Abstract
To compare the performance of two contrast agents, barium sulfate and lipiodol, in the performance of 3D CT-DCG. A prospective interventional radiological study was performed on 15 lacrimal drainage systems of 14 patients who clinically presented with nasolacrimal duct obstructions. All patients underwent CT-DCG, traditionally first using lipiodol and a week later by barium sulfate. CT-DCG data was used to construct 3D models as per standard published protocols. A 3D software was used to calculate the volume (which reflects the filling ability of the dye) and the number of details (counted by the number of different faces creating the object) of the 3D CT-DCG of the studied lacrimal drainage systems. The analysis was performed in R, version 4.3.2. The overall mean volume of the dye (filling feature) in the 3D CTDCG reconstructed models of the lacrimal system following lipiodol injection was 55.2 mm3 vs. 244.2 mm3 with barium sulfate (p < 0.001). A minimum of 94% gain was noted in volume with barium sulfate contrast (p < 0.001). The barium sulfate was superior to lipiodol for the same lacrimal drainage system and provided a better appreciation of lacrimal drainage details. The visualization of the stenosis and obstructions of the nasolacrimal duct were better delineated. The filling defects in cases of dacryoliths were more pronounced with barium sulfate with intricate visualization of the presumed shape and size of the dacryolith. The present study is the first to highlight the advantages of barium sulfate over the traditional contrast agent, lipiodol, for the acquisition of 3D CT-DCG images. Barium sulfate is a viable alternative and an economically more feasible option for performing 3D CT-DCG.
Similar content being viewed by others
Introduction
Dacryocystography was first performed in 1909 using bismuth subnitrate for assessing the lacrimal sac abscess cavity1. It took 81 years since then (1990) when the first computed tomography-dacryocystography (CT-DCG) was performed2. The rapid-paced evolution of three-dimensional imaging techniques using CT and MRI has helped surgeons create 3-dimensional models (3D) which gives them a significant edge in planning and performing complex surgeries.
Several contrast agents are used in dacryocystography, including bismuth subnitrate, lipiodol, iohexol, iopamidol, sinograffin, and gadolinium3. An ideal contrast agent is expected to be non-irritant, non-toxic, and homogenous with optimal viscosity. Lipiodol is a common agent used for CT-DCG, but its oil-soluble nature makes it immiscible with tears and can rarely cause granulomas3. Barium sulfate is a common agent used for contrast studies of the gastrointestinal tract via barium swallow or barium enema4,5,6. To the best of the authors’ knowledge, its use for lacrimal drainage imaging has been reported in veterinary literature in a single case of a horse7. The present study aimed to evaluate the techniques and outcomes of performing 3D CT-DCG using barium sulfate and comparing the imaging obtained in the same patients with the traditional contrast agent, lipiodol.
Methods
The study was conducted at the tertiary Ophthalmology Care unit at the Military Institute of Medicine- National Research Institute, Warsaw, Poland. The study followed the Tenets of the Declaration of Helsinki and was approved by the bioethics committee at the Military Medical Chamber. Informed consent was obtained from all the patients. A prospective interventional radiological study was performed on 15 lacrimal drainage systems of 14 patients who clinically presented with nasolacrimal duct obstructions. All patients underwent CT-DCG, traditionally first using lipiodol and a week later by barium sulfate. CT-DCG data was used to construct 3D models as per standard published protocols8.
Technique
After application of topical anesthesia, Lipiodol (Lipiodol Ultra Fluide, 480 mg/ml, Guerbet, France) (Fig. 1A) was slowly injected into the lacrimal sac through a lacrimal cannula mounted on a luer-lock syringe. The endpoint was the first sign of regurgitation of the dye from the punctum. A CT scan was performed, and 0.6 mm axial, coronal, and reconstructed sagittal images were obtained. The lacrimal system was then irrigated with saline to remove the contrast. A week later, the same lacrimal system was injected with barium sulfate (Barium sulfuricum medana, 1 gm/ml, Polpharma SA, Starogard Gdanski, Poland), and similar CT imaging was obtained. The modified preparation of barium sulfate (Fig. 1B) was a mixture of one part each of commercial barium sulfate and sodium hyaluronate (1.4%, EyefillR C Cohesive, Bausch & Lomb, Berlin, Germany). The sodium hyaluronate is commonly used in several ophthalmic preparations.
