Abstract
Fertilizer application is a common agricultural practice that enhances soil fertility but can also increase heavy metal mobility in contaminated soils. This study used a pot experiment with four vegetables (water spinach, Chinese cabbage, lettuce, and garland chrysanthemum) to evaluate the impact of BC, ZE, and their combination (CO) on Cd and Pb levels under different fertilization schemes. Results showed that CO treatment significantly enhanced enzyme activities, increasing urease by 2.6–31.6% and catalase by 1.37–14.24% under varying fertilizer conditions. However, sucrase activity increased only with compound fertilizers. The use of compound fertilizers alone raised Cd and Pb levels in vegetable shoots by 0.65 mg·kg⁻¹ and 12.76 mg·kg⁻¹, respectively, while the CO amendment effectively mitigated these increases. BCR sequential extraction indicated that BC and CO shifted Cd and Pb into more stable soil fractions, reducing their bioavailability. Specifically, CO reduced Pb accumulation in shoots by 24.8–49.7%, with BC showing particular efficacy in reducing Cd levels. These findings highlight that BC and ZE, particularly in combination, offer an effective strategy for remediating heavy metal-contaminated soils in agricultural systems, especially when chemical fertilizers are used.
Similar content being viewed by others
Introduction
Soil contamination by heavy metals, such as cadmium (Cd) and lead (Pb), is a significant environmental issue, posing risks to ecosystems and human health1. In China, it is estimated that over 19% of agricultural lands are contaminated with heavy metals, resulting in approximately 12 million tons of contaminated grain annually2,3. Consuming food crops contaminated with Cd and Pb poses serious health risks4. Cd can accumulate in the kidneys, leading to kidney dysfunction and bone damage caused by oxidative stress and apoptosis5. Pb exposure, even at low levels, is associated with neurotoxicity and cognitive impairment, particularly in children, as it interferes with enzyme function and induces oxidative damage6. Cd and Pb disrupt cellular functions by replacing essential metals, generating reactive oxygen species, and inhibiting key enzymes7. This contamination is often exacerbated by industrial activities, particularly in mining regions, where heavy metals can accumulate to hazardous levels in the soil.
Various strategies have been employed to address heavy metal contamination in soils, including physical removal, chemical washing, and immobilization. However, physical methods are resource-intensive, requiring substantial amounts of clean soil, while chemical washing can lead to the depletion of essential soil nutrients and structural components8,9. Among the chemical immobilization techniques, the use of natural minerals like zeolite and organic materials such as biochar has gained attention due to their potential to reduce heavy metal bioavailability and transfer to plants10,11,12.
Zeolite, characterized by its aluminosilicate composition and high cation exchange capacity (CEC), and biochar, known for its ability to improve soil pH and organic carbon content, have been shown to immobilize heavy metals effectively13,14. Despite numerous studies demonstrating the benefits of these amendments individually, there remains a lack of comprehensive research exploring the combined effects of biochar and zeolite under different fertilization regimes. Furthermore, the influence of these amendments on soil physicochemical properties, enzyme activities, and heavy metal uptake by crops in contaminated soils has not been fully elucidated, especially under varying fertilization conditions.
Currently, soil heavy metal pollution poses a significant threat to ecosystems and human health. Although various strategies have been implemented to immobilize heavy metals in soil and reduce their mobility and plant uptake, there is still a lack of systematic research on the specific effects of different fertilizer application schemes on these strategies. In particular, the interaction effects of different soil amendments (such as biochar and zeolite) under different fertilizer applications in Cd and Pb contaminated soils have not been fully explored. This research gap limits our comprehensive understanding of the effectiveness of soil heavy metal remediation technologies in actual agricultural production.
Compared to other commonly used soil amendments, such as organic composts or synthetic stabilizers, the combination of biochar and zeolite offers several advantages. Biochar, with its high surface area and porous structure, effectively adsorbs heavy metal ions, reducing their bioavailability in the soil14. Zeolite, on the other hand, enhances soil nutrient availability and ion-exchange capacity, further immobilizing heavy metals through its unique molecular structure. Together, these amendments offer a synergistic effect that not only stabilizes metals but also improves soil properties in a more sustainable manner compared to organic composts, which may carry trace amounts of heavy metals, or synthetic stabilizers15. Therefore, the objectives of this study were as follows: (1) To evaluate the impact of biochar and zeolite amendments, individually and combined, on soil physicochemical properties (such as pH, soil organic matter, and cation exchange capacity) and nutrient availability (N, P, and K). (2) To assess the effects of these amendments on soil enzyme activities (urease, catalase, and sucrase) and the bioavailability and chemical forms of Cd and Pb in the soil. (3) To quantify the accumulation of Cd and Pb in the roots and shoots of vegetables grown in amended soils under varying fertilization regimes. (4) To provide practical guidelines for the combined use of biochar and zeolite in remediating heavy metal-contaminated soils, especially in agricultural systems that apply chemical fertilizers. By conducting a pot experiment with these amendments and evaluating their impact on soil properties, nutrient availability, enzyme activity, and metal accumulation in vegetables, we seek to provide a more comprehensive understanding of their potential benefits and limitations. This research is crucial for developing effective soil remediation strategies, particularly in agricultural systems where chemical fertilizers are commonly used, and for minimizing the risks associated with heavy metal contamination in food crops.
Materials and methods
Soil, amendments, and fertilizer
The soil sample was collected from the surface layer (0–20 cm) of farmland adjacent to the tungsten mining area in Ganzhou, Jiangxi province, China (114.3191°E, 25.4647°N). The soil was passed through a 2 mm sieve after air-drying for pot experiments and property analysis. The soil is a typical red clay contaminated with cadmium and lead, with concentrations of 2.32 mg·kg− 1 and 105.60 mg·kg− 1, respectively. Both concentrations exceed the risk thresholds specified in the Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land (GB 15618 − 2018), which are 0.3 mg·kg− 1 for cadmium and 90 mg·kg− 1 for lead16. Soil pH was 5.52, measured by a pH meter at a soil/water of 1:2.5. The soil organic matter (SOM) was detected by bulk density method, and the cation exchange capacity (CEC) was detected by ammonium acetate exchange method1.The SOM was 38.06 g·kg− 1 and The CEC was 9.75 cmol·kg− 1.
Zeolite was sourced from Yusong Environmental Equipment Factory in Gongyi City, China, subsequently crushed and sieved through a #60 mesh. Biochar, derived from rice husk, was produced via pyrolysis at 400 °C in an oxygen-free environment for 4 h, then similarly crushed and sieved through a #60 mesh. Following our previous research17, zeolite and biochar were combined at a 1:1 mass ratio. The characteristics and elemental compositions of the amendments are detailed in Table 1 and S1, respectively. Fig S1 and S2 show the SEM, XRD, and FTIR spectra of biochar and zeolite. The characterization analysis of the amendment can be found in Supplementary Material S1.
