Abstract
Melatonin is known to delay postharvest nutrient loss. However, its specific roles in regulating cell wall and sugar metabolism to maintain postharvest quality in flowering Chinese cabbage remain unclear. In this study, postharvest flowering Chinese cabbage was treated with melatonin (100 µmol L− 1) to investigate the cell wall and sugar metabolisms. Our research found that melatonin supplementation increased levels of important nutrients like ascorbic acid, sugars, soluble protein, carotenoid, and glucosinolates. It also enhanced various cell wall components (cellulose, hemicellulose, lignin, and protopectin) while reducing water-soluble pectin accumulation. Melatonin helped stabilize cell wall by reducing the expression of genes related to cell wall degradation (BrBGAL4, BrPG, BrCEL3, and BrPME3), inhibiting activities of cell wall-degrading enzymes like pectin methylesterase and cellulase, and improving expressions of cellulose biosynthesis-related genes (BrCesA1, BrCesA3.1, and BrCesA3.2). Additionally, it boosted sugar accumulation by increasing activities of sucrose synthetase and sucrose phosphate synthetase, upregulating expressions of BrSUS1, BrSUS5, BrSPS1F, BrSPS3F, BrSPS4F, and suppressing activities of neutral invertase and acidic invertase and expressions of genes involved in sugar metabolism (BrSUS3, BrINV, BrSWEET2, BrSWEET4, and BrSWEET17). This study highlights melatonin’s vital roles in maintaining postharvest quality and offers practical insights for improving market performance of flowering Chinese cabbage.
Similar content being viewed by others
Introduction
Flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis Tsen et Lee) is a popular cruciferous vegetable, which is commercially cultivated in Southeast Asia1. It is esteemed for their nutritional richness and bioactive constituents, including vitamins, amino acids, sugars, carotenoids, flavonoids, and glucosinolates2. However, as an edible part of flowering Chinese cabbage, the tender leaves and stems are prone to cause water lose, yellowing, wilting and rapid degradation of nutrients after harvest, which seriously affect the postharvest quality and commercial value3,4. Therefore, it is an issue of concern to maintain the stability of nutritional constituents in harvested flowering Chinese cabbage.
The structural integrity of plant cell walls is pivotal, significantly influencing the quality of agricultural products. Extensive researches have demonstrated the crucial role of enzymes involved in cell wall degradation, such as cellulase (Cx), polygalacturonase (PG), and pectin methylesterase (PME), in modifying cell wall structure and maintaining postharvest quality in crops5,6. Alterations and degradation of cell walls impact the firmness and crispness of fruits during senescence and ripening7, with fruit softening closely linked to the regulation of cellulose, hemicellulose, and pectin by enzymes such as Cx, PG, PME, and β-glucosidase (β-Glu)8. Studies have manifested that inhibiting softening of fruit, sustaining higher firmness, and repressing the activities of β-Glu, PME, PG, and Cx, as well as the transcripts of cell wall degradation genes (Miβ-glu, MiPME, MiPG14, and MiCel) in mango, significantly improve postharvest quality9. Additionally, the research reveals the involvement of cell wall degradation and lignin accumulation in the aging process of Chinese flowering cabbage10. Cellulose, a predominant component of primary and secondary cell walls in plant, is synthesized by cellulose synthase11. However, the effects of regulating cellulose biosynthesis-related genes on the postharvest quality of horticultural products remain largely understudied. Further advancement in the systematic analysis of cell wall metabolism and its impacts on postharvest quality in flowering Chinese cabbage are imperative.
Sugar serves not only as a critical quality determinant in agricultural products but also as a signaling molecule involved in various biological processes12. Numerous studies indicated that enhancing sugar metabolism leads to superior flavor and quality in horticultural crops due to increased soluble sugar accumulation13,14. Sugar metabolism is regulated by a range of enzymes, such as sucrose synthetase (SS), sucrose phosphate synthetase (SPS), neutral invertase (NI), and acid invertase (AI)15,16,17. In sugarcane, the contents of sucrose and fructose correlate positively with the activity of AI, NI, and SS, whereas the content of glucose exhibits opposite trends18. NI and AI are pivotal for soluble sugar accumulation, and changes in SS activity correspond with alterations in glucose content in pea sprouts16. Studies indicated that sucrose content correlates with SPS activity, with SPS activity primarily regulated post-transcriptionally. Glucose/fructose content is influenced by vacuolar invertase (VIN) activity, and the transcript of IbVIN1 correlates with VIN activity and sweetness index, suggesting IbVIN1 as a potential candidate gene in sweetpotato varieties with high sweetness19. Sugars significantly contribute to the flavor and sweetness of fleshy horticultural products, with their allocation and translocation largely dependent on sugar transporters such as SUTs and SWEETs20. SUTs, primarily locate in the plasma membrane, are involved in phloem loading, unloading, and carbohydrate distribution21. CitERF16 serves as a positive regulator, promoting sucrose accumulation by trans-activating CitSWEET11 expression in citrus. Additionally, overexpression of the pear sugar transporter protein gene, PbSWEET4, leads to decreased leaf sugar content and chlorophyll, resulting in leaf senescence22. Furthermore, the potato sugar transporter protein StTST1 regulates sugar accumulation in stem pieces during storage23. However, our understanding of the relationship between changes in postharvest quality and sugar metabolism in flowering Chinese cabbage remains limited.
Melatonin (n-acetyl-5-methoxytryptamine) is an indole amine that plays a crucial positive role in regulating the maturity and senescence to sustain the postharvest quality of horticultural products24. For example, the application of melatonin delays fruit softening by repressing the activities of β-Glu, PME, Cx, and PG and the content of water-soluble pectin, maintaining production of water-insoluble pectin25,26, and downregulating the transcriptional expressions of related genes like PE, PG, BG6, PME, Cx, and GAL127. Moreover, melatonin treatment increases the accumulation of sugars including soluble sugar, fructose, glucose, and sucrose by modulating the activities of SPS, SS, NI and AI, enzymes as well as their transcriptions, thereby delaying the senescence and maintaining quality of kiwi berry during storing period28. However, melatonin treatment reduces NI activity and gene expression, while increasing both SPS and SS activities and gene expression in postharvest pear fruit29. Nevertheless, few studies are available on the postharvest quality of melatonin involved in regulating cellulose biosynthesis-related genes and sugar transporters (SWEETs) in horticultural crops. Therefore, the regulatory mechanisms of melatonin involved in regulating postharvest quality through the cell wall metabolism and sugar transport still need to be further improved. To date, the studies on melatonin in harvested flowering Chinese cabbage are mainly focused on the metabolism and regulation of reactive oxygen species and phytohormone hormones30,31. Our recent study has shown that melatonin can delay senescence by inhibiting flavonoid degradation of harvested flowering Chinese cabbage4, but it is vague whether it impacts the metabolism of cell walls and sugar.
The objective of this work is to examine the influence of melatonin on cell wall and sugar metabolisms in harvested flowering Chinese cabbage. Our outcomes demonstrated that melatonin upregulated the expression of sugar biosynthesis-related genes (BrSUS1, BrSUS5, BrSPS1F, BrSPS3F, and BrSPS4F) and inhibited the expression of sugar metabolism-related genes ( BrSUS3, BrINV, BrSWEET2, BrSWEET4, and BrSWEET17), leading to the downregulation of cell wall degradation-related genes (BrBGAL4, BrPG, BrCEL3, and BrPME3) and the upregulation of cellulose biosynthesis-related genes (BrCesA1, BrCesA3.1, and BrCesA3.2), thereby maintaining cell wall stability and postharvest quality. These findings can provide valuable insights into the mechanisms by which melatonin contributes to maintaining postharvest quality through modulation of cell wall and sugar metabolisms in flowering Chinese cabbage.