3D reconstructions
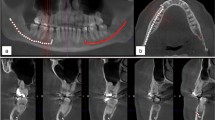
These are performed using earlier published protocols8. In brief, once the CT-DCG images are obtained in axial, coronal, and sagittal sections, they were analyzed in HorosTM radiology software (Purview, USA) (Fig. 2A). 3D reconstructions were then performed and exported from the HorosTM in a 3D file format (.STL) for further processing with the MeshLabR software (Visual Computing Lab & ISTI-CNR, Italy) for model cleaning and simplification. The final processing is using the software BlenderR (Blender Foundation, Netherlands), where materials, colors, and textures are assigned before rendering high-resolution 3D images as .PNG image files (Fig. 2B). Four 3D images with both contrast agents were constructed for each of the fifteen lacrimal systems. They were assigned letters A-D (A-anterior view, B-posterior view, C-medial view, and D-lateral view) (Fig. 3). Barium sulfate and lipiodol images were assigned blue and yellow colors, respectively (Fig. 3).
3D reconstructed CT-DCG of a patient with nasolacrimal duct stenosis (Left Panel, C12 A-D). Note the blue images are with barium sulfate and the yellow ones with lipiodol. Reconstructed images of a patient with dacryolith (Right Panel, C13 A-D). Compare the superiority of barium sulfate over lipiodol.
Parameters assessed
A three-dimensional object consists of a mesh of vertices (orange and black lines, Fig. 4A and B) connected by edges (red arrow, Fig. 4A). Edges create numerous defined faces on the surface of the 3D object (blue area, Fig. 4A; red area, Fig. 4B). The described structure enables the 3D software (BlenderR) to calculate the object volume (which reflects the filling ability of the dye) and the number of details (counted by the number of different faces creating the object) of the lacrimal drainage system (Fig. 4B), which were compared using lipiodol and barium for each of the fifteen lacrimal drainage system enrolled in the current study. Other parameters assessed include demographics, clinical presentation, complexities of the nasolacrimal duct obstruction, radiological features of CTDCG, and comparison outcomes between the contrast agents.
Statistical analysis
The analysis was performed in R, version 4.3.2. The analysis of quantitative variables (expressed in numbers) was performed by calculating descriptive statistics such as mean, standard deviations, median, quartiles, and minimum and maximum values. A comparison of the values in two repeated measurements was performed using the Wilcoxon test for pairs. The analysis of qualitative variables (not expressed in numbers) was carried out by calculating the absolute frequencies and percentages of all values that these variables could assume. A p-value of < 0.05 was considered statistically significant.
Results
Fifteen lacrimal drainage systems (LDS) of 14 patients were assessed in the study. The mean age at presentation was 54.27 years (range: 18–83 years). There was no gross predisposition to gender or laterality (Table 1). Most patients (60%, 9/15) had varying degrees of nasolacrimal duct stenosis and obstruction; the remaining 40% (6/15) of the lacrimal systems demonstrated dacryoliths. The overall mean volume of the dye (filling feature) in the 3D CTDCG reconstructed models of the lacrimal system following lipiodol injection was 55.2 mm3 vs. 244.2 mm3 with barium sulfate (p < 0.001) (Table 2). A minimum of 94% gain was noted in volume with barium sulfate contrast (p < 0.001). The overall mean ‘faces’ on the surface of the LDS reconstructed models with lipiodol and barium were 607.1 and 1779.9, respectively (Table 2). A minimum of 54.9% gain was noted with barium sulfate compared to lipiodol (p < 0.001). Figure 5A and B demonstrate improvement in the filling and surface details with barium sulfate and show several lines, with each line representing individual LDS that were studied using both contrast agents.