Ternary compound fertilizer (TCF), a type of chemical fertilizer, was purchased from Youyu Fertilizer Company in Guizhou, China, which is a commonly used compound fertilizer in the local area for agriculture production. The basic properties of fertilizer are listed in Table S2, and the mass percentages of N, P and K in fertilizer were 15, 15, and 15%, respectively. In our study, we used four commonly cultivated local vegetable species: water spinach (WS), Chinese cabbage (CC), lettuce (LE), and garland chrysanthemum (CH). The vegetable seeds were all purchased from the official flagship store of Wotian Agriculture.
Treatment
To assess the impact of amendments on Cd and Pb in varying fertilizer application scenarios, we conducted a pot experiment. Each plastic pot, measuring 20 cm in diameter and 11 cm in height, was filled with 2 kg of soil. The application of ternary compound fertilizer was categorized into two levels: 0 g·kg− 1 (no fertilizer) and 1 g·kg− 1 (ternary compound fertilizer), resulting in two distinct groups based on the fertilizer regime. Amendments were incorporated at a 5% mass ratio (amendment to soil)17, with a control group receiving no amendments for comparison. Consequently, four treatments were established: CK (control), BC (biochar), ZE (zeolite), and CO (combination of biochar and zeolite), each treatment was conducted under two fertilizer conditions: no fertilizer (NF) and ternary compound fertilizer (TCF). Furthermore, four commonly cultivated local vegetables were selected for the experiment: WS, CC, LE, and CH. Following sterilization with 3% H2O2, seeds of these vegetables were sown and thinned to five plants per pot, with each treatment replicated thrice. The plants were grown in a greenhouse maintained at 20% soil moisture and 25 °C, with 10 h of daily light exposure. After two months, all plants were harvested, and growth parameters including plant height, root length, fresh weight, and dry weight were meticulously recorded.
Sample analysis
The plants were collected and separated into shoot and root parts for determination. 0.2 g air-dried plant was digested in a microwave-accelerated digestion system with 8 mL HNO3 and 1 mL H2O2. The digestion progress was listed in Table S3, and the Cd and Pb contents in the digestion solution were determined by inductively coupled plasma mass spectrometry (ICP-MS). Soils were air-dried for analysis after plant harvest. The activities of soil enzymes (sucrase, urease, and catalase) in air-dried soil samples were measured using commercial test kits from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The measurements were conducted based on ultraviolet-visible spectrophotometry18. Determination of soil available phosphorus content using sodium bicarbonate dissolution molybdenum blue colorimetric method, the ammonium acetate extraction method was used for available potassium content19, and the alkaline diffusion method was used for available nitrogen content20. The soil was digested in a microwave-accelerated digestion system, and the digestion progress is listed in Table S4. The contents of Cd and Pb in the digestion solution were determined by ICP-MS. The chemical forms of heavy metals in soil samples were analyzed using the modified European Community Bureau of Reference (BCR) sequential extraction method (see Supplementary Material S2). According to this method, soil heavy metals are divided into four forms: acid extracted (F1), reducible (F2), oxidizable (F3), and residual (F4) forms17. Using HF-HClO4-HNO3 to digest the residual (F4) state of the soil samples (see Supplementary Material S3). In this study, inductively coupled plasma mass spectrometry (ICP-MS) was used to determine Cd and Pb in plant and soil samples. The limits of detection (LOD) and limits of quantification (LOQ) for Cd were 0.01 µg·L− 1 and 0.05 µg·L− 1, respectively, while those for Pb were 0.02 µg·L− 1 and 0.1 µg·L− 1. Additionally, the calibration of ICP-MS was performed using a series of standard solutions to construct a working curve, with correlation coefficients (R2) all exceeding 0.999, ensuring the accuracy and repeatability of the measurement data.
Statistical analysis
All experiments were performed in triplicate. Data are presented as mean ± standard deviation (SD). The enrichment factor of heavy metals in vegetables (BF) can express the metals uptake capacity of the plants from the soil. Transport factor (TF) is used to evaluate the mobility of heavy metals from soil to vegetables. The calculated methods of BF and TF as shown in Table 2. Statistical significance was determined by one-way analysis of variance (ANOVA) using R4.0.0. Conduct correlation analysis using SPSS 26. During the analysis, various quality assurance/quality control (QA/QC) methods were employed, which involved the establishment of duplicate, blank, and standard samples. The QA/QC analysis was conducted on soil-certified reference materials (GBW07430), with a recovery rate ranging from 93.6 to 105.2%. All figures were created using Origin 2022.
Results and discussion
The effects of amendments on soil nutritive elements
The impact of amendments on soil nutritional elements (AP, AK, and AN) is depicted in Fig. S3. The application of BC and CO has significantly increased AP. As shown in Fig S3a, under NF conditions, compared with CK, BC and CO increased AP by 25.0-46.2% and 16.7–42.3%, respectively. This growth is consistent with the findings of Hartley et al.21, who observed that the high affinity of biochar for aluminum and iron ions promotes the release of dissolved phosphorus, thereby increasing AP levels in the soil. In addition, the ability of biochar to affect soil AP is consistent with similar studies emphasizing the role of biochar in enhancing nutrient retention and availability22. The research results also indicate that although ZE does not contain nutrients such as nitrogen, phosphorus, and potassium, due to its soil regulating properties, it can indirectly affect AP under specific crop conditions23. This effect is particularly evident in the LE planting scenario, where ZE supports root development and nutrient absorption.
Compared with NF, TCF significantly increased the levels of AP, AK, and AN in the soil, attributed to the composition of the fertilizer (refer to Table S2). As shown in Fig S3b, under TCF conditions, the effects of BC and CO treatment are more significant, with an average increase of 43.1% in AP compared to NF. Previous studies have shown that the combined use of amendments and fertilizers produces a synergistic effect, which is more effective in improving nutrient utilization efficiency than using a single amendment alone24. This synergistic effect may be due to the physical and chemical properties of biochar (refer to Table 1), which improves soil structure and nutrient retention capacity25. Overall, the combined application of amendments and fertilizers has had a significant effect on the optimization of soil nutrients.
The effect of amendments on soil physical and chemical properties
Soil pH
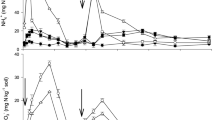
In the NF condition, Fig. 1a illustrates the influence of amendments on soil pH. Relative to the CK, the incorporation of BC, ZE, and CO led to an average soil pH increase of 0.30, 0.14, and 0.21 units for four plant types, respectively. The alkaline properties of biochar play an important role in neutralizing soil acidity, while its porous structure helps improve soil aeration and enhance microbial activity, thereby contributing to increased pH stability26. Zeolite, as an alkaline hydrated aluminosilicate, has negatively charged sites that can exchange cations with soil solution, thereby increasing soil pH and improving nutrient retention, consistent with the findings of Cui et al.13. and Wu et al.14. Moreover, the combined use of biochar and zeolite showed a synergistic effect in increasing soil pH, likely due to their complementary mechanisms in altering soil chemical properties. This is in agreement with the study by Ibrahim et al.27, which indicated that the combined application of biochar and zeolite can effectively improve soil quality by enhancing pH stability. The extent of pH change was also influenced by the type of vegetable planted. The study found that soils planted with WS and CC had a higher increment in pH compared to LE and CH, particularly under treatments such as BC + WS, CO + WS, BC + CC, and CO + CC. This finding suggests that soils planted with LE and CH have better acid-base buffering capacity. This observation merits further exploration in future research to fully understand the interaction between crop types and soil amendment effects.
