Abstract
RNA interference (RNAi) is a sequence-specific gene silencing mechanism triggered by double-stranded RNA (dsRNA). Exploiting the RNAi mechanism to silence essential genes in insects has emerged as a promising new pest control strategy, and RNAi-based products are being developed for plant protection. RNAi has proven effective in silencing genes and causing mortality in the highly invasive emerald ash borer (EAB) (Agrilus planipennis) (Coleoptera: Buprestidae); however, a practical delivery method remains a barrier to its deployment. In this study, we evaluate the systemic distribution and retention of exogenously applied dsRNA in green ash (Fraxinus pennsylvanica) tissues to investigate the feasibility of dsRNA delivery through the host plant. To evaluate the distribution and persistence of dsRNA, seedings are exposed to EAB-specific dsRNA as a root soak, sampled 3, 7, 14, 21, and 30 d after exposure, and sectioned into root, woody-stem, soft-stem, and leaf tissues. Total RNA is extracted and evaluated by RT-PCR. Gel images and Sanger sequencing confirm the presence of exogenously applied dsRNAs, demonstrating successful uptake and translocation of dsRNAs throughout plant tissues. Our findings demonstrate that root application represents a viable delivery method for dsRNA in ash seedlings, supporting the potential of this technology in providing ash protection against EAB.
Similar content being viewed by others
Introduction
Emerald ash borer (EAB, Agrilus planipennis Coleoptera: Buprestidae) is a destructive wood-boring pest of forest, shade, and ornamental ash trees (Fraxinus spp.). Native to Northeastern Asia, EAB’s accidental introduction into North America is believed to have occurred in the mid-1990’s1, and it has since spread to 36 US states and 5 Canadian provinces2. EAB has killed millions of ash trees throughout its invaded range3, devastating ecosystems4, compromising biodiversity5, and overwhelming the budgets of affected municipalities6,7. Female beetles oviposit on ash bark, and neonate larvae bore through the bark to feed on the phloem, excavating serpentine galleries and destroying the cambial tissue before pupation and adult emergence. When populations are high larval feeding girdles trees, eliminating their capacity to translocate water and nutrients and leading to rapid tree mortality8.
Mitigating the EAB invasion in the US has been a challenge, with enormous expenditures for research and management. Insecticidal suppression has been successful9, but costs are high and the prevalence and distribution of ash in the eastern US make this impractical outside of urbanized areas. Classical biological control has provided the most hope and has been the primary management approach. Four hymenopteran parasitoids native to Asia, Spathius agrili (Braconidae), S. galinae (Braconidae), Tetrastichus planipennisi (Eulophidae), and Oobius agrili (Encyrtidae), have been intensively screened and are now laboratory reared in the USA for incorporation into biological control releases10. Natural enemy releases have potential to contribute to EAB suppression, but parasitoid efficacy is lower in the invaded range relative to the native range11, establishment is a critical step, and the effects of biocontrol agents on EAB populations is slow11,12,13,14. Systemic insecticides are widely deployed in highly managed areas and can be applied as soil drenches or trunk injections. Emamectin benzoate applied through trunk injections at approximately 2 to 3-year intervals is considered the gold standard15, causing up to 100% EAB mortality16. However, emamectin benzoate is a restricted use insecticide, is expensive, and chemical insecticides can affect non-target and beneficial species.
The continuing challenges associated with mitigating the EAB invasion demonstrate the need for additional management tools. One such tool that’s shown to be effective in laboratory and greenhouse research is the use of RNA interference or RNAi technology. RNAi is a natural cellular regulatory mechanism mediated by small RNAs that direct post-transcriptional gene silencing. Beyond its natural function in the cellular immune response, RNA-induced gene silencing is emerging as a next generation pest management technology, offering a highly specific, non-chemical approach to pest management17,18. The RNAi pathway is triggered by exogenous double stranded RNAs (dsRNAs) that the cell perceives as viruses; these are processed by the cellular RNAi machinery to silence target genes. The RNAi mode of action is based on sequence complementarity; by carefully designing dsRNAs targeting specific genes in a given species, this technology offers a more specific pest management approach than traditional broad-spectrum insecticides19,20.
RNAi technology works in EAB21; effective target genes that cause insect mortality have been identified22, its specificity has been demonstrated23, as has proof of concept for in planta delivery as a foliar spray24. However, practical deployment will require delivery methods beyond foliar spray. Here we investigate in planta behavior of EAB dsRNAs in greenhouse grown ash seedlings following hydroponic exposure to assess its systemic distribution and retention and to investigate the feasibility of root exposure as a dsRNA delivery approach for tree protection against EAB.
