/nu13093170 (2021).
Yan, C. et al. Pathogenic mechanisms and regulatory factors involved in alcoholic liver disease. J. Transl Med. 21, 300. https://doi.org/10.1186/s12967-023-04166-8 (2023).
Wu, X. et al. Recent advances in understanding of Pathogenesis of Alcohol-Associated Liver Disease. Annu. Rev. Pathol. 18, 411–438. https://doi.org/10.1146/annurev-pathmechdis-031521-030435 (2023).
Singal, A. K., Shah, V. H. & Malhi, H. Emerging targets for therapy in ALD: lessons from NASH. Hepatology 80, 223–237. https://doi.org/10.1097/hep.0000000000000381 (2024).
Wang, X., Wang, J., Peng, H., Zuo, L. & Wang, H. Role of immune cell interactions in alcohol-associated liver diseases. Liver Res. 8, 72–82. https://doi.org/10.1016/j.livres.2024.06.002 (2024).
Slevin, E. et al. Kupffer cells: inflammation pathways and cell-cell interactions in Alcohol-Associated Liver Disease. Am. J. Pathol. 190, 2185–2193. https://doi.org/10.1016/j.ajpath.2020.08.014 (2020).
Zeng, T., Zhang, C. L., Xiao, M., Yang, R. & Xie, K. Q. Critical roles of Kupffer Cells in the Pathogenesis of Alcoholic Liver Disease: from Basic Science to clinical trials. Front. Immunol. 7, 538. https://doi.org/10.3389/fimmu.2016.00538 (2016).
Brahadeeswaran, S., Dasgupta, T., Manickam, V., Saraswathi, V. & Tamizhselvi, R. NLRP3: a new therapeutic target in alcoholic liver disease. Front. Immunol. 14, 1215333. https://doi.org/10.3389/fimmu.2023.1215333 (2023).
Knorr, J., Wree, A., Tacke, F. & Feldstein, A. E. The NLRP3 inflammasome in alcoholic and Nonalcoholic Steatohepatitis. Semin Liver Dis. 40, 298–306. https://doi.org/10.1055/s-0040-1708540 (2020).
Szabo, G. & Petrasek, J. Inflammasome activation and function in liver disease. Nat. Rev. Gastroenterol. Hepatol. 12, 387–400. https://doi.org/10.1038/nrgastro.2015.94 (2015).
Petrasek, J. et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Invest. 122, 3476–3489. https://doi.org/10.1172/jci60777 (2012).
Shen, Y. et al. MLKL deficiency alleviates acute alcoholic liver injury via inhibition of NLRP3 inflammasome. Toxicology 506, 153864. https://doi.org/10.1016/j.tox.2024.153864 (2024).
Zeqiraj, E. et al. Higher-Order Assembly of BRCC36-KIAA0157 is required for DUB activity and biological function. Mol. Cell. 59, 970–983. https://doi.org/10.1016/j.molcel.2015.07.028 (2015).
Ren, G. et al. ABRO1 promotes NLRP3 inflammasome activation through regulation of NLRP3 deubiquitination. Embo j. 38 https://doi.org/10.15252/embj.2018100376 (2019).
Py, B. F., Kim, M. S., Vakifahmetoglu-Norberg, H. & Yuan, J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol. Cell. 49, 331–338. https://doi.org/10.1016/j.molcel.2012.11.009 (2013).
Rabl, J. BRCA1-A and BRISC: multifunctional molecular machines for Ubiquitin Signaling. Biomolecules 10 https://doi.org/10.3390/biom10111503 (2020).
Ren, G. M. et al. Pharmacological targeting of NLRP3 deubiquitination for treatment of NLRP3-associated inflammatory diseases. Sci. Immunol. 6 https://doi.org/10.1126/sciimmunol.abe2933 (2021).
Zheng, H. et al. A BRISC-SHMT complex deubiquitinates IFNAR1 and regulates interferon responses. Cell. Rep. 5, 180–193. https://doi.org/10.1016/j.celrep.2013.08.025 (2013).
Zhang, W. et al. BRISC is required for optimal activation of NF-κB in Kupffer cells induced by LPS and contributes to acute liver injury. Cell. Death Dis. 14, 743. https://doi.org/10.1038/s41419-023-06268-z (2023).
Bertola, A., Mathews, S., Ki, S. H., Wang, H. & Gao, B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 8, 627–637. https://doi.org/10.1038/nprot.2013.032 (2013).
Kitamura, H. Ubiquitin-specific proteases (USPs) and metabolic disorders. Int. J. Mol. Sci. 24 https://doi.org/10.3390/ijms24043219 (2023).
Lv, X. Y., Duan, T. & Li, J. The multiple roles of deubiquitinases in liver cancer. Am. J. Cancer Res. 10, 1647–1657 (2020).
Aryapour, E. & Kietzmann, T. Mitochondria, mitophagy, and the role of deubiquitinases as novel therapeutic targets in liver pathology. J. Cell. Biochem. 123, 1634–1646. https://doi.org/10.1002/jcb.30312 (2022).