Results
Melatonin delayed the degradation of soluble protein, carotenoid, ascorbic acid, and glucosinolates in postharvest cabbage
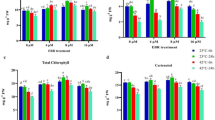
The changes in nutritional quality significantly influence the commercial value of postharvest agricultural products. Our previous studies have demonstrated the ability of melatonin to delay aging of harvested flowering Chinese cabbage (Fig. S1)4. Building upon this foundation, we further investigated the effects of melatonin on nutritional quality parameters, including carotenoid, soluble protein, ascorbic acid, and glucosinolates contents in postharvest flowering Chinese cabbage. As depicted in Fig. 1A-D, after storing for 20 d, compared to 0 d, the contents of carotenoid, soluble protein, and ascorbic acid in the control decreased by 32.64%, 35.68%, and 64.21%, respectively, whereas the content increased by 7.61%. The levels of carotenoid, soluble protein, and glucosinolates in the melatonin-treated samples did not significantly differ from the control during the initial 0 to 5 d of storage. However, the content of ascorbic acid increased by 26.16% during this period. Subsequently, after 10, 15, and 20 d of storage under melatonin treatment, notable increases were observed in carotenoid content by 21.89%, 29.98%, and 50.57%, soluble protein content by 27.78%, 33.24%, and 44.59%, ascorbic acid by 24.21%, 58.22%, and 61.04%, and glucosinolates content by 33.04%, 29.48%, and 52.25% compared to control, respectively. These findings suggested that soluble protein, carotenoid, and ascorbic acid degraded gradually during storage, while glucosinolates levels displayed an increasing trend. Melatonin exhibited a capacity to impede the degradation of carotenoid, soluble protein, and ascorbic acid during storage while promoting the accumulation of glucosinolates.
Effect of melatonin on quality of harvested flowering Chinese cabbage. (A) Carotenoid content. (B) Soluble protein content. (C) Ascorbic acid content. (D) Glucosinolate content. Control: Double distilled water. Melatonin: 100 µmol L− 1 melatonin treatment. The vertical bars represent the standard error of the mean (n = 3). The asterisk above the column represents a significant difference of P < 0.05.
The effects of melatonin on cell wall metabolism
Analysis of cell wall components under melatonin treatment
The degradation of cell walls significantly influences product quality. Apart from the consistent increase in water-soluble pectin, the levels of cellulose, hemicellulose, lignin, and protopectin were observed to decrease in the control group over the course of 20 d of storage (Fig. 2A-E). After 20 d of storage, the contents of cellulose, hemicellulose, lignin, and protopectin in the control decreased by 65.75%, 53.60%, 39.64%, and 40.92% compared to 0 d of storage, respectively, while the water-soluble pectin content increased by 76.27%. These findings indicated that the cellulose, hemicellulose, lignin, and protopectin were decomposed during storage, while water-soluble pectin exhibited continuous accumulation.
Effects of melatonin on cell wall components of harvested flowering Chinese cabbage (A) cellulose content. (B) Hemicellulose content. (C) Lignin content. (D) Water soluble pectin content. (E) Protopectin content. Control: Double distilled water. Melatonin: 100 µmol L− 1 melatonin treatment. The vertical bars represent the standard error of the mean (n = 3). The asterisk above the column represents a significant difference of P < 0.05.
Interestingly, melatonin suppressed the accumulation of water-soluble pectin during storage. Compared to control, the cellulose content in melatonin-treated group increased by 19.18%, 17.97%, 66.75%, and 64.06% after storing for 5, 10, 15, and 20 d, respectively; the hemicellulose content in melatonin-treated group increased by 28.46%, 45.28%, 13.88%, and 8.16% after storing for 5, 10, 15, and 20 d, respectively; the lignin content in melatonin-treated group increased by 38.77%, 44.38%, and 35.18% after storing for 10, 15, and 20 d, respectively, but there was no obvious difference on 5 d of storage; the protopectin content increased by 6.39%, 4.75%, 28.00%, and 24.02%, while the water-soluble pectin content decreased by 4.78%, 18.25%, 15.11%, and 11.48% after 5, 10, 15, and 20 d of storage, respectively. These findings displayed that melatonin could inhibit cell wall degradation through enhancing the accumulation of cellulose, hemicellulose, lignin, and protopectin to maintain the stability of cell wall components of harvested flowering Chinese cabbage.
Impacts of melatonin on gene transcript and enzyme activity involved in cell wall metabolism
To examine the regulatory impacts of melatonin on cell wall metabolism, we assessed the transcripts of genes involved in cell wall degradation (BrBGAL2, BrBGAL4, BrBGAL5, BrBGAL17, BrPG, BrCEL3, and BrPME3) and the activities of associated enzymes (PME and Cx). Throughout the study, the expression level of BrBGAL2 gradually decreased in the control and melatonin treatments over the storage period, with melatonin treatment showing an enhanced transcript level after 15 d of storage (Fig. 3A). Notably, BrBGAL4 expression was upregulated in both treatments during the 20 d of storage period, yet the transcript in the melatonin treatment was consistently lower than that of the control, suggesting a suppressive effect of melatonin on BrBGAL4 transcript (Fig. 3B). Conversely, BrBGAL5 expression decreased in both treatments over the storage period, with the melatonin treatment exhibiting higher levels compared to the control after 5, 10, 15, and 20 d of storage, indicating an activation of BrBGAL5 transcript by melatonin (Fig. 3C). While BrBGAL17 expression was initially inhibited in the melatonin treatment on the 5th d of storage, no obvious changes were found between the melatonin and control treatments on subsequent days (Fig. 3D). Compared to the control, melatonin treatments downregulated the transcripts of BrPG, BrCEL3, and BrPME3 over the 20 d storage period, indicating an inhibitory effect of melatonin on the expressions of these cell wall degradation-associated genes (Fig. 3E and G). Furthermore, the activities of cell wall degradation-related enzymes CX and PME increased in both groups, yet melatonin treatment attenuated their activities compared to control over the 20 d storage period (Fig. 3H and I).
Effects of melatonin on gene expression and enzyme activity involved in cell wall metabolism of harvested flowering Chinese cabbage. BrBGAL2 (A), BrBGAL4 (B), BrBGAL5 (C), BrBGAL17 (D), BrPG (E), BrCEL3 (F), and BrPME3 (G), (H) Pectin methylase (PME) activity, (I) Cellulase (Cx) activity. Control: Double distilled water. Melatonin: 100 µmol L− 1 melatonin treatment. The vertical bars represent the standard error of the mean (n = 3). The asterisk above the column represents a significant difference of P < 0.05.
In addition, we correspondingly noticed that melatonin treatment could affect the transcriptions of cellulose biosynthesis-related genes (BrCesA1, BrCesA3.1, BrCesA3.2, BrCesA5, BrCesA6, BrCslB3.1, BrCslB3.2, BrCslB4, and BrCslD1). As shown in Fig. 4, the expressions of BrCesA1, BrCesA3.1, BrCesA3.2, BrCesA5, and BrCesA6 were found to downregulate, however, the expressions of BrCslB3.1, BrCslB3.2, BrCslB4, and BrCslD1 were observed to upregulate in the control over the 20 d of storage period. Compared to control, melatonin treatment could upregulate the expressions of BrCesA1, BrCesA3.1, and BrCesA3.2, but downregulate the expressions of BrCesA6, BrCslB3.2, and BrCslB4 during storage, respectively. No obvious changes were observed in BrCesA5 transcript between control and melatonin treatments during 20 d of storage. Moreover, the BrCslB3.1 expression of melatonin treatment was exhibited to be lower than that of the control during 0 to 10 d of storing period, but higher than that of control during 15 to 20 d of storage. The change of BrCslD1 expression between control and melatonin treatments presented the opposite result of BrCslB3.1 expression. These outcomes displayed that the transcript patterns of different cellulose biosynthesis-associated genes were different, and melatonin played crucial roles in regulating the cellulose biosynthesis of flowering Chinese cabbage.