Direct and head-on comparison of the filling and detail characteristics between lipiodol and barium for all the cases together (A and B) and those patients with dacryoliths (C and D). Each of the blue lines represents one lacrimal drainage system. Note how barium sulfate improved the filling and detail characteristics compared to lipiodol.
If the LDS with dacryoliths is separately analyzed, the mean volume of filling following lipoidol injection was 65 mm3 vs. 297.9 mm3 (Table 3). A minimum of 172.2% gain for LDS filling and 126.5% for LDS faces was noted with barium sulfate compared to lipiodol (p < 0.031) (Table 3). Figure 5C and D demonstrate the improvement in the filling and surface details with barium sulfate compared to lipiodol for LDS with dacryoliths. For the same LDS, barium sulfate was superior to lipiodol and provided a better appreciation of lacrimal drainage details (Figs. 3 and 6). The visualization of the stenosis and obstructions of the nasolacrimal duct were better delineated (Fig. 6). The filling defects in cases of dacryoliths were more pronounced with barium sulfate, with intricate visualization of the presumed shape and size of the dacryolith (Fig. 3). The details of the dacryolith could be reconstructed using barium sulfate, which was inaccurate when using lipiodol (Fig. 2B).
3D reconstructed CT-DCG of a patient with distal severe nasolacrimal duct stenosis (Left Panel, C10 A-D). Note the blue images are with barium sulfate and the yellow ones with lipiodol. Reconstructed images of a patient with a complete post-saccal obstruction (Right Panel, C11 A-D). Compare the superiority of barium sulfate over lipiodol.
Discussion
Dacryocystography aided by computed tomography and magnetic resonance imaging has significantly aided in the management of lacrimal drainage disorders secondary to maxillofacial trauma, diverticula, and malignancy. Several contrast agents have been used to highlight the details of the lacrimal drainage. The commonly used lipiodol is an oil-soluble agent that provides good-quality CT-DCG images but has a few disadvantages. It is immiscible in tears, can cause granuloma formation, and lead to persistent tissue reaction in cases of accidental extravasation9,10. The other contrast agents used are those with high iodine content like iohexol and iopamidol 300, which provide good images but are known to cause patient discomfort3. Low-iodine water-soluble contrast agents like omnipaque and amipaque have been successfully employed for digital subtraction DCG11.
Barium sulfate is an odourless white crystalline substance found in the natural mineral barite and is a good contrast agent because of its ability to absorb X-rays. It was first used for gastrointestinal imaging in 1904 by Cannon. It was in clinical use as a standard by 19105. Barium sulfate is available in powder or liquid (suspension) forms and is considered superior to water-soluble contrast agents because of its resistance to dilution and the desirable ability to coat the mucosal surfaces for better delineation4. Several refinements in the past 100 years have enabled it to become one of the frequently used contrast agents during imaging. Latimer et al.7 used barium sulfate to obtain a dacryocystorhinography of a horse and found it to provide good radiographic contrast and a precise delineation of anatomical features of the nasolacrimal duct. The potential advantage of barium sulfate to coat the lacrimal drainage mucosa and highlight the abnormalities was assessed in humans in the present study and compared with the traditional and routine contrast agent, lipiodol.
The present study demonstrated that the comparative dye filling and facets assessment reflected the superiority of barium sulfate over lipiodol in assessing lacrimal drainage disorders (Fig. 5A and D). Anatomical details were better delineated with barium sulfate, and the dacryolith filling defects could be better evaluated (Figs. 3 and 6). Both these features would aid in subsequent patient management. Besides the imaging advantages (as demonstrated in the present study) of barium sulfate, it is also significantly cheaper (more than ten times) than lipiodol. This may have a bearing on the lacrimal drainage system where the volume to be injected is much less and makes barium sulfate a better economic product. It would make more economic sense in Eastern European countries like Poland and developing countries like India.