Xu, M. et al. The deubiquitinating enzyme 13 retards non-alcoholic steatohepatitis via blocking inactive rhomboid protein 2-dependent pathway. Acta Pharm. Sin B. 13, 1071–1092. https://doi.org/10.1016/j.apsb.2022.12.006 (2023).
Baek, J. H. et al. Ablation of the deubiquitinase USP15 ameliorates nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Exp. Mol. Med. 55, 1520–1530. https://doi.org/10.1038/s12276-023-01036-7 (2023).
Ke, X., Hu, H., Peng, Q., Ying, H. & Chu, X. USP33 promotes nonalcoholic fatty acid disease-associated fibrosis in gerbils via the c-myc signaling. Biochem. Biophys. Res. Commun. 669, 68–76. https://doi.org/10.1016/j.bbrc.2023.05.100 (2023).
Ji, Y. X. et al. The deubiquitinating enzyme cylindromatosis mitigates nonalcoholic steatohepatitis. Nat. Med. 24, 213–223. https://doi.org/10.1038/nm.4461 (2018).
Zhou, B. et al. Amelioration of nonalcoholic fatty liver disease by inhibiting the deubiquitylating enzyme RPN11. Cell. Metab. https://doi.org/10.1016/j.cmet.2024.07.014 (2024).
Yan, R. et al. Ubiquitin-specific protease 22 ameliorates chronic alcohol-associated liver disease by regulating BRD4. Pharmacol. Res. 168, 105594. https://doi.org/10.1016/j.phrs.2021.105594 (2021).
Lei, H., Wang, J., Hu, J., Zhu, Q. & Wu, Y. Deubiquitinases in hematological malignancies. Biomark. Res. 9, 66. https://doi.org/10.1186/s40364-021-00320-w (2021).
Lange, S. M., Armstrong, L. A., Kulathu, Y. & Deubiquitinases From mechanisms to their inhibition by small molecules. Mol. Cell. 82, 15–29. https://doi.org/10.1016/j.molcel.2021.10.027 (2022).
Harrigan, J. A., Jacq, X., Martin, N. M. & Jackson, S. P. Deubiquitylating enzymes and drug discovery: emerging opportunities. Nat. Rev. Drug Discov. 17, 57–78. https://doi.org/10.1038/nrd.2017.152 (2018).
Donaghy, R. et al. The BRISC deubiquitinating enzyme complex limits hematopoietic stem cell expansion by regulating JAK2 K63-ubiquitination. Blood 133, 1560–1571. https://doi.org/10.1182/blood-2018-10-877563 (2019).
Miskinyte, S. et al. Loss of BRCC3 deubiquitinating enzyme leads to abnormal angiogenesis and is associated with syndromic moyamoya. Am. J. Hum. Genet. 88, 718–728. https://doi.org/10.1016/j.ajhg.2011.04.017 (2011).
Shen, H. et al. BRCC3 regulation of ALK2 in vascular smooth muscle cells: implication in Pulmonary Hypertension. Circulation 150, 132–150. https://doi.org/10.1161/circulationaha.123.066430 (2024).
Wang, T. et al. ABRO1 arrests cardiomyocyte proliferation and myocardial repair by suppressing PSPH. Mol. Ther. 31, 847–865. https://doi.org/10.1016/j.ymthe.2023.01.011 (2023).
Uesugi, T., Froh, M., Arteel, G. E., Bradford, B. U. & Thurman, R. G. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology 34, 101–108. https://doi.org/10.1053/jhep.2001.25350 (2001).
de Carvalho Ribeiro, M. et al. Alcohol-induced extracellular ASC specks perpetuate liver inflammation and damage in alcohol-associated hepatitis even after alcohol cessation. Hepatology 78, 225–242. https://doi.org/10.1097/hep.0000000000000298 (2023).
Irizarry, R. A. et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264. https://doi.org/10.1093/biostatistics/4.2.249 (2003).
Funding
This work was supported by grants from the National Key Research and Development Program of China (2020YFA0113500), the National Natural Science Foundation of China (82470633, 82200701), Beijing Nova Program (20220484086).
Author information
Authors and Affiliations
Contributions
T.W. and W.Z. contributed most experiments with the assistance of K.L. and G.-M.R.; S.-S.X., Y.-Q.Z., H.C., and H.-Y.G. contributed reagents and materials. C.-Y.L., M.Y., and K.Z. helped to design the experiments and contributed methodology; T.W., W.Z., and X.L. analyzed the data. R.-H.Y. and T.W. designed the experiments. X.-M.Y., R.-H.Y., and T.W. wrote the manuscript. X.-M.Y. and R.-H.Y. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, T., Zhang, W., Liu, X. et al. BRISC inactivation alleviates alcohol-induced liver injury in mice. Sci Rep 15, 5154 (2025). https://doi.org/10.1038/s41598-025-89796-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-89796-2