Under TCF conditions, the incorporation of BC, ZE, and CO led to pH increases of 0.18, 0.07, and 0.11 units, respectively, as shown in Fig. 1b. The most pronounced increase occurred in the BC + CC treatment, where pH surged by 0.29 units. Notably, the soil pH under TCF conditions was lower than that under NF conditions. Compared to CK in NF, the CK pH under TCF decreased by 0.76 units after fertilization. Chemical fertilizers are commonly used in agricultural production to address soil degradation and enhance crop yields; however, long-term use is associated with soil acidification, which can lower soil pH28. In contrast, the range of pH changes under TCF conditions was less pronounced than that under NF. It is noteworthy that the soil pH for WS cultivation significantly decreased under both fertilization conditions (p > 0.05), indicating that WS may have a different impact on soil acidification dynamics compared to other crops. The above results indicate that BC and ZE, especially when combined, effectively increase soil pH, helping to counteract soil acidification from fertilizer use.
The change of soil pH, SOM, and CEC. (a), (c), and (e) represented NF condition; (b), (d), and (f) represented TCF condition. Note: Data were presented as the mean ± SD (n = 3). p < 0.05 was considered significant, and groups of the same plant with different superscript letters show significant differences.
Soil SOM
Soil organic matter (SOM) is a pivotal physical and chemical attribute of soil, with its variations depicted in Fig. 1c and d. Our results showed that the application of TCF did not significantly impact SOM levels compared to treatments under NF conditions, which is likely due to the low organic matter content in TCF (refer to Table S2). However, under both NF and TCF conditions, BC and CO treatments resulted in substantial increases in SOM, ranging from 52.42 to 57.22% and 30.93–42.51%, respectively, compared to their controls. In contrast, ZE had minimal effects on SOM. This indicates that the increase in SOM in CO treatments is primarily attributed to biochar rather than zeolite. Previous studies have shown that biochar can enhance SOM through mechanisms such as stabilization and reduced mineralization. For instance, Glab et al.29 and Peng et al.30 demonstrated biochar’s efficacy in increasing SOM through stabilization of organic-mineral complexes and by improving soil aggregate stability. Our findings align with these studies, showing that biochar’s high organic matter content directly boosts SOM and reinforces soil stability. Furthermore, although plants are usually a significant SOM source through root exudates and litter, the short experiment duration and complete plant harvesting limited these contributions, which is consistent with Ruf et al.31 findings on the importance of longer-term plant interactions for SOM accrual. These findings suggest that BC, due to its high organic content, substantially raises SOM levels, with combined applications further enhancing soil stability. These findings suggest that BC, due to its high organic content, substantially raises SOM levels, with combined applications further enhancing soil stability.
Soil CEC
Cation exchange capacity (CEC) is a critical characteristic for nutrient retention in agricultural soils. The variation in soil CEC is depicted in Fig. 1e and f. Our data showed that BC, ZE, and CO treatments enhanced CEC by 13.6–14.6%, 2.0-5.4%, and 7.9–9.3%, respectively, under NF conditions, with similar increases under TCF conditions. Notably, biochar and zeolite’s contributions to CEC are supported by their structural properties. For example, Atkinson et al.32 highlighted that biochar’s porous structure and functional groups facilitates ion exchange, while Akbar et al.33 explained that zeolite’s aluminosilicate framework provides high CEC due to its negatively charged tetrahedral structures. Post-amendment application, a comparative increase of approximately 4.6% in CEC was observed against the control, with the order being BC > CO > ZE > CK. Under NF conditions, BC, ZE, and CO contributed to average CEC enhancements of 13.9%, 3.5%, and 8.6%, respectively, reflecting the intrinsic high CEC values of biochar and zeolite (refer to Table 1). Remediation materials, to a certain degree, may serve as alternatives to fertilizers in augmenting soil CEC effects. BC and ZE jointly improve soil CEC, enhancing nutrient retention and providing a supportive environment for plant growth.
The effects of amendments on soil enzyme
Urease enzyme activity
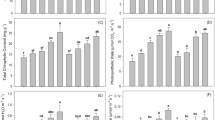
Ammonia, produced by the enzymatic reaction of urease, serves as an effective nitrogen source for plants. Moreover, urease activity acts as an indicator of soil nutritional status and nitrogen fertilizer efficiency34. Figure 2a illustrates that urease activity experienced an increment of 0.013 mg·g-1 and 0.005 mg·g− 1 following BC and CO treatments, respectively. This enhancement aligns with the findings of Zhao et al.35, where biochar increased SOM, thereby enhancing nitrogen availability in the soil. The stability of the organic-mineral complexes provided by BC and CO may support increased urease activity, consistent with the SOM correlation discussed in Chen et al.‘s36 studies. In contrast, ZE treatment had no significant effect on urease activity and even showed an inhibitory effect in soils planted with WS and LE, as shown in Fig. 2b. This is consistent with the findings of Tzanakakis et al.37, who reported that zeolite effectively inhibited soil urease activity. Figure 2b demonstrates that soils under TCF exhibited a lower average urease activity (0.061 mg·g-1) compared to NF (0.104 mg·g-1), which can be attributed to soil acidification and the mobilization of heavy metals induced by fertilizer application, potentially mitigated by amendments38. The above results indicate that CO applications enhance urease activity, supporting nitrogen availability and cycling in the soil.
The change of enzyme activity. (a), (c), and (e) represented NF condition; (b), (d), and (f) represented TCF condition. Note: Data were presented as the mean ± SD (n = 3). p < 0.05 was considered significant, and groups of the same plant with different superscript letters show significant differences.