Effects of melatonin on the expressions of cellulose synthesis-related genes BrCesA1 (A), BrCesA3.1 (B), BrCesA3.2 (C), BrCesA5 (D), BrCesA6 (E), BrCSLB3.1 (F), BrCSLB3.2 (G), BrCSLB4 (H), and BrCSLD1 (I) in harvested flowering Chinese cabbage during storage. Control: Double distilled water. Melatonin: 100 µmol L− 1 melatonin treatment. The vertical bars represent the standard error of the mean (n = 3). The asterisk above the column represents a significant difference of P < 0.05.
Effects of melatonin on sugar content
Effects of melatonin on sugar content
The content of soluble sugar in the control exhibited a decreasing trend during storage, and that of melatonin treatment was found to increase by 14.81%, 46.38%, 43.05%, and 73.44% after storing for 5, 10, 15, and 20 d compared with control, respectively (Fig. 5A). The sucrose content was increased in both control and melatonin treatments, reached a maximum value on the 10 d and 15 d of storing period, respectively, and then gradually decreased after that. The sucrose content of melatonin treatment was increased by 15.96%, 22.44%, 109.14%, and 108.30% after storing for 5, 10, 15, and 20 d compared to control, respectively (Fig. 5B). Furthermore, the glucose content of melatonin treatment was increased by 49.23%, 36.71%, 85.99% and 28.89% after storing for 5, 10, 15, and 20 d compared with control, respectively (Fig. 5C). Similarly, the fructose content of melatonin treatment was found to be higher 15.40%, 26.53%, 34.31%, and 25.01% than that of control after storing for 5, 10, 15, and 20 d, respectively (Fig. 5D). These findings indicated that the longer storage time caused the decrease in the contents of sucrose and soluble sugar, and the contents of glucose, fructose, sucrose, and soluble sugar of melatonin treatment were found to elevate compared to control, suggesting that melatonin could accelerate the sugar accumulation during storage in flowering Chinese cabbage.
Impacts of melatonin on sugar content and activities of enzymes in harvested flowering Chinese cabbage. (A) Soluble sugar content. (B) Sucrose content. (C) Glucose content. (D) Fructose content. (E) sucrose synthase (SS) activity. (F) sucrose phosphate synthetase (SPS) activity. (G) Neutral invertase (NI) activity. (H) Acid convertase (AI) activity. Control: Double distilled water. Melatonin: 100 µmol L− 1 melatonin treatment. The vertical bars represent the standard error of the mean (n = 3). The asterisk above the column represents a significant difference of P < 0.05.
Impacts of melatonin on the activities of sugar metabolism-associated enzymes
To elucidate the mechanism behind melatonin-induced sugar accumulation, we further investigated its effects on the activities of four sugar metabolism-associated enzymes (SPS, SS, NI, and AI). As depicted in Fig. 5E-H, melatonin application altered the activities of these enzymes in flowering Chinese cabbage. Initially, no obvious changes were observed in the activities of SPS and SS between the melatonin and control treatments during the initial 5 d of storage. However, after 5 d of storage, melatonin treatment led to enhance activities of both SS and SPS. Relative to the control, SS activity of melatonin treatment increased by 34.49%, 338.96%, and 112.32% after 10, 15, and 20 d of storage, respectively, while SPS activity increased by 29.51%, 22.95%, and 23.98% after the same durations, suggesting that melatonin could promote sucrose accumulation by improving activities of SS and SPS over time. Interestingly, no changes were observed in the activities of AI and NI between the control and melatonin treatments during the initial 5 d of storage. However, after 5 d of storage, melatonin treatment inhibited the activities of both AI and NI. Compared with control, NI activity of melatonin treatment decreased by 24.86%, 44.86%, and 22.05% after storing for 10, 15, and 20 d, respectively, whereas AI activity decreased by 54.03%, 32.96%, and 44.83% over the same storage durations. These results implied that melatonin could suppress sucrose hydrolysis by improving sugar metabolism during storing period in flowering Chinese cabbage.
Impact of melatonin on the expressions of sugar metabolism-associated genes
We further explored the impacts of melatonin treatment on transcripts of genes involved in sugar metabolism (BrSUS1, BrSUS3, BrSUS5, BrSPS1F, BrSPS3F, BrSPS4F, BrINV, BrSWEET1, BrSWEET2, BrSWEET3, BrSWEET4, BrSWEET11, BrSWEET15, and BrSWEET17). As shown in Fig. 6, the expressions of BrSUS1, BrSUS3, and BrINV were found to upregulate, however, the expressions of BrSUS5, BrSPS1F, BrSPS3F, and BrSPS4F were observed to downregulate in the control during storage, especially in the later stage of storing period. Compared to control, melatonin treatment could increase the expressions of BrSUS1, BrSUS5, BrSPS1F, BrSPS3F, and BrSPS4F, but suppress the transcripts of BrSUS3 and BrINV during 20 d of storing period, respectively.
Effects of melatonin on sugar metabolism genes BrSUS1 (A), BrSUS3 (B), BrSUS5 (C), BrSPS1F (D), BrSPS3F (E), BrSPS4F (F), and BrINV (G) in harvested flowering Chinese cabbage at 4℃ during storage. Control: Double distilled water. Melatonin: 100 µmol L− 1 melatonin treatment. The vertical bars represent the standard error of the mean (n = 3). The asterisk above the column represents a significant difference of P < 0.05.
SWEET sugar transporters play a crucial role in sugar unloading and accumulation in crops. As depicted in Fig. 7, compared to control, application of melatonin repressed the expressions of BrSWEET2, BrSWEET4, and BrSWEET17 over the 20 d of storage, respectively. However, the expression patterns of BrSWEET1, BrSWEET3, BrSWEET11, and BrSWEET15 differed from those of BrSWEET2, BrSWEET4, and BrSWEET17 under melatonin treatment during storing period. BrSWEET1 expression was initially inhibited in the melatonin-treated group on the 5th d of storage, while no changes were observed between the control and melatonin treatments on subsequent days. Melatonin treatment enhanced the expressions of BrSWEET3 during storage, particularly in the later stages. While the expressions of BrSWEET11 and BrSWEET15 under melatonin treatment were lower than those of the control on 5 d, they were subsequently enhanced, especially on 10 and 15 d of storage. These findings highlight the differential regulation of different sugar transporters by melatonin.
Effects of melatonin on sugar transport genes BrSWEET1 (A), BrSWEET2 (B), BrSWEET3 (C), BrSWEET4 (D), BrSWEET11 (E), BrSWEET15 (F), and BrSWEET17 (G) in harvested flowering Chinese cabbage during storage. Control: Double distilled water. Melatonin: 100 µmol L− 1 melatonin treatment. The vertical bars represent the standard error of the mean (n = 3). The asterisk above the column represents a significant difference of P < 0.05.