The present study first assessed a lacrimal drainage system with lipiodol and irrigated the system following imaging. Since lipiodol is immiscible in tears, a gap of one week was given so that it does not interfere with the subsequent CT-DCG. In one week, the same lacrimal system was reassessed with barium sulfate to obtain a direct comparison with the same patient.
The present study has several limitations. These include a smaller sample size, lack of standardization of the ideal concentration of barium sulfate for CT-DCG, the utility of adding hyaluronate, and the unknown complications of barium sulfate CT-DCG (although none were noted in the present). The strengths of the present study are the head-on comparison between a new contrast agent and a traditional one in the same lacrimal drainage system, the exploration of more sustainable and economically viable options, and comparative three-dimensional CT-DCG models. The future research can be directed towards standardization of the ideal concentration of barium sulfate for CTDCG and testing in larger cohorts from different geographical regions and exploring the feasibility in several lacrimal drainage disorders like dacryolithiasis.
Conclusions
The present study is the first to demonstrate the utility of barium sulfate as a viable alternative contrast agent for CT-DCG. Further exploration is required to assess the optimal concentration of barium sulfate needed for dacryocystography and compare it with iodine containing contrast agents.
Data availability
“Data is provided within the manuscript file”.
References:
Ewing, A. E. Roentgen ray demonstrations of the lacrymal abscess cavity. Am. J. Ophthalmol. 26, 1–4 (1909).
Mahesh, L. & Ali, M. J. Imaging modalities for lacrimal disorders. In Principles and Practice of Lacrimal Surgery 2nd edn (ed. Ali, M. J.) 111–121 (Springer, 2018).
Singh, S., Ali, M. J. & Paulsen, F. Dacryocystography: From theory to current practice. Ann. Anat. 224, 33–40 (2019).
O’Connor, S. D. & Summers, R. M. Revisiting oral barium sulfate contrast agents. Acad. Radiol. 14(1), 72–80 (2007).
Levine, M. S. & Rubesin, S. E. History and evolution of the barium swallow for evaluation of the pharynx and esophagus. Dysphagia 32(1), 55–72 (2017).
Beckett, K. R., Moriarity, A. K. & Langer, J. M. Safe use of contrast media: What the radiologist needs to know. Radiographics 35(6), 1738–1750 (2015).
Latimer, C. A., Wyman, M., Diesem, C. D. & Burt, J. K. Radiographic and gross anatomy of the nasolacrimal duct of the horse. Am. J. Vet. Res. 45(3), 451–458 (1984).
Nowak, R., Nowak-Gospodarowicz, I., Rękas, M. & Ali, M. J. Virtual reality and mixed reality-assisted endoscopic DCR in extremely complex lacrimal obstructions. Laryngoscope 6, 66 (2024).
Delaney, Y. & Khooshabeh, R. Lipogranuloma following traumatic dacryocystography in a 4-year-old boy. Eye 15(Pt 5), 683–684 (2001).
Mansfield, D. C., Zeki, S. M. & Mackenzie, J. R. Case report: Extravasation of lipiodol–a complication of dacryocystography. Clin. Radiol. 49(3), 217–218 (1994).
El Gammal, T. & Brooks, B. S. Amipaque dacryocystography Biplane magnification and subtraction technique. Radiology 141(2), 541–542 (1981).
Funding
Hyderabad Eye Research Foundation and SERB, India.
Author information
Authors and Affiliations
Contributions
“RN and MJA designed the research. ING, RN, MN, and AKK collected the data. ING, RN, MR, MN, AKK, and MJA analyzed the data. RN, and MJA wrote the manuscript. RN and MN contributed with images. RN, MR, and MJA supervised the project. All authors contributed to manuscript revision, read, and approved the submitted version.”
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nowak, R., Nowak-Gospodarowicz, I., Kicinska, A.K. et al. 3D computed tomography-dacryocystography (3D CT-DCG) and the contrast agents: direct comparison of Lipiodol and barium sulfate. Sci Rep 15, 5695 (2025). https://doi.org/10.1038/s41598-025-89894-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-89894-1