Catalase enzyme activity
Soil catalase activity is a crucial indicator of soil’s oxidation-reduction potential and significantly influences the impact of toxic elements on soil health13. As shown in Fig. 2c and d, under NF conditions, the application of CO led to an increase in catalase activity by 1.37–14.24% across four different vegetable types, suggesting that CO can play a significant role in enhancing soil quality. This increase indicates that CO can significantly support soil health by promoting microbial stability and reducing oxidative stress. Previous studies, such as those by Xu et al.39 and Chang et al.40, have shown that organic amendments, including compost and biochar, can enhance soil catalase activity by providing essential nutrients that facilitate microbial growth and function, aligning well with our results. In the case of solitary applications, BC and ZE showed varied impacts on catalase activity depending on the vegetable type. For instance, during CH cultivation, ZE application did not lead to a significant increase in catalase activity, which may be attributed to unique soil-microbe interactions influenced by the crop type. Conversely, BC application in CC cultivation improved catalase activity by 1.37%. TCF treatments led to an average increase in catalase activity of 23.31%, attributed to the addition of nutritive elements such as AP, AK, and AN41. Combined treatments involving fertilizers with BC, ZE, and CO resulted in increases in soil catalase activity by 1.88 to 7.52%, 2.45 to 15.52%, and 2.59 to 10.47% respectively, underscoring the crucial role of fertilizers in catalase activity enhancement. The enhanced catalase activity resulting from the combined application of amendments and fertilizers suggests a potential reduction in soil oxidative stress, which may mitigate the adverse effects of toxic elements on soil health. This finding highlights the effectiveness of BC and ZE in synergistically boosting catalase activity, thereby potentially reducing oxidative damage in contaminated soils.
Sucrase enzyme activity
Soil sucrase catalyzes the hydrolysis of sucrose into glucose and fructose, serving as an important indicator of soil fertility and nutrient availability42. Figure 2e and f illustrate that under NF conditions, CO application did not significantly affect sucrase activity, indicating that the synergistic effect may not be sufficient in the absence of fertilizers. Notably, ZE application enhanced sucrase activity in soils planted with CC and CH, with increases of 8.4% and 22.8%, respectively, compared to CK. This enhancement can be attributed to ZE’s ability to improve soil structure and microbial habitat, facilitating greater microbial activity and enzyme production, as highlighted in studies by Mondal et al.43. BC treatment boosted sucrase activity in soils planted with LE and CH by 36.7% and 22.5%, respectively, but had an adverse effect on soils planted with WS and CC. Furthermore, when comparing TCF with NF conditions, there was an average increase of 6.06% in sucrase activity with fertilizer application, likely due to the added nutrients (AP, AK, and AN) in the fertilizer36. These results suggest that BC and ZE, particularly under fertilized conditions, can effectively enhance sucrase activity, thereby supporting soil microbial health and promoting carbon cycling, which is essential for soil fertility.
The effects on morphology of heavy metals in soil
Following a two-month period of potted plant experiments, we measured the cadmium and lead concentrations in various treatment groups (CK, BC, ZE, CO) of unfertilized water spinach soil, as presented in Table S5. The different treatments significantly impacted the concentrations of Cd and Pb, with the CO group showing the most effective reduction. This finding suggests that the combination of biochar and zeolite has substantial potential for remediating heavy metal-contaminated soil. According to the Soil Environmental Quality—Risk Control Standard for Soil Contamination of Agricultural Land (GB 15618—2018), although the Cd concentration in each treatment group exceeded the standard, the Pb concentration was below the risk screening value16.
The form of heavy metals significantly impacts their bioavailability, migration, and phytotoxicity. The acid-extractable (F1) and reducible (F2) forms are more accessible to plants, in contrast to the oxidizable (F3) and residual (F4) forms, which are more stable and exhibit lower bioavailability44. The forms of Cd and Pb in the soil were analyzed using the BCR sequential extraction procedure, as illustrated in Fig. 3.
Figure 3a illustrates that in the CK group, untreated, Cd predominantly occurs in the F1 form, characterized by high mobility and potential environmental harm. Upon the addition of BC, ZE, and CO, there was a noticeable reduction in the F1 fraction, decreasing to 45%, 46%, and 35%, respectively, with CO exhibiting the most pronounced effect. This result is consistent with previous research, where BC, due to its high surface area, can increase soil pH and adsorb heavy metals, thereby reducing their mobility45. Meanwhile, ZE, with its high cation exchange capacity, immobilizes heavy metals through ion exchange, converting them into forms with lower bioavailability46. The combination of BC and ZE enhances these immobilization mechanisms, resulting in a more stable distribution of heavy metal forms and reducing bioavailability in soil17. The addition of BC, ZE, and CO significantly increased the F4 fraction from 12 to 30%, 26%, and 29%, respectively, with CO’s impact surpassing that of ZE alone. BC specifically influenced the morphology of F2, resulting in a 13% decrease compared to the CK group, while ZE significantly affected F3, reducing it by 4%. The modifications in Cd’s morphology due to BC and CO can be primarily attributed to biochar’s ability to transform soluble metals into insoluble forms that bind with organic matter47,48.
Figure 3b demonstrates that in the control group, the F1 form of Pb concentration was 7.65 mg/kg, constituting 6% of the total Pb. Upon application of BC, ZE, and CO, the F1 fraction of Pb decreased to 4%, 3%, and 2%, respectively. Notably, the application of ZE resulted in a significant reduction of the F2 fraction, primarily transforming it into the more stable F4 fraction (increasing from 15 to 37%), consistent with the findings of Ye et al.49, which demonstrated that ZE application effectively reduces the bioavailability of Pb in soil. Similarly, BC treatment contributed to an increase in the F4 fraction from 15 to 20%, aligning with previous research that shows biochar enhances heavy metal immobilization through its large adsorption surface area and negative charge content50. Thus, the introduction of these amendments effectively lowered the bioavailable fractions (F1 and F2) while increasing the proportion of heavy metals in the more stable F4 fraction, a similar pattern observed in related studies51. Among the BC and CO treatments, the CO group exhibited a higher percentage of F4, likely due to the elevated soil pH in the CO group facilitating the formation of Ca2Pb8(PO4)6(OH)2 and Pb5(PO4)3OH. The findings emphasize that applying CO can transform the bioavailable forms of Cd and Pb into more stable, less mobile forms, thereby reducing environmental risks.
Heavy metals in vegetable plants
Cd accumulation
The absorption of Cd by plants is governed by several factors, including Cd’s mobility and the plant species. Our prior research indicates that biochar, zeolite, and their combination can effectively reduce Cd’s mobility in soil17. Our results are consistent with previous studies, showing that both BC and ZE treatments significantly reduced cadmium mobility, leading to decreased cadmium concentrations in roots and stems under NF and TCF conditions. Except in specific cases such as NF + LE and NF + CC, BC and CO treatments were particularly effective in reducing cadmium content. The study by Kim et al.52 also indicated that biochar effectively immobilized heavy metals and reduced plant uptake. In contrast, ZE treatment also reduced cadmium levels, but was less effective than BC and CO treatments. The effectiveness ranking was as follows: BC > CO > ZE. Cd accumulation was predominantly higher in the roots than in the shoots across all vegetable types, except for LE. Compared to NF, TCF usage resulted in a mean increase of 0.65 mg·kg− 1 in shoot Cd content, attributed to soil acidification due to fertilization53. This is further corroborated by significant Cd increases in WS and CC alongside notable pH rises (Fig. 4a and b).