Discussion
The postharvest senescence seriously affects the nutritional quality and commercial value of agricultural products. Melatonin treatment can delay the senescence to maintain the postharvest quality in several horticultural crops including blueberry, barley, kiwiberry, sweet cherry, and flowering Chinese cabbage4,24,26,28. Previous reports showed that melatonin can improve ascorbic acid biosynthesis to increase its accumulation in pear fruit and flowering Chinese cabbage29,31. Melatonin can increase glucosinolates content through modulating the glucosinolates biosynthesis in Chinese cabbage32. In addition, melatonin can also promote the contents of soluble protein and soluble sugar in kiwifruit24. In this study, melatonin application was found to enhance the contents of soluble sugar, ascorbic acid, soluble protein, and glucosinolates in harvested flowering Chinese cabbage. These results were consistent with the previous reports24,29,31,32. Interestingly, melatonin treatment increases α, β-carotene, and lycopene levels, and carotenoid biosynthetic gene expression in tomatoes33. However, the contents of β-carotene, β-cryptoxanthin, zeaxanthin and lutein are inhibited in melatonin-treated group in broccoli34. Our present result displayed in melatonin treatment increased carotenoid content postharvest flowering Chinese cabbage, which was similar to the finding reported33. These results show that the carotenoid biosynthesis in responding to melatonin remains distinct in different crops. Overall, melatonin can effectively sustain the quality by retarding the nutrient degradation of postharvest horticultural crops.
The primary cell wall in most plants primarily contains three kinds of polysaccharides, including cellulose, hemicellulos, and pectin. The changes of cell wall composition influence shelf life and postharvest quality of horticultural products5,9. Studies have displayed that postharvest quality is intricately linked to the activities of cell wall catabolism-related enzymes including Cx, PG, and PME that catalyze the decomposition of cell wall substances including hemicellulose, cellulose, and pectin7,35,36. In the process of cell wall metabolism, melatonin plays important regulating roles. Previous studies have indicated that melatonin decreases the degradation of cellulose and pectin by diminishing the activities of cell wall degradation-associated enzymes such as β-galactosidase (β-Gal). PME, PG, and Cx in the later stage of storing period, and downregulating the transcripts of associated genes such as VcGAL1, VcPG, VcPE, and VcBG6, thereby preventing senescence and softening of postharvest blueberry fruit26. Additionally, the fruit of melatonin treatment displays decreased water-soluble pectin accompanied with higher cellulose, hemicellulose, protopectin, sodium carbonate soluble pectin, and chelate soluble pectin by suppressing the activities of β-Gal, PME, Cx, and PG35. Similarly, melatonin treatment also maintains the quality of diverse horticultural crops including jujube, kiwifruit, blueberry, and eggplant through delaying the decrease in contents of protopectin, cellulose, and hemicellulose and fruit firmness and the increase in content of water-soluble pectin, as well as inhibiting the gene transcript and activity of PG, Cx, β-Glu, and β-Gal enzymes6,9,25,27,37. Interestingly, melatonin treatment reduces the hardness of fruit and pulp, fruit elasticity, contents of covalently-bound pectin, ion-bound pectin, hemicellulose, and cellulose, and improves the content of water-soluble pectin and the activities of pectin lyase, PME, PG, and β-Gal, thus promoting the fruit softening of Chinese plum38. In addition, melatonin enhances lignin content in various plants including herbaceous peony and tea39,40. However, the study indicated that melatonin application reduces the firmness and lignin content and repressed the decomposition of ascorbic acid and total phenols through diminishing the activities of peroxidase and phenylalanine ammonia-lyse, cinnamyl alcohol dehydrogenase, as well as lignin synthesis-associated genes transcript levels in water bamboo shoot41. A similar finding is reported that observed melatonin treatment retarded the activities of peroxidase and phenylalanine ammonia-lyase and decreased the content of lignin, thereby procrastinating the increase in firmness in green asparagus42. It can be seen that the impacts of melatonin on postharvest quality by regulating cell wall metabolism are distinct in different crops. Our study demonstrated that melatonin inhibited the transcripts of BrBGAL4, BrPG, BrCEL3, and BrPME3 genes, along with the activities of PME and Cx enzymes, while also enhancing the transcript of BrBGAL5 during the 20 d of storage period in harvested flowering Chinese cabbage. However, we did not observe consistent expression patterns in other related genes such as BrBGAL2 and BrBGAL17 under melatonin treatment. Additionally, we found that melatonin promoted the contents of cellulose, hemicellulose, lignin, and protopectin, but decreased the water-soluble pectin content during storing period. These findings suggested that BrBGAL4, BrPG, BrCEL3, and BrPME3 genes, along with PME and Cx enzymes, are pivotal candidates involved in inhibiting cell wall decomposition to maintain cell wall stability mediated by melatonin in harvested flowering Chinese cabbage. The cellulose content in plant cell wall accounts for 10–30% of dry matter mass in primary cell wall and 50–60% of dry matter mass in secondary cell wall43. Cellulose biosynthesis genes encode the enzymes involved in cell wall metabolism. However, little is known about how melatonin impacts the cellulose synthase family genes to regulate cell wall metabolism in the postharvest quality maintenance of horticultural products. Therefore, we analyzed the transcripts of cellulose biosynthesis-associated genes including BrCesA1, BrCesA3.1, BrCesA3.2, BrCesA5, BrCesA6, BrCslB3.1, BrCslB3.2, BrCslB4, and BrCslD1 during storage. Our findings showed that melatonin treatment upregulated the expressions of BrCesA1, BrCesA3.1, and BrCesA3.2, while downregulating the expressions of BrCesA6, BrCslB3.2, and BrCslB4, respectively. Additionally, we did not observe significant difference in BrCesA5 transcript between control and melatonin treatments during the 10 to 20 d of storage. Moreover, the transcript patterns of BrCslB3.1 and BrCslD1 differed between the early and late stages of storage. These results suggest that BrCesA1, BrCesA3.1, and BrCesA3.2 may be crucial genes involved in promoting cellulose biosynthesis to maintain cell wall stability mediated by melatonin in harvested flowering Chinese cabbage. Taken together, the transcript patterns of different cell wall degradation-associated genes and enzymes, as well as cellulose biosynthesis-related genes, varied under melatonin treatment. BrBGAL4, BrPG, BrCEL3, BrPME3, BrCesA1, BrCesA3.1, and BrCesA3.2 genes, along with PME and Cx enzymes, emerge as potential candidates involved in maintaining cell wall stability to preserve quality in postharvest flowering Chinese cabbage.