Results
Seedling measurements
The average length of experimental seedlings (n = 45) was 92.28 ± 2.14 cm and the average root collar diameter (RCD) was 0.95 cm ± 0.02. Seedling length did not vary among replicates (F2,42 = 0.15, p = 0.85), but differences among time intervals were detected (F4,40 = 2.84, p = 0.03), with seedlings sampled at day 21 d being shorter than their counterparts. Seedling RCD differed among replicates (F2,42 = 10.32, p = 0.0002), with seedlings from replicate 3 having higher RCD; however, no differences in RCD were observed among time intervals within replicates (F4,40 = 0.85, p = 0.50).
Recovery of exogenous dsRNA
Presence/absence of dsRNA was verified by the detection of an amplicon corresponding to the length of the EAB target sequence (302 bp) (Fig. 1a), along with the amplification of the endogenous control, the ash-specific gene ef1β (Fig. 1b), thus confirming dsRNA recovery; the absence of such EAB hsp amplicon in a given sample indicated lack of the dsRNA. Non-specific products (primer dimers) were present in some samples, but they did not interfere with the analysis of the results. Tissues from untreated control seedlings exposed only to water showed no amplification of the treatment (EAB hsp), and successful amplification of the endogenous control sequence (ef1β) (Figs. 2a and 2b), as expected.
Gel image demonstrating (a) successful amplification of EAB-specific hsp (302 bp) from ash treated tissues at different time-points, and (b) amplification of the endogenous control gene ef1β (116 bp) from the same samples; lanes labeled with M represents 1000 bp DNA marker (D.3, 14, and 30 on the top gel) or 100 bp DNA marker (D.7, 21 on the top gel and all time-points on the bottom gel). R = root, WS = woody stem, SS = soft-stem, L = leaf.
Gel images demonstrating (a) water negative control samples from different timepoints and tissue types showing no amplification of the target gene (hsp) on lanes 1–12, and (b) amplification of the endogenous control gene ef1β (116 bp) from the same samples. Lanes 13 and 14 represent no-template cDNA and no-template PCR respectively, and lanes labeled with M represent 100 bp DNA marker. R = root, WS = woody stem, SS = soft-stem, L = leaf.
Gel images indicate recovery of exogenous dsRNA in 98.3% of the samples (Table 1) following hydroponic treatment, and recovery across time points did not differ (Fig. 3a) (χ21,180 = 1.15, p = 0.28). There were differences in recovery between tissue types across time (Fig. 3b), with a slight increase in the probability of dsRNA recovery in woody stem tissue after day 3, and a slight reduction in dsRNA recovery in soft-stem at day 30. However, the overall dsRNA recovery in both tissue types was 95.5% and our statistical analysis showed no significant association between recovery and tissue type (χ23,180 = 4.56, p = 0.20).
Finally, our logistic regression modeling detected no association between dsRNA recovery and the multiple predictors (time, RCD, height, tissue, and replicate) (χ29, 180 = 11.73 p = 0.16), and none of the parameters were significant when each predictor variable was individually assessed (Table 2). The odds ratios were ≤ 1.0, indicating a negative relationship between the predictor and the response, however the p-values were non-significant in our model.
Sanger sequencing
The Sanger sequences of our samples were aligned to generate a consensus sequence of 283 bp. Pairwise alignments comparing the consensus sequence of the amplicons recovered from dsRNA treated seedlings and the EAB hsp annotated gene sequence resulted in ~ 94% similarity with 18 gaps (6%) (Supplementary Material S1).
Discussion
RNAi-induced gene silencing is emerging as a next-generation pest management approach. RNAi-based technologies for plant protection have undergone significant advances over the last decades, with improvements in delivery methods that do not rely on plant transformation25. Mitigation and management of EAB in North America continues to pose challenges; development of novel and innovative approaches that could include genetically based methods such as gene silencing using RNAi would be a welcome addition to an IPM toolbox. The ability to manipulate and trigger the RNAi pathway in planta is key to its eventual deployment for tree protection. In this study we demonstrate successful uptake, systemic movement, and long-term persistence of exogenous dsRNA applied through the roots to ash seedlings, providing proof of concept of root application as a potential delivery method for tree protection against EAB. Previously, dsRNA uptake, vascular translocation, and systemic distribution of labeled dsRNA in ~ 5 cm length ash seedlings were shown using confocal microscopy26. Here we take it a step further and use Reverse Transcription Polymerase Chain Reaction (RT-PCR), a semi-qualitative approach, to detect exogenous dsRNA applied through the roots to ~ 92 cm greenhouse-grown ash seedlings over a 30-day time interval.