For the NF condition, the Cd accumulation capacities of four vegetables were ranked as follows: LE > CH > WS > CC. In the CK group, the mean Cd concentrations in the roots and shoots were 0.75 and 0.64 mg·kg-1, respectively. These concentrations exceed the Chinese Food Safety Standard (GB 2762 − 2017), which sets the maximum allowable limit for Cd in leafy vegetables at 0.2 mg·kg-1, indicating a potential health risk54. Following treatments with BC, ZE, and CO, Cd accumulation decreased significantly (p < 0.05). Relative to CK, the reductions in shoot Cd concentration were 40.59%, 14.53%, and 31.19% for BC, ZE, and CO treatments, respectively, while in the roots, decreases of 30.53%, 7.91%, and 20.63% were observed. However, despite these reductions, the Cd levels in all treatments still approached or exceeded the food safety threshold, highlighting the persistent contamination issues and the need for more effective or prolonged treatments. The application of fertilizers led to an increased risk of Cd accumulation in the edible parts of four types of local vegetables, a finding consistent with previous studies55. Under TCF conditions, Cd concentrations in the roots and shoots increased by 32.0% and 62.5%, respectively, compared to the NF condition, due to soil acidification caused by fertilization. Although the amendments reduced Cd accumulation significantly, the levels remained above the safety standard.
The bioconcentration factor (BF) and translocation factor (TF) of Cd in vegetables under NF conditions are presented in Table 3. The results indicate that the amendments significantly reduce the BF for all treatments, indicating their effectiveness in limiting Cd uptake from soil to the roots, potentially leading to cleaner production practices. Specifically, BC and ZE treatments increased the TF for CC and CH, respectively. Conversely, CO decreased the TF for all vegetables, highlighting the promising application potential of combined amendments. This aligns with Paul et al.56, who suggested that combined amendments could offer a more balanced approach to immobilizing heavy metals. This study suggests that CO application is the most effective strategy for reducing Cd accumulation in vegetables.
Pb accumulation
Under NF conditions, the mean Pb accumulations in the roots and shoots without any amendment application (CK) were 4.26 and 8.98 mg·kg-1, respectively, as shown in Fig. 5a. These concentrations significantly exceed the Chinese Food Safety Standard (GB 2762 − 2017), which sets the maximum allowable limit for Pb in leafy vegetables at 0.3 mg·kg-154. The application of amendments reduced Pb accumulation in plants, with the greatest reduction in shoots observed in the CO group at 32.4%, followed by BC at 31.6%, and ZE at 16.2%. These results suggest that combining amendments is more effective than using a single agent in decreasing Pb levels in plants, a mechanism attributed to the enhanced efficiency of the combination in immobilizing Pb in the soil17,28. Despite these reductions, Pb concentrations remained above the permissible limit, indicating that while the amendments are effective, they are insufficient on their own to bring levels below hazardous thresholds. Except for CC, few changes in Pb accumulation in the roots were observed after the application of amendments. Under TCF conditions, as presented in Fig. 5b, the mean Pb concentrations in the shoots and roots with CK treatment were 5.16 and 21.16 mg·kg-1, respectively, indicating that the Pb concentration in roots was four times higher than in shoots. The application of BC, ZE, and CO significantly reduced Pb levels in plant shoots, with mean reductions of 28.2%, 20.1%, and 32.4%, respectively. Notably, the CO treatment led to the greatest reduction in Pb in the shoots of LE and CH, with decreases of 52.6% and 36.6%, respectively. Among the treatments, BC was the most effective at reducing Pb accumulation in roots, with a mean reduction of 42.8% across all four vegetable types. However, despite these reductions, Pb levels still exceeded the Chinese Food Safety Standard (GB 2762 − 2017) limits, underscoring the need for further optimization of amendment application. Furthermore, a comparison of Pb accumulation under NF and TCF conditions revealed a noticeable increase in Pb concentration due to fertilizer application, consistent with Cd accumulation patterns, with the largest increase observed in the roots of CC. This is closely related to soil acidification caused by fertilization28. Although current treatments can mitigate heavy metal accumulation, prolonged application may be required to achieve levels that ensure food safety and protect public health.
The BF and TF of Pb in vegetables under NF conditions are detailed in Table 4. After applying amendments, all TF values for Pb in vegetables decreased, except for CC. As illustrated in Fig. 5a, the application of amendments significantly reduced the Pb concentration in the roots of CC, which likely explains the observed results. The BF1 and BF2 values for WS, LE, and CH also saw reductions compared to their respective controls. Numerous studies have verified that amendments can mitigate the risk of Pb transfer from soil to the edible parts of vegetables52,56. The results indicate that BC and ZE reduce Cd and Pb accumulation in vegetables, with the greatest reduction observed in the CO treatment, thus enhancing food safety.
The effects on growth indices of vegetables
The growth indices of vegetables were showed in Table S6. Numerous studies affirm that soil amendments can positively impact vegetable yields by enhancing soil properties such as pH, CEC, SOM, and nutrient availability, as well as by reducing heavy metal migration rates47,57,58. Our findings are consistent with these previous studies, as we observed that amendments such as CO and BC improved vegetable growth indices through alterations in soil characteristics. For example, under NF conditions, CO increased the dried weight of LE by 33.5%, showcasing its effectiveness in promoting growth by improving soil quality. However, BC, while beneficial for WS and CC, did not significantly enhance the growth of LE, and even inhibited certain indices such as plant height and fresh weight. This outcome suggests that the exclusive application of BC may have limitations for specific crops like LE, aligning with Steiner et al.’s24 findings, which emphasized biochar’s role as an amendment rather than a direct nutrient source due to its low inherent nutrient content. For WS and CC, BC treatment showed a significant positive effect on plant growth, which increased their dried weight by 52.7 and 29.7%, respectively. In addition, amendments reduced the dried weight of CH, and the use of them for planting CH need to be re-estimated for field production.
Zeolite, a natural mineral, is not enriched in N, P, and K. Despite this, it contributes to plant growth by mitigating toxic effects59. In our study, the combined application of these amendments with fertilizers under TCF conditions improved plant growth indices more significantly than under NF conditions, suggesting a synergistic effect. For instance, compost increased the dried weights of WS, LE, and CH by 33.3%, 32.1%, and 14.5%, respectively, demonstrating its potential to promote healthier and higher-yielding crops. The above results indicate that the application of CO improved the growth index of vegetables, promoting healthier and higher-yielding crops.
Correlation between soil properties and heavy metal accumulation in vegetables
To explore the interactions between soil physicochemical properties and heavy metal accumulation in plants, we conducted a correlation analysis using SPSS software. The analysis results are presented in Table 5. The results indicate that the enrichment of Cd and Pb in vegetables shows a negative correlation with soil pH, CEC, and SOM.