Sugar content serves as a critical indicator for evaluating the postharvest quality of horticultural crops. Glucose, sucrose, and fructose are the primary soluble carbohydrates in fruit, which undergo mutual transformation and serve as substrates for the metabolism of respiratory, thereby influencing shelf life, flavor, and quality15. Soluble sugar metabolism is regulated by multiple enzymes and genes. Enzymes such as SPS, SS, NI, and AI modulate sucrose biosynthesis and degradation into fructose and glucose within various cellular compartments17. The accumulation of sucrose and fructose correlates with increased activities of SS, NI, and AI, while the content of glucose shows contrasting data in sugarcane18. Additionally, sucrose levels correlate with SPS activity, while glucose and fructose content correlate with VIN activity, thereby enhancing sweetness parameters in sweetpotato19. Previous studies have displayed that melatonin plays different roles in regulating sugar metabolism. For example, in pear fruit, melatonin increases the contents of glucose, sucrose, and soluble sugars, while having no effect on fructose content14. Similarly, melatonin application enhances the ratio of soluble solids to acid and the contents of soluble sugar, fructose, glucose, and sucrose in kiwiberry fruit by upregulating the activities and transcript levels of NI, SPS, and SS enzymes and downregulating AI activity and expression28. In peach fruits, melatonin treatment increases the levels of sucrose and total soluble sugar by upregulating the activities and gene transcripts of NI, AI, SS, and SPS enzymes44. However, in pear fruit, melatonin-treated fruit exhibits reduced NI activity and gene expression while elevating both SPS and SS activities and gene transcripts29. Similarly, in apple fruit, postharvest melatonin treatment increases the contents of ascorbic acid, soluble sugar, soluble solid and flesh firmness, while inhibiting NI and AI activities and improving SPS and SS activities15. Studies have exhibited that melatonin accelerates the content of soluble sugar content (glucose, sucrose, and sorbitol) primarily in fruit by improving the activities of SPS and SSs and repressing the activities of NI, AI, and SSc14,15. In our study, melatonin treatment promoted the levels of fructose, glucose, sucrose, and total soluble sugar in postharvest flowering Chinese cabbage. Additionally, melatonin treatment enhanced the activities of SPS and SS and the transcripts of BrSUS1, BrSUS5, BrSPS1F, BrSPS3F, and BrSPS4F but suppressed the activities of AI and NI and the transcripts of BrSUS5, and BrINV. These findings highlight the diverse impacts of melatonin on the activities and gene expressions of sugar metabolism-associated enzymes in different crops. SWEETs represent a relatively described class of small transporters that mediate sugar transport45. Previous reports have demonstrated the importance of SWEET gene family members in sugar metabolism. For instance, ossweet1b knockout plants display alterations in glucose, fructose, sucrose, galactose, starch, and chlorophyll contents, suggesting the crucial role of OsSWEET1b in sugar metabolism and leaf senescence in rice46. In tobacco, the upregulation of PgSWEET17a increases fructose content while decreasing glucose and sucrose contents, indicating the involvement of PgSWEET17a in sugar metabolism in pomegranate47. AcSWEET2a/2b and AcSWEET16b are implicated in sugar transport during fruit ripening of Averrhoa carambola48. To date, there is limited literature available on the impacts of melatonin on postharvest quality in relation to SWEET gene family members. In our study, seven detected SWEET genes (BrSWEET1, BrSWEET2, BrSWEET3, BrSWEET4, BrSWEET11, BrSWEET15, and BrSWEET17) exhibited distinct expression patterns after melatonin treatment. Notably, melatonin treatment suppressed the expressions of BrSWEET2, BrSWEET4, and BrSWEET17 but improved BrSWEET3 transcript during the 20 d of storage period. However, the impacts of melatonin treatment on expressions of BrSWEET1, BrSWEET11, and BrSWEET15 differed between the early and later stages of storage. Taken together, the transcript patterns of different sugar metabolism-associated genes and enzymes varied during storage. It is reasonable to believe that melatonin treatment promotes the contents of fructose, glucose, sucrose, and total soluble sugar by increasing the activities of SPS and SS and the expressions of BrSUS1, BrSUS5, BrSPS1F, BrSPS3F, BrSPS4F, and inhibiting the activities of NI and AI as well as the expressions of BrSUS3, BrINV, BrSWEET2, BrSWEET4, and BrSWEET17.
It is obvious that melatonin plays an important role in regulating sugar metabolism and cell wall metabolism. Studies have shown that the increase of sucrose content in cotton can promote secondary cell wall biosynthesis and fiber initiation49. In transgenic bananas overexpressing MaNAC1, glucose levels are found to be much higher than those of the wild type, resulting in increased cellulose content and cell wall thickness50. In this study, melatonin promoted sucrose biosynthesis and accumulation through enhancing the activities of SS and SPS. Simultaneously, melatonin upregulated the expression of genes related to sucrose biosynthesis (BrSUS1, BrSUS5, BrSPS1F, BrSPS3F, BrSPS4F), further strengthening sugar synthesis capacity. Additionally, melatonin inhibited the activities of NI and AI and downregulated the expression of genes involved in sugar metabolism (BrSUS3, BrINV, BrSWEET2, BrSWEET4, BrSWEET17), thereby reducing sugar breakdown and maintaining intracellular sugar homeostasis. The enhancement of sugar metabolism provided sufficient substrates for the biosynthesis of cell wall components. Melatonin upregulated the expression of cellulose biosynthesis-related genes (BrCesA1, BrCesA3.1, BrCesA3.2), promoting the biosynthesis of cellulose, hemicellulose, lignin, and protopectin. Increase in these cell wall components helps strengthen the structural stability of the cell wall, delaying its degradation during postharvest senescence. Additionally, melatonin downregulated the expression of genes related to cell wall degradation (BrBGAL4, BrPG, BrCEL3, BrPME3), inhibiting the activities of cell wall-degrading enzymes such as Cx and PEM, thereby reducing the breakdown of cell wall components. Notably, melatonin significantly reduced the accumulation of water-soluble pectin, which helped maintain cell wall integrity and delayed cell wall softening. In summary, melatonin regulates sugar metabolism, promoting sugar accumulation and providing substrates for the biosynthesis of cell wall components, while simultaneously delaying cell wall degradation through inhibiting the activity of enzymes and expression of genes related to cell wall degradation. This dual regulatory mechanism not only maintained the mechanical strength and stability of cell wall but also significantly improved the texture and postharvest nutritional quality of flowering Chinese cabbage (Fig. 8). This research provided a theoretical basis for the application of melatonin in postharvest preservation of flowering Chinese cabbage.
Materials and methods
Materials and treatments
In this study, the ‘60-day’ variety of flowering Chinese cabbage was utilized as the experimental material. Postharvest fresh plant samples (35 days after sowing) were collected from Huachuang vegetable Farm in Zhongshan City, China. Based on our previous findings indicating that 100 µmol L− 1 melatonin exhibited the most effective delay in postharvest senescence of flowering Chinese cabbage4, the same concentration of melatonin was employed in this study. Controls were sprayed with double distilled water. Each replicate sample was then packed in a polyethylene (PE) bag (40 × 30 cm2) and sealed following spraying. Each treatment consisted of triplicate samples with 20 plants per replicate. Following treatment, all samples were stored for 20 d at 4 °C, and relative data were analyzed at 5, 10, 15, and 20 d of storage. All the reagents were purchased from Shanghai Sangon Biotechnology Co., LTD.
Determination of quality index
The assessment of carotenoid content was carried out using spectrophotometry51. 0.3 g of fresh leaves was placed into 15 mL of extraction solution, consisting of a mixture of acetone and ethanol with a volume ratio of 1. Absorbance was measured using a spectrophotometer (UV-1800, Shimadzu, Shanghai, China) at wavelengths of 645 nm, 663 nm, and 440 nm. The result is expressed as mg g− 1 on the basis of fresh weight (FW).
The ascorbic acid and soluble protein contents were assessed following our previous method16. Briefly, fresh plant samples (1 g) was ground with oxalic acid solution (25 mL), then centrifuged at 7500xg (10 min, 4 °C), collected the supernatant, and stood for 15 min. Ascorbic acid content was measured at 705 nm using an ultraviolet (UV)-2410PC spectrophotometer. In addition, 1 g of fresh plant samples were ground with 10 mL of 50mM phosphate buffer containing 1 mg mL− 1 Crosslinked Polyvinylpyrrolidone (PVPP) and 1.33mM Ethylene Diamine Tetraacetic Acid (EDTA) (pH 7.8), then centrifuged at 12000xg for 10 min (4 °C), and the supernatant was collected for assessment of protein content. The results are expressed as mg g− 1 on the basis of fresh weight (FW).