Following total RNA extraction and cDNA synthesis, each sample collected from different tissue types and time points was evaluated in PCRs with primers targeting both the EAB-specific hsp gene to evaluate the presence of exogenous dsRNA, and the plant-specific ef1β gene, which served as a positive control for our protocol and methods. Our RT-PCR results indicate recovery of the exogenous dsRNA applied hydroponically to ash seedlings. The gel image (Fig. 1a) shows the presence of an amplicon corresponding to the EAB hsp gene (302 bp) in different tissue types and time points. Combined with our inability to amplify such amplicon using the same EAB-specific primers from the negative controls (Fig. 2a), the RT-PCR results provide initial evidence of the recovery of exogenous dsRNA using gel visualization, and Sanger sequencing confirms that the recovered material is our EAB-specific dsHSP.
The detection of EAB-hsp in tissues not directly exposed demonstrates plant uptake and systemic movement of dsRNA throughout the ash seedling. Overall, the exogenously applied dsRNA was detected in ~ 98% of the samples (Table 1), indicating consistent distribution and persistence over the 30-day bioassay. Using similar methodology, Bragg and Rieske (2022a)27 demonstrated systemic movement and persistence of dsRNAs in white oak seedlings following hydroponic exposure over a 7-day study; Hunter et al. (2012)28 used RT-qPCR to demonstrate recovery of dsRNA applied to citrus trees as a root drench 57 days post application.
Although the majority of work on topical application of dsRNA has been conducted in herbaceous plants29, there is mounting evidence of the uptake, systemic movement, and persistence of dsRNAs applied to woody plants through a) soil/root drench in grapevines and citrus28,30, b) trunk injection and petiole absorption in apple29, c) root soak in seedlings of white oak27 and loblolly pine31, and d) foliar application in ash seedlings24. Collectively, these findings support the occurrence of systemic spread and long-term persistence of dsRNAs applied hydroponically to woody plants, demonstrating consistent potential for in planta dsRNA delivery for tree pests.
We found overall dsRNA recovery was slightly lower in stem tissues, with 2 samples from woody-stem and 2 samples from soft-stem having no dsRNA detection at days 3 and 30, respectively. We speculate this was due to methodological limitations or stochastic dsRNA degradation rather than plant processing. A tissue-specific response in stem tissues leading to dsRNA degradation would prevent translocation of the molecule to distal leaf tissues, which was not observed here, as dsRNA was detected in 100% of the leaf samples evaluated. This hypothesis is supported by our logistic regression modeling showing no association between dsRNA recovery and the predictor variables (time, RCD, length, tissue, and replicate), suggesting that dsRNA could be recovered from any tissue type and time point.
The plant vascular system, particularly the xylem, is an RNase free environment32,33 so any dsRNA accessing this tissue should remain unprocessed and available for target pest consumption for long periods. Dalakouras et al. (2018)29 evaluated systemic movement of exogenous dsRNAs in woody plants following petiole absorption and trunk injection; northern blot and confocal imagery showed that hairpin RNA (hpRNA) is transported exclusively in woody plant xylem. This translocation path prevents the hpRNA from reaching the cell interior and from being processed by the plant RNAi machinery, thus remaining intact and available for pest consumption. This corroborates our RT-PCR and Sanger sequencing results and suggests that in our study the recovered dsRNA remained stable and unprocessed by the plant RNAi machinery. However, additional assessment of the presence of small RNAs is needed to investigate plant processing of exogenous dsRNAs.
Beyond the long-term persistence, exogenous dsRNA was also detected throughout plant tissues 3 days post application. This relatively rapid dissemination, likely via the plant vascular system, suggests that intact dsRNAs can reach distal, untreated plant tissues, fairly rapidly after exposure, potentially offering whole plant protection against target pests27. The quick dsRNA dissemination is particularly relevant for root application since dsRNA can be rapidly degraded in as little as 35 h under different soil types and environmental conditions34,35. Although in our study the first time point evaluated was 3 days post treatment, Bragg and Rieske (2022a, 2022b)27,31 provide evidence of recovery of exogenous dsRNA applied hydroponically in woody plant tissues in 24 h. This rapid dissemination suggests dsRNA is taken up by the plant vascular system and remains intact within plant tissues, thus protected from environmental degradation. However, further investigations addressing environmental degradation of dsRNAs under various soil conditions are needed to determine the success of soil drench as a delivery method in the ash/EAB system.