The analysis indicates that the accumulation of Cd in WS shows a significant negative correlation with soil pH, CEC, and OM (P < 0.05), suggesting that an increase in these soil properties can reduce Cd accumulation in plants. Studies have shown that soil pH and organic matter are key factors in the migration and transformation of heavy metals such as cadmium and lead. Siddique et al.60. found that higher pH levels promote the formation of insoluble metal complexes, thereby reducing cadmium bioavailability. Kwiatkowska-Malina et al.61. demonstrated that an increase in SOM content through biochar addition enhances complexation with Cd ions, reducing their mobility and subsequent accumulation in vegetables, which is consistent with the findings of this study. In CH, Cd accumulation shows a significant negative correlation with AK and AP (P < 0.05), indicating that higher levels of these nutrients decrease Cd bioavailability. This observation aligns with the findings of some scholars, who reported that available potassium and phosphorus can reduce Cd uptake by plants through competitive inhibition in the soil matrix62,63.
In terms of Pb accumulation, Pb content in CC shows a significant negative correlation with catalase activity (P < 0.05), indicating that higher catalase activity in soil can reduce Pb mobility. Studies have shown that Pb adversely affects catalase activity, possibly due to Pb inhibiting the oxidation reactions facilitated by catalase in the soil64. Additionally, the analysis indicates a significant negative correlation between Pb accumulation in lettuce and available phosphorus (P < 0.01). The addition of amendments increased available phosphorus in the soil, promoting the formation of lead-phosphate complexes, thereby reducing Pb bioavailability to plants65.
Conclusion
This study confirms that the combined use of BC and ZE is an effective strategy for remediating Cd and Pb contaminated soils, particularly under fertilization conditions. The amendments enhanced soil pH, organic matter, cation exchange capacity, and nutrient availability, leading to reduced bioavailability of Cd and Pb. This stabilization effect was reflected in lower heavy metal accumulation in vegetable crops, demonstrating the amendments’ ability to mitigate metal uptake and promote safer agricultural production. The findings suggest that applying BC and ZE together can counteract soil acidification and heavy metal mobilization caused by fertilizers, making this combined amendment a promising approach for sustainable management of heavy metal-contaminated agricultural soils.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- SOM:
-
soil organic matter
- CEC:
-
cation exchange capacity
- SSA:
-
specific surface area
- OM:
-
organic matter
- TCF:
-
ternary compound fertilizer
- NF:
-
no fertilizer
- CK:
-
control
- BC:
-
biochar
- ZE:
-
zeolite
- CO:
-
combination of biochar and zeolite
- WS:
-
water spinach
- CC:
-
Chinese cabbage
- LE:
-
lettuce
- CH:
-
garland chrysanthemum
- BF:
-
enrichment factor
- TF:
-
transport factor
- AP:
-
available phosphorus
- AK:
-
available potassium
- AN:
-
available nitrogen
- F1:
-
acid extracted form
- F2:
-
reducible form
- F3:
-
oxidizable form
- F4:
-
residual form
- Cd:
-
cadmium
- Pb:
-
lead
- SD:
-
standard deviation
- LOD:
-
limits of detection
- LOQ:
-
limits of quantification
- QA:
-
quality assurance
- QC:
-
quality control
- ANOVA:
-
analysis of variance
References
Zheng, X. J. et al. Ecological risk assessment of heavy metals in the vicinity of tungsten mining areas, southern Jiangxi province. Soil. Sediment. Contamination: Int. J. 29, 665–679. https://doi.org/10.1080/15320383.2020.1763912 (2020).
Abbas, T. et al. Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol. Environ. Saf. 140, 37–47. https://doi.org/10.1016/j.ecoenv.2017.02.028 (2017).
Teng, Y., Ni, S., Wang, J., Zuo, R. & Yang, J. A geochemical survey of trace elements in agricultural and non-agricultural topsoil in Dexing area, China. J. Geochem. Explor. 104, 118–127. https://doi.org/10.1016/j.gexplo.2010.01.006 (2010).
Yimthiang, S. et al. Effects of environmental exposure to cadmium and lead on the risks of diabetes and kidney dysfunction. Int. J. Environ. Res. Public Health. 19, 2259. https://doi.org/10.3390/ijerph19042259 (2022).
Du, B. et al. Environmental and human health risks from cadmium exposure near an active lead-zinc mine and a copper smelter, China. Sci. Total Environ. 720, 137585. https://doi.org/10.1016/j.scitotenv.2020.137585 (2020).
Šljivić Husejnović, M., Janković, S., Nikolić, D. & Antonijević, B. Human health risk assessment of lead, cadmium, and mercury co-exposure from agricultural soils in the Tuzla Canton (Bosnia and Herzegovina). Arh. Hig. Rada Toksikol. 72, 268–279. https://doi.org/10.2478/aiht-2021-72-3533 (2021).
Satarug, S., Gobe, C., Vesey, G. A. & Phelps, R. D., K., Cadmium and Lead Exposure, Nephrotoxicity, and Mortality. Toxics 8, 86. (2020). https://doi.org/10.3390/toxics8040086
Alazaiza, M. Y. et al. Recent advances of nanoremediation technologies for soil and groundwater remediation: a review. Water 13, 2186. https://doi.org/10.3390/w13162186 (2021).
Yao, Z., Li, J., Xie, H. & Yu, C. Review on remediation technologies of soil contaminated by heavy metals. Procedia Environ. Sci. 16, 722–729. https://doi.org/10.1016/j.proenv.2012.10.099 (2012).
Oste, L. A., Lexmond, T. M. & Van Riemsdijk, W. H. Metal immobilization in soils using synthetic zeolites. J. Environ. Qual. 31, 813–821. https://doi.org/10.2134/jeq2002.8130 (2002).
Castaldi, P., Santona, L. & Melis, P. Heavy metal immobilization by chemical amendments in a polluted soil and influence on white lupin growth. Chemosphere 60, 365–371. https://doi.org/10.1016/j.chemosphere.2004.11.098 (2005).
Bashir, S. et al. Cadmium immobilization potential of rice straw-derived biochar, zeolite and rock phosphate: extraction techniques and adsorption mechanism. Bull. Environ Contam. Toxicol. 100, 727–732. https://doi.org/10.1007/s00128-018-2310-z (2018).
Cui, L. et al. The reduction of wheat cd uptake in contaminated soil via biochar amendment: a two-year field experiment. Bioresources 7, 5666–5676. https://doi.org/10.15376/biores.7.4.5666-5676 (2012).
Wu, J. et al. Magnetic biochar reduces phosphorus uptake by Phragmites australis during heavy metal remediation. Sci. Total Environ. 758, 143643. https://doi.org/10.1016/j.scitotenv.2020.143643 (2021).
Ge, Q. L., Tian, Q., Wang, S. F. & &Zhu, F. Synergistic effects of phosphoric acid modified hydrochar and coal gangue-based zeolite on bioavailability and accumulation of cadmium and lead in contaminated soil. Chin. J. Chem. Eng. 46, 150–160. https://doi.org/10.1016/j.cjche.2021.03.029 (2022).