The total glucosinolates content was measured by palladium chloride method52. Took 0.3 g fresh sample, added 0.5 mL 60% (v/v) methanol after grinding, then bathed in water at 80 °C for 5 min, cooled and let stand. After absorbing 1 mL supernatant and reacting with 2 mL 4 M palladium chloride solution for 2 h, the absorbance was determined by ultraviolet spectrophotometer at 540 nm. The total glucosinolates content was expressed as µmol g− 1 on the basis of fresh weight (FW).
Assessment of cell wall components
The content of pectin was evaluated by carbazole colorimetry53. Briefly, fresh samples (0.5 g) were put into a test tube, ground with 15 mL 95% (v/v) ethanol, put it into in a boiling water bath (20 min), then cooled at room temperature, and soaked in ultra-double distilled water to extract water-soluble pectin. The remaining residues were mixed with 50 mM HCl, then placed in boiling water bath for 1 h, cooled to room temperature, and soaked in ultra-double distilled water to extract protopectin. The water-soluble pectin and protopectin were evaluated by ultraviolet spectrophotometer at 540 nm. Both water-soluble pectin content and protopectin content are expressed as mg g− 1 on the basis of fresh weight (FW).
Cellulose, hemicellulose and lignin were determined by colorimetric kit (Shenggong Bioengineering Co., LTD. Shanghai, China). The specific operation was carried out according to the instructions. The content of Cellulose, hemicellulose and lignin were expressed as mg g− 1 on the dry weight (DW) basis.
Determination of cell wall degradation-associated enzyme activities (PEM and cx)
The activities of PME and Cx were measured following previously reported method53. For crude enzyme extraction, fresh plant samples (1.0 g) were ground with 3.5 mL of pre-cooled extraction buffer (prepared by dissolving 10.5 g NaCl in 50 mM sodium acetate buffer (pH 5.5) and diluting to 100 mL). After centrifugating, the supernatants were collected as the enzyme extraction, then stored at 4 °C. 0.5 mL of enzyme extraction were mixed with 1.0 mL of sodium acetate buffer (pH 5.5, 50 mM) and 0.5 mL 1% (w/v) pectin, then incubated in a water bath for 1 h (37 °C). After incubation, PME activity was evaluated at 540 nm. The activity was expressed as U mg− 1 protein. 1 U was defined as the ability of 1 mg protein to produce 1 µg of galacturonic acid per minute in the reaction system. Additionally, For Cx activity determination, 0.5 mL of enzyme extraction were mixed with 1.0 mL of 50 mM, pH 5.5 sodium acetate buffer and 1% (w/v) sodium carboxymethyl cellulose, then incubated in a 37 °C water bath (1 h). After incubation, Cx activity was evaluated at 540 nm. The activity was expressed as U mg− 1 protein. 1 U was defined as the ability of 1 mg protein to produce 1 µg of glucose acid per minute.
Determination of soluble sugar, sucrose, glucose and fructose
Soluble sugar content was evaluated by the previously reported method16. Briefly, fresh samples (0.3 g) were heated in boiling water and bathed twice with 10 mL of distilled water. The extracted solution was combined and then reacted with zinc acetate (1 mL) and potassium ferrocyanide (1 mL) for 15 min. The absorption value was determined by an ultraviolet spectrophotometer at 630 nm. The soluble sugar content was expressed as mg g− 1 on the basis of fresh weight (FW).
The contents of fructose, glucose, and sucrose were confirmed by high performance liquid chromatography (HPLC)54,55. Briefly, freeze-dried samples (0.5 g) were finely ground with 80% (v/v) ethanol solution (5 mL), then incubated in 80 °C water (1 h). After centrifugation at room temperature at 9000xg (20 min), the supernatants were dried in 80 °C water bath, dissolved in 1 mL deionized water, then filtered via a 0.22 μm membrane filter. Chromatographic separation was achieved using a HPLC system (Waters Alliance E2695) with an Agilent NH2 column (4.6 mm × 250 mm) and an acetonitrile-water mobile phase (volume ratio: 75:25). The flow rate was fixed in 1 mL min− 1 and the column temperature was sustained at 35 °C. Detection was carried out using a refractive index detector with a detector temperature of 35 °C. The results are expressed as mg g− 1 on the basis of fresh weight (FW).
Assessment of enzyme activity of sugar metabolism
The evaluation of enzymes associated with sugar metabolism was conducted following previously reported methods56. Briefly, fresh samples (0.3 g) were mixed with 1 mL of pre-cooled extraction buffer (100 mM pH 7.5 phosphate buffer containing 5 mM MgCl2, 0.1% (v/v) TritonX-100, 0.1% (v/v) β-mercaptoethanol, and 1 mM EDTA), the ground into a homogenate at 4 °C, centrifuged at 7500xg for 15 min (4 °C), and the supernatants were collected to evaluate the activities of sucrose synthase (SS), sucrose phosphate synthase (SPS), neutral invertase (NI), and acid invertase (AI). The enzyme activity of SS, SPS, NI, and AI were expressed as U g− 1 on the FW basis. The SS and SPS activity units are expressed in terms of the ability to produce 1 mg of sucrose per hour. NI and AI activity units are expressed in terms of the ability to produce 1 mg of glucose per hour.
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
The total RNA was extracted using the TRIzol kit (Magen, Guangzhou, China) from leaves of plant. Then, the first strand cDNA was synthesized through reverse-transcription of total RNA utilizing the RT Master Mix kit (Accurate Biotechology Co. Ltd, Hunan, China). qRT-PCR reaction was carried out utilizing SYBR® qPCR Master Mix kit (Accurate Biotechology Co. Lid, Hunan, China) with Bio-Rad CFX96 Real-Time PCR System. The Actin was used as an internal reference gene. The 2−ΔΔCt method was performed for relative expression level of genes57. Three biological replicates were performed for each experiment. The primers were listed in Table S1.
Statistical analysis
All data were presented as mean ± SD. Statistical analysis was conducted using R v4.1.1 and IBM SPSS Statistics 24 (SPSS Inc., Chicago, IL, USA). Tukey’s test (p ≤ 0.05) was employed to assess treatment effects. Figures were created using R v4.1.1 and Origin 2021 (OriginLab Corp., Northampton, MA, USA).
Conclusion
In this work, we found that melatonin could promote the levels of important nutrients like ascorbic acid, sugars (soluble sugar, fructose, glucose, and sucrose), soluble protein, carotenoid, and glucosinolates in postharvest flowering Chinese cabbage. It also enhanced various cell wall components (cellulose, hemicellulose, lignin, and protopectin) while reducing water-soluble pectin accumulation. Melatonin effectively maintained cell wall stability by downregulating the transcripts of genes involved in cell wall degradation (BrBGAL4, BrPG, BrCEL3, and BrPME3), inhibiting the activities of cell wall degradation-associated enzymes like Cx and PME, and improving the transcripts of genes involved in cellulose biosynthesis (BrCesA1, BrCesA3.1, and BrCesA3.2). Additionally, melatonin could improve the sugar contents by increasing the activities of SPS and SS and expressions of BrSUS1, BrSUS5, BrSPS1F, BrSPS3F, and BrSPS4F, and inhibiting the activities of NI and AI and expressions of BrSUS3, BrINV, BrSWEET2, BrSWEET4, and BrSWEET17. Our findings not only contribute to a deeper understanding of melatonin-maintained postharvest quality via regulating the metabolisms of cell wall and sugar but also provide practical implications for improving the commodity performance of horticultural products after harvest.