Previously, we used fluorescently labeled dsRNA and confocal microscopy to demonstrate uptake and systemic dissemination of exogenous dsRNA in small ash seedlings26, and in a recent study, we demonstrate systemic distribution and insecticidal activity of EAB-dsHSP delivered through foliar application to greenhouse-grown ash seedlings (~ 95 cm average height); our bioassay resulted in significant sublethal effects and target gene silencing in EAB larvae exposed to dsRNA sprayed seedlings24. Here we demonstrate systemic movement and long-term retention of dsRNAs applied to ash seedlings as a root soak, and in an un-replicated associated pilot study, RT-PCR and Sanger sequencing also provided evidence of dsRNA systemic dissemination and persistence up to 30 days following trunk application (Supplementary Material S2).
Further investigations of the environmental fate and stability of dsRNA applied as a soil drench as well as potential dsRNA degradation by the plant RNAi machinery are needed as we move forward with RNAi technology targeting EAB. Collectively, our findings of systemic movement and long-term persistence of exogenous applied dsRNAs, support our assertion of root soak as a potential exogenous dsRNA delivery method targeting EAB, demonstrating that appropriately timed applications represent a feasible means of providing season long protection of ash trees against EAB.
Conclusions
RNAi technology combines effectiveness with minimal environmental impact due to its sequence-specific mode of action. dsRNA, the key trigger molecule of RNAi, has been shown to provide plant protection without the need for integration of dsRNA-expressing constructs as transgenes25, stimulating the development of RNA-based biopesticides as a potential alternative to chemical-based control.
Limitations for a commercially viable product to overcome include cost-effective and large-scale production, stable delivery of the topically applied dsRNA and extension of the duration of protection36. Current methods for large-scale dsRNA production consist of in vivo production (E. coli, symbiotic bacteria, yeast, bacteriophage) and in vitro synthesis using cell-free production platforms17. Over the past decade, numerous biotech start-ups have invested in research and development of RNAi-based technology. Pebble Labs (https://www.pebblelabs.com) and Renaissance Bioscience (https://www.renaissancebioscience.com/) are examples of companies working on cost-effective mass production of dsRNAs/siRNAs via microbial fermentation; companies like AgroSpheres (https://www.agrospheres.com/) and Trillium Ag (https://trillium.ag/) are focused on RNA delivery platforms to improve RNAi stability37. Additionally, Ledprona™ (www.greenlightbiosciences.com), the first sprayable dsRNA product targeting Colorado potato beetle (Leptinotarsa decemlineata) has been approved by the U.S. Environmental Protection Agency (EPA)38. With advances in mass production and stability of RNAi-based technologies, non-transgenic RNAi-based products are expected to reach global markets soon17.
This study serves as a proof of concept and demonstrates plant uptake, systemic movement and long-term persistence of EAB-specific dsRNA applied hydroponically to greenhouse grown ash seedlings. Combined with previous studies showing similar results following foliar spray and trunk application, our findings support the feasibility of the efficacy of exogenous dsRNA application as a potential means to deliver RNAi to EAB.
Materials and methods
Ash seedlings
Dormant green ash seedlings (mean = 70 cm from root tip to terminal bud, average RCD = 1 cm), identified and grown by the PA Dept. Conservation and Natural Resources, Spring Mills, PA, were obtained and potted in general purpose Promix BX growing medium (Premier Tech Horticulture, 92 Rivière-du-Loup, QC) in 10.16 × 35.56 cm tall tree pots (Stuewe & Sons Inc., OR, USA) 40–45 days before use and maintained in the greenhouse (~ 18–22 ºC, 15:9 L:D) for the duration of the experiments. Seedlings were potted in groups of ~ 30 to ensure developmental uniformity between replicates.