MEEPRC (Ministry of Ecology and Environment of the People’s Republic of. China) Soil Environmental Quality–Risk Control Standard for Soil Contamination of Agricultural Land. GB 15618–2018. (2018). http://down.foodmate.net/standard/sort/3/53200.html
Zheng, X. J. et al. Assessment of Zeolite, biochar, and their combination for stabilization of multimetal-contaminated soil. ACS Omega. 5, 27374–27382. https://doi.org/10.1021/acsomega.0c03710 (2020).
Jiang, Y. et al. Dynamic responses of soil enzymes at key growth stages in rice after the in situ remediation of paddy soil contaminated with cadmium and arsenic. Sci. Total Environ. 830, 154633. https://doi.org/10.1016/j.scitotenv.2022.154633 (2022).
Sun, X. et al. Microbial sulfur and arsenic oxidation facilitate the establishment of biocrusts during reclamation of degraded mine tailings. Environ. Sci. Technol. acs. https://doi.org/10.1021/acs.est.3c10945 (2024).
Liu, Y., Wang, H., Zhang, H. & Liber, K. A comprehensive support vector machine-based classification model for soil quality assessment. Soil Tillage. Res. 155, 19–26. https://doi.org/10.1016/j.still.2015.07.006 (2016).
Hartley, W., Dickinson, N. M., Riby, P. & Lepp, N. W. Arsenic mobility in brownfield soils amended with green waste compost or biochar and planted with Miscanthus. Environ. Pollut. 157, 2654–2662. https://doi.org/10.1016/j.envpol.2009.05.011 (2009).
Lehmann, J. & Joseph, S. E. Biochar for environmental management: science, technology and implementation. (2024). https://doi.org/10.4324/9781003297673
Glaser, B., Lehmann, J. & Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal – a review. Biol. Fertil. Soils. 35, 219–230. https://doi.org/10.1007/s00374-002-0466-4 (2002).
Steiner, C. et al. Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered central amazonian upland soil. Plant. soil. 291, 275–290. https://doi.org/10.1007/s11104-007-9193-9 (2007).
Hossain, M. Z. et al. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2 (4), 379–420. https://doi.org/10.1007/s42773-020-00065-z (2020).
Lu, K. et al. Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals (cd, Cu, Pb and Zn) in contaminated soil. J. Environ. Manage. 186, 285–292. https://doi.org/10.1016/j.jenvman.2016.05.068 (2017).
Ibrahim, E. A., El-Sherbini, M. A. & Selim, E. M. M. Effects of biochar, zeolite and mycorrhiza inoculation on soil properties, heavy metal availability and cowpea growth in a multi-contaminated soil. Sci. Rep. 13, 6621. https://doi.org/10.1038/s41598-023-33712-z (2023).
Cai, Z. et al. Intensified soil acidification from chemical N fertilization and prevention by manure in an 18-year field experiment in the red soil of southern China. J. Soils Sediments. 15, 260–270. https://doi.org/10.1007/s11368-014-0989-y (2015).
Gląb, T. & Gondek, K. Mierzwa–Hersztek, M. Biological effects of biochar and zeolite used for remediation of soil contaminated with toxic heavy metals. Sci. Rep. 11, 1–11 (2021).
Peng, Z. et al. Heavy metal leachability in soil amended with zeolite-or biochar-modified contaminated sediment. Environ. Monit. Assess. 190, 1–13. https://doi.org/10.1007/s10661-018-7124-2 (2018).
Ruf, A., Kuzyakov, Y. & Lopatovskaya, O. Carbon fluxes in soil food webs of increasing complexity revealed by 14 C labelling and 13 C natural abundance. Soil Biol. Biochem. 38, 2390–2400. https://doi.org/10.1016/j.soilbio.2006.03.008 (2006).
Atkinson, C. J., Fitzgerald, J. D. & Hipps, N. A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant. soil. 337, 1–18. https://doi.org/10.1007/s11104-010-0464-5 (2010).
Akbar, S., Khatoon, S., Shehnaz, R. & Hussain, T. Natural zeolites: structures, classification, origin, occurrence and importance. Sci. Int. (Lahore). 11, 73–78 (1999). (1999).
Mierzwa-Hersztek, M. et al. Effect of coapplication of poultry litter biochar and mineral fertilisers on soil quality and crop yield. Zemdirb Agric. 105, 203–210. https://doi.org/10.13080/z-a.2018.105.026 (2018).
Zhao, H. et al. Nitrogen combined with biochar changed the feedback mechanism between soil nitrification and cd availability in an acidic soil. J. Hazard. Mater. 390, 121631. https://doi.org/10.1016/j.jhazmat.2019.121631 (2020).
Chen, H. et al. Reduced tillage and increased residue retention increase enzyme activity and carbon and nitrogen concentrations in soil particle size fractions in a long-term field experiment on Loess Plateau in China. Soil Tillage. Res. 194, 104296. https://doi.org/10.1016/j.still.2019.104296 (2019).
Tzanakakis, V. A., Monokrousos, N. & Chatzistathis, T. Effects of clinoptilolite zeolite and vermiculite on nitrification and nitrogen and phosphorus acquiring enzymes in a nitrogen applied agricultural soil. J. Soil. Sci. Plant. Nutr. 21, 2791–2802. https://doi.org/10.1007/s42729-021-00566-1 (2021).
Blake, L. & Goulding, K. Effects of atmospheric deposition, soil pH and acidification on heavy metal contents in soils and vegetation of semi-natural ecosystems at Rothamsted Experimental Station, UK. Plant. soil. 240, 235–251. https://doi.org/10.1023/A:1015731530498 (2002).
Xu, Y. X. et al. Effects of biochar addition on enzyme activity and fertility in paddy soil after six years. Ying Yong Sheng Tai Xue bao = J. Appl. Ecolog. 30, 1110–1118. https://doi.org/10.13287/j.1001-9332.201904.002 (2019).
Chang, E. H., Chung, R. S. & Tsai, Y. H. Effect of different application rates of organic fertilizer on soil enzyme activity and microbial population. Soil. Sci. Plant. Nutr. 53, 132–140. https://doi.org/10.1111/j.1747-0765.2007.00122.x (2007).
Liu, Z., Xie, W., Yang, Z., Huang, X. & Zhou, H. Effects of Manure and Chemical Fertilizer on Bacterial Community structure and soil enzyme activities in North China. Agronomy 11 (1017). https://doi.org/10.3390/agronomy11051017 (2021).
Yang, R. et al. Effects of interaction between enrofloxacin and copper on soil enzyme activity and evaluation of comprehensive toxicity. Chemosphere 268, 129208. https://doi.org/10.1016/j.chemosphere.2020.129208 (2021).
Mondal, M. et al. Zeolites Enhance Soil Health, Crop Productivity and Environmental Safety. Agronomy 11, 448. https://doi.org/10.3390/agronomy11030448 (2021).