Data availability
All data are available in the main text or the supplementary material.
References
Zhang, L. et al. Transcriptomic and metabolomic analyses reveal the mechanism of uniconazole inducing hypocotyl dwarfing by suppressing BrbZIP39–BrPAL4 module mediating lignin biosynthesis in flowering Chinese cabbage. Front. Plant. Sci. 13, 1014396. https://doi.org/10.3389/fpls.2022.1014396 (2022).
Yue, L. et al. 1-Methylcyclopropene promotes glucosinolate biosynthesis through BrWRKY12 mediated jasmonic acid biosynthesis in postharvest flowering Chinese cabbage. Postharvest Biol. Technol. https://doi.org/10.1016/j.postharvbio.2023.112415 (2023).
Kuang, L. et al. The roles of Salvia miltiorrhiza-derived carbon dots involving in maintaining quality by delaying senescence of postharvest flowering Chinese cabbage. Food Chem. https://doi.org/10.1016/j.foodchem.2022.134704 (2023).
Yue, L. et al. Melatonin delays Postharvest Senescence through suppressing the inhibition of BrERF2/BrERF109 on Flavonoid Biosynthesis in Flowering Chinese Cabbage. Int. J. Mol. Sci. 24(3), 2933. https://doi.org/10.3390/ijms24032933 (2023).
Chen, Y. et al. The role of cell wall polysaccharides disassembly in Lasiodiplodia theobromae-induced disease occurrence and softening of fresh longan fruit. Food Chem. https://doi.org/10.1016/j.foodchem.2021.129294 (2021).
Tang, Q. et al. Exogenous application of melatonin maintains storage quality of jujubes by enhancing anti-oxidative ability and suppressing the activity of cell wall-degrading enzymes. Lwt. https://doi.org/10.1016/j.lwt.2020.109431 (2020).
Zhai, R. et al. Melatonin limited ethylene production, softening and reduced physiology disorder in pear (Pyrus communis L.) fruit during senescence. Postharvest Biol. Technol. 139, 38–46. https://doi.org/10.1016/j.postharvbio.2018.01.017 (2018).
Lin, Y. et al. Effects of hydrogen peroxide treatment on pulp breakdown, softening, and cell wall polysaccharide metabolism in fresh longan fruit. Carbohydr. Polym. https://doi.org/10.1016/j.carbpol.2020.116427 (2020).
Njie, A. et al. Melatonin treatment inhibits mango fruit (cv. ‘Guiqi’) softening by maintaining cell wall and reactive oxygen metabolisms during cold storage. Postharvest Biol. Technol. https://doi.org/10.1016/j.postharvbio.2023.112500 (2023).
Wang, L. et al. Involvement of lignin deposition and cell wall degradation in stem senescence of Chinese flowering cabbage during storage. Postharvest Biol. Technol. https://doi.org/10.1016/j.postharvbio.2023.112256 (2023).
Schneider, R., Hanak, T., Persson, S. & Voigt, C. A. Cellulose and callose synthesis and organization in focus, what’s new? Curr. Opin. Plant. Biol. 34, 9–16. https://doi.org/10.1016/j.pbi.2016.07.007 (2016).
Durán-Soria, S., Pott, D. M., Osorio, S. & Vallarino, J. G. Sugar Signaling during Fruit Ripening. Front. Plant. Sci. https://doi.org/10.3389/fpls.2020.564917 (2020).
Ge, Y. et al. Changes in the sucrose metabolism in apple fruit following postharvest acibenzolar-S‐methyl treatment. J. Sci. Food Agric. 99, 1519–1524. https://doi.org/10.1002/jsfa.9326 (2018).
Liu, J. et al. Effects of Exogenous Application of Melatonin on Quality and Sugar Metabolism in ‘Zaosu’ Pear Fruit. J. Plant. Growth Regul. 38, 1161–1169. https://doi.org/10.1007/s00344-019-09921-0 (2019).
Fan, Y. et al. Postharvest melatonin dipping maintains quality of apples by mediating sucrose metabolism. Plant. Physiol. Biochem. 174, 43–50. https://doi.org/10.1016/j.plaphy.2022.01.034 (2022).
Tan, C. et al. Effects of exogenous sucrose and selenium on plant growth, quality, and sugar metabolism of pea sprouts. J. Sci. Food Agric. 102, 2855–2863. https://doi.org/10.1002/jsfa.11626 (2021).
Zhang, J. et al. Effects of trisodium phosphate treatment after harvest on storage quality and sucrose metabolism in jujube fruit. J. Sci. Food Agric. 99, 5526–5532. https://doi.org/10.1002/jsfa.9814 (2019).
Lobo, A. K. M. et al. Exogenous sucrose supply changes sugar metabolism and reduces photosynthesis of sugarcane through the down-regulation of Rubisco abundance and activity. J. Plant. Physiol. 179, 113–121. https://doi.org/10.1016/j.jplph.2015.03.007 (2015).
Ru, L. et al. Role of sucrose phosphate synthase and vacuolar invertase in postharvest sweetening of immature sweetpotato tuberous roots (Ipomoea batatas (L.) Lam Cv ‘Xinxiang’). Sci. Hortic. https://doi.org/10.1016/j.scienta.2021.110007 (2021).
Eom, J. S. et al. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr. Opin. Plant. Biol. 25, 53–62. https://doi.org/10.1016/j.pbi.2015.04.005 (2015).
Sun, L. et al. Down-regulation of the sucrose transporter CsSUT1 causes male sterility by altering Carbohydrate Supply. Plant. Physiol. 180, 986–997. https://doi.org/10.1104/pp.19.00317 (2019).
Ni, J. et al. Overexpression of sugar transporter gene PbSWEET4 of pear causes sugar reduce and early senescence in leaves. Gene https://doi.org/10.1016/j.gene.2020.144582 (2020).
Liu, T. et al. Potato tonoplast sugar transporter 1 controls tuber sugar accumulation during postharvest cold storage. Hortic. Res..https://doi.org/10.1093/hr/uhad035 (2023).
Liang, D. et al. Exogenous melatonin application delays senescence of Kiwifruit leaves by regulating the antioxidant capacity and biosynthesis of flavonoids. Front. Plant. Sci. https://doi.org/10.3389/fpls.2018.00426 (2018).
Cao, S. et al. Effects of melatonin treatment on the physiological quality and cell wall metabolites in kiwifruit. Food Sci. Technol. https://doi.org/10.1590/fst.85421 (2022).
Liu, R. et al. Melatonin treatment delays the softening of blueberry fruit by modulating cuticular wax metabolism and reducing cell wall degradation. Food Res. Int. https://doi.org/10.1016/j.foodres.2023.113357 (2023).
Song, L. et al. Melatonin alleviates chilling injury and maintains postharvest quality by enhancing antioxidant capacity and inhibiting cell wall degradation in cold-stored eggplant fruit. Postharvest Biol. Technol. https://doi.org/10.1016/j.postharvbio.2022.112092 (2022).
Zhang, Y. et al. Exogenous melatonin maintains postharvest quality in kiwiberry fruit by regulating sugar metabolism during cold storage. Lwt. https://doi.org/10.1016/j.lwt.2022.114385 (2023).
Liu, L. et al. Melatonin mobilizes the metabolism of sugars, ascorbic acid and amino acids to cope with chilling injury in postharvest pear fruit. Sci. Hortic. https://doi.org/10.1016/j.scienta.2023.112548 (2024).
Tan, X. L. et al. Melatonin delays leaf senescence of Chinese flowering cabbage by suppressing ABFs-mediated abscisic acid biosynthesis and chlorophyll degradation. J. Pineal Res. https://doi.org/10.1111/jpi.12570 (2019).
Tan, X. L. et al. Melatonin delays leaf senescence of postharvest Chinese flowering cabbage through ROS homeostasis. Food Res. Int. https://doi.org/10.1016/j.foodres.2020.109790 (2020).
Teng, Z. et al. Melatonin regulated glucosinolate profile via modulation of genes related with sulfur and nitrogen metabolism in Brassica rapa ssp. pekinensis. Ind. Crops Prod. https://doi.org/10.1016/j.indcrop.2022.114538 (2022).
Sun, Q. et al. Melatonin promotes carotenoid biosynthesis in an ethylene-dependent manner in tomato fruits. Plant. Sci. https://doi.org/10.1016/j.plantsci.2020.110580 (2020).
Lou, J. et al. Melatonin treatment delays postharvest senescence of broccoli with regulation of carotenoid metabolism. Food Chem. https://doi.org/10.1016/j.foodchem.2022.135185 (2023).
Ali, S. et al. Exogenous melatonin mitigates chilling injury in zucchini fruit by enhancing antioxidant system activity, promoting endogenous proline and GABA accumulation, and preserving cell wall stability. Postharvest Biol. Technol. https://doi.org/10.1016/j.postharvbio.2023.112445 (2023).
Hou, Y., Wu, F., Zhao, Y., Shi, L. & Zhu, X. Cloning and expression analysis of polygalacturonase and pectin methylesterase genes during softening in apricot (Prunus armeniaca L.) fruit. Sci. Hortic. https://doi.org/10.1016/j.scienta.2019.108607 (2019).
Sun, Y. et al. Effect of exogenous melatonin treatment on quality and softening of jujube fruit during storage. J. Food Process. Preserv. https://doi.org/10.1111/jfpp.16662 (2022).
Li, Z. et al. Transcriptome profiles reveal the promoting effects of Exogenous Melatonin on Fruit Softening of Chinese Plum. Int. J. Mol. Sci. https://doi.org/10.3390/ijms241713495 (2023).
Han, M. et al. Exogenous melatonin positively regulates lignin biosynthesis in Camellia sinensis. Int. J. Biol. Macromol. 179, 485–499. https://doi.org/10.1016/j.ijbiomac.2021.03.025 (2021).
Zhao, D. et al. Melatonin enhances stem strength by increasing lignin content and secondary cell wall thickness in herbaceous peony. J. Exp. Bot. 73, 5974–5991. https://doi.org/10.1093/jxb/erac165 (2022).
Yang, B. et al. Application of melatonin delays lignification in postharvest water bamboo shoots in association with energy metabolism. Postharvest Biol. Technol. https://doi.org/10.1016/j.postharvbio.2022.112149 (2023).
Boonsiriwit, A., Lee, M., Kim, M., Itkor, P. & Lee, Y. S. Exogenous melatonin reduces lignification and retains quality of Green Asparagus (Asparagus officinalis L). Foods https://doi.org/10.3390/foods10092111 (2021).
Juraniec, M. & Gajda, B. Cellulose biosynthesis in plants - the concerted action of CESA and non-CESA proteins. Biol. Plant. 64, 363–377. https://doi.org/10.32615/bp.2020.065 (2020).
Zhou, K. et al. Effects of exogenous melatonin on sugar and organic acid metabolism in early-ripening peach fruits. PLoS One. https://doi.org/10.1371/journal.pone.0292959 (2023).
Chen, L. et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468, 527–532. https://doi.org/10.1038/nature09606 (2010).
Zhang, Q. et al. Plasma membrane-localized hexose transporter OsSWEET1b, affects sugar metabolism and leaf senescence. Plant. Cell. Rep. https://doi.org/10.1007/s00299-023-03125-3 (2024).
Zhang, X. et al. Identification, Analysis and Gene Cloning of the SWEET Gene Family Provide Insights into Sugar Transport in Pomegranate (Punica granatum). Int. J. Mol. Sci. https://doi.org/10.3390/ijms23052471 (2022).
Lin, Q., Zhong, Q. & Zhang, Z. Identification and functional analysis of SWEET gene family in Averrhoa carambola L. fruits during ripening. PeerJ https://doi.org/10.7717/peerj.11404 (2021).
Ding, X. et al. Sucrose enhanced reactive oxygen species generation promotes cotton fibre initiation and secondary cell wall deposition. Plant. Biotechnol. J. 19 (6), 1092–1094. https://doi.org/10.1111/pbi.13594 (2021).
Yin, Q. et al. Banana MaNAC1 activates secondary cell wall cellulose biosynthesis to enhance chilling resistance in fruit. Plant. Biotechnol. J. 22, 413–426. https://doi.org/10.1111/pbi.14195 (2024).
Tomsone, L. & Kruma, Z. Spectrophotometric analysis of pigments in horseradish by using various extraction solvents. FoodBalt https://doi.org/10.22616/FoodBalt.2019.023 (2019).
Ishida, M. et al. Small variation of glucosinolate composition in Japanese cultivars of radish (Raphanus sativus L.) requires simple quantitative analysis for breeding of glucosinolate component. Breed. Sci. 62, 63–70. https://doi.org/10.1270/jsbbs.62.63 (2012).
Ji, Y. et al. Ethanol vapor delays softening of postharvest blueberry by retarding cell wall degradation during cold storage and shelf life. Postharvest Biol. Technol. https://doi.org/10.1016/j.postharvbio.2021.111538 (2021).
Wang, L. et al. Glycine betaine reduces chilling injury in peach fruit by enhancing phenolic and sugar metabolisms. Food Chem. 272, 530–538. https://doi.org/10.1016/j.foodchem.2018.08.085 (2019).
Zheng, H., Zhang, Q., Quan, J., Zheng, Q. & Xi, W. Determination of sugars, organic acids, aroma components, and carotenoids in grapefruit pulps. Food Chem. 205, 112–121. https://doi.org/10.1016/j.foodchem.2016.03.007 (2016).
Yu, L. et al. Effects of hot air and methyl jasmonate treatment on the metabolism of soluble sugars in peach fruit during cold storage. Postharvest Biol. Technol. 113, 8–16. https://doi.org/10.1016/j.postharvbio.2015.10.013 (2016).
Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, 45e–445. https://doi.org/10.1093/nar/29.9.e45 (2001).
Acknowledgements
This work was financially supported by 2022 Seed Industry Revitalization Project of Rural Revitalization Strategy Special Fund of Guangdong Province {Yue Cai Nong (2022) No. 184}, GuangDong Basic and Applied Basic Research Foundation (2023A1515010505), Guangzhou Basic and Applied Basic Research Foundation (2023A04J0114).
Author information
Authors and Affiliations
Contributions
Dengjin Kang: Conceptualization, Investigation, Methodology, Writing – original draft. Jiajing Zeng: Methodology, Writing – original draft, Formal analysis – review & editing. Dalian Lu: Methodology, Formal analysis– review & editing. Lingqi Yue: Methodology – review & editing. Yunyan Kang: Methodology, Investigation – review & editing. Min Zhong: Conceptualization, Methodology– review & editing. Juxian Guo: Methodology, Formal analysis – review & editing. Xian Yang: Supervision, Project administration, Writing – review & editing, Funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kang, D., Zeng, J., Lu, D. et al. Melatonin maintains postharvest quality by modulating the metabolisms of cell wall and sugar in flowering Chinese cabbage. Sci Rep 15, 5396 (2025). https://doi.org/10.1038/s41598-025-89811-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-89811-6