Li, X. et al. Combined magnetic biochar and ryegrass enhanced the remediation effect of soils contaminated with multiple heavy metals. Environ. Int. 185, 108498. https://doi.org/10.1016/j.envint.2024.108498 (2024).
Puga, A. P., Abreu, C. A., Melo, L. C. A. & Beesley, L. Biochar application to a contaminated soil reduces the availability and plant uptake of zinc, lead and cadmium. J. Environ. Manage. 159, 86–93. https://doi.org/10.1016/j.jenvman.2015.05.036 (2015).
Senila, M., Cadar, O., Senila, L. & Angyus, B. S. Simulated bioavailability of heavy metals (Cd, Cr, Cu, Pb, Zn) in contaminated soil amended with natural zeolite using diffusive gradients in thin-films (DGT) technique. Agriculture 12, 321. (2022). https://doi.org/10.3390/agriculture12030321
Uchimiya, M., Lima, I. M., Klasson, K. T. & Wartelle, L. H. Contaminant immobilization and nutrient release by biochar soil amendment: roles of natural organic matter. Chemosphere 80, 935–940. https://doi.org/10.1016/j.chemosphere.2010.05.020 (2010).
Zheng, X. J., Qiu, S. Y., Zhou, B. C., Li, Q. & Chen, M. Leaching of heavy metals from tungsten mining tailings: a case study based on static and kinetic leaching tests. Environ. Pollut. 342, 123055. https://doi.org/10.1016/j.envpol.2023.123055 (2024).
Ye, S., Wang, L. & Liu, T. Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil. Open. Chem. 20, 1–9. https://doi.org/10.1515/chem-2021-0101 (2022).
Fadil, H. & Fahmi, A. H. Biochar enhanced chemical and biological properties of contaminated soils with lead. IOP Conference Series: Earth and Environmental Scienc 1259, 012024. (2023). https://doi.org/10.1088/1755-1315/1259/1/012024
Ma, Y. et al. Stabilization of Pb, Cd, and Zn in soil by modified-zeolite: mechanisms and evaluation of effectiveness. Sci. Total Environ. 814, 152746. https://doi.org/10.1016/j.scitotenv.2021.152746 (2022).
Kim, H. S. et al. Effect of biochar on heavy metal immobilization and uptake by lettuce (Lactuca sativa L.) in agricultural soil. Environ. Earth Sci. 74, 1249–1259. https://doi.org/10.1007/s12665-015-4116-1 (2015).
Zhang, Y. et al. Soil acidification caused by excessive application of nitrogen fertilizer aggravates soil-borne diseases: evidence from literature review and field trials. Agric. Ecosyst. Environ. 340, 108176. https://doi.org/10.1016/j.agee.2022.108176 (2022).
NHFPC, NMPA (National Health and Family Planning Commission of the People’s Republic of China and National Medical Products Administration) Foodstuff Safety National Criteria-max-imum Levels for Contaminants in Foodstuff. GB2762-2017 (in Chinese). Beijing:Standards Press of China. 1–17. (2017).
Lei, Z., Yang, L. & Yang, Z. Research progress on effects of common fertilizers on heavy metal accumulation in crops and its mechanism. J. Agricultural Sci. Technol. 22, 123 (2020).
Paul, S., Kauser, H., Jain, M. S., Khwairakpam, M. & Kalamdhad, A. S. Biogenic stabilization and heavy metal immobilization during vermicomposting of vegetable waste with biochar amendment. J. Hazard. Mater. 390, 121366. https://doi.org/10.1016/j.jhazmat.2019.121366 (2020).
Lwin, C. S., Seo, B. H., Kim, H. U., Owens, G. & Kim, K. R. Application of soil amendments to contaminated soils for heavy metal immobilization and improved soil quality—A critical review. Soil. Sci. Plant. Nutr. 64, 156–167. https://doi.org/10.1080/00380768.2018.1440938 (2018).
Derakhshan Nejad, Z., Jung, M. C. & Kim, K. H. Remediation of soils contaminated with heavy metals with an emphasis on immobilization technology. Environ. Geochem. Health. 40, 927–953. https://doi.org/10.1007/s10653-017-9964-z (2018).
de Campos Bernardi, A. C., Oliviera, P. P. A., de Melo Monte, M. B. & Souza-Barros, F. Brazilian sedimentary zeolite use in agriculture. Microporous Mesoporous Mater. 167, 16–21. https://doi.org/10.1016/j.micromeso.2012.06.051 (2013).
Siddique, A. B., Rahman, M. M., Islam, M. R. & Naidu, R. Influences of soil pH, iron application and rice variety on cadmium distribution in rice plant tissues. Sci. Total Environmen. 810, 152296. https://doi.org/10.1016/j.scitotenv.2021.152296 (2022).
Kwiatkowska-Malina, J. Functions of organic matter in polluted soils: the effect of organic amendments on phytoavailability of heavy metals. Appl. Soil. Ecol. 123, 542–545. https://doi.org/10.1016/j.apsoil.2017.06.021 (2018).
Li, Y. et al. Effect of phosphorus supplementation on growth, nutrient uptake, physiological responses, and cadmium absorption by tall fescue (Festuca arundinacea Schreb.) Exposed to cadmium. Ecotoxicol. Environ. Saf. 213, 112021. https://doi.org/10.1016/j.ecoenv.2021.112021 (2021).
Wang, K. et al. Effects of different potassium fertilizers on cadmium uptake by three crops. Environ. Sci. Pollut. Res. 26, 27014–27022. https://doi.org/10.1007/s11356-019-05930-4 (2019).
Sun, X. et al. Effects of lead pollution on soil microbial community diversity and biomass and on invertase activity. Soil. Ecol. Lett. 5, 118–127. https://doi.org/10.1007/s42832-022-0134-6 (2023).
Zhao, J. et al. Formation and aggregation of lead phosphate particles: implications for lead immobilization in Water Supply systems. Environ. Sci. Technol. 52, 12612–12623. https://doi.org/10.1021/acs.est.8b02788 (2018).
Funding
This work was funded by the National Key R&D Program of China [No. 2019YFC1805100], the National Natural Science Foundation of China [No.51664025], the Jiangxi Provincial Natural Science Foundation [No. 20232ACB203026], the Science and Technology Project of Ganzhou City [No. 2023PNS27982], and the Jiangxi Provincial Key Laboratory of Environmental Pollution Prevention and Control in Mining and Metallurgy [No. 2023SSY01071].
Author information
Authors and Affiliations
Contributions
Z.B.C: Project administration, Writing – original draft, Writing – review & editing. L.Y.Q: Writing – review & editing, Methodology, Conceptualization. Z.X.J: Writing – review & editing. W.Z.L: Writing – review & editing. L.Q: Writing – review & editing. C.M: Funding acquisition, Supervision.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, B., Liao, Y., Zheng, X. et al. The effects of amendments on cd and pb under different fertilizer application conditions. Sci Rep 15, 5385 (2025). https://doi.org/10.1038/s41598-025-90063-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-90063-7