Selected genes
The EAB heat shock 70-kDa protein gene (hsp)22 was selected to evaluate uptake, translocation and distribution, and persistence of exogeneous EAB specific dsRNA in ash tissues following root application. Elongation factor β (ef1β)39, an ash specific gene, served as an endogenous and quality control gene for the RT-PCR methods.
dsRNA synthesis
Total RNA was isolated from EAB larvae using Trizole reagent (ThermoFisher, USA), followed by cDNA synthesis using M-MLV reverse transcriptase (ThermoFisher, USA) according to the manufacturer’s instructions. PCR templates for in vitro synthesis of dsRNA were generated using hsp specific primers22. PCR cycle conditions were 94 °C for 2 min, 30 cycles at 94 °C for 30 s, 60 °C for 30 s, and 72°C for 45 s, finishing with an extension step at 72 °C for 10 min. The PCR template was purified using a PCR purification kit (Qiagen Inc., Valencia, CA, USA). After PCR purification, dsRNA synthesis was performed using the MEGAscript RNAi Kit (Ambion Inc., Foster City, CA, USA), following the manufacturer’s instructions. The quality of the dsRNA was checked by electrophoresis and quantified using a spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
dsRNA exposure
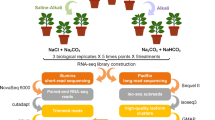
Seedlings were gently removed from the pots and potting medium, and the root system rinsed for 1–2 min with tap water followed by soaking in deionized water (dd H2O) to ensure removal of any remaining soil. Seedlings were individually placed into clear glass cylinders (7.5 × 40.5 cm, WGV International, CA, USA) containing either 200 µg of EAB specific dsHSP diluted in 1 L of dd H2O, or water only to serve as the negative control27,31. Seedlings were randomly assigned to receive either the dsRNA or to serve as a negative control, as well as to exposure intervals of 3, 7, 14, 21, and 30 days. Seedling assay cylinders were topped with aluminum foil to prevent evaporation and maintained in the greenhouse (18–22 °C, 15:9 L:D) for the duration of the study. At 7-day intervals dd H2O was added to maintain a total volume of 1 L; after 14 days the dsRNA solution was replaced by dd H2O. There were three seedlings per time interval per replicate for the dsHSP treatment (n = 15 per replication) and one negative control seedling per time interval (n = 5 total). Three replicates were performed at approximately 2-week intervals in June and July 2021. The experimental design and workflow are illustrated on Supplementary Materials S3.
Plant processing and RNA isolation
At the established time intervals, seedlings were removed from assay cylinders, rinsed with tap water for 30 s and measured (total seedling length (cm)) from root tip to the apical meristem, and root collar diameter (mm))27. Seedlings were then sectioned into a) root; b) woody stem, representing the previous year’s growth; c) soft stem, representing the stem tissue grown during the current season; and d) leaf. Subsequently, each tissue type was soaked in a 1% bleach solution for 30 s to remove any remaining dsRNA and rinsed for 30 s in dd H2O. Following protocols from Bragg and Rieske (2022a,b)27,31 samples were processed, total RNA was isolated and used for the cDNA synthesis.
Recovery of exogenously applied dsRNA
Reverse transcription-polymerase chain reaction (RT-PCR) and gel electrophoresis
RT-PCR was used to detect the presence of exogenously applied dsRNA in each tissue type and each time interval. Following total RNA extraction, sample concentration and quality were analyzed via absorbance measurements of 260/280 nm and 260/230 nm (NanoDrop Technologies, Wilmington, DE, US) to determine the RNA concentration and check for the presence of contaminants such as proteins, carbohydrates, and phenol. 1000 ng of RNA was used for cDNA synthesis using a M-MLV reverse transcriptase kit (ThermoFisher, USA). To increase the specificity of the reverse transcription, we used a combination of both Oligo(dT) and EAB-hsp reverse primers. After the reverse transcription reaction, each cDNA sample served as a template for PCRs targeting both the EAB hsp gene, and the ash ef1β gene that served as a positive control to confirm the success of RNA extraction, cDNA synthesis, and PCR amplification. To increase the sensitivity of the PCR, we selected nested primers flanking a 302 bp region inside the original dsHSP sequence (468 bp) (Table 3). PCR cycle conditions were 2 min at 94 °C followed by 40 cycles of 30 s at 94 °C, 1 min at 50 °C, and 1 min at 68 °C, finishing with an extension step at 68 °C for 5 min. PCR amplification was visualized by gel electrophoresis to evaluate the presence of the target amplicons in the different tissues (roots, woody and soft stem, leaf) and the different time points (3, 7, 14, 21, 30 days).
A logistic regression model was used to estimate the factors that influenced dsRNA recovery in treated tissues. The dsRNA recovery in each sample was treated as a binary dependent variable, with samples equal to 1 representing successful amplification of the EAB gene, and samples equal to 0 representing unsuccessful recovery and no detection of the target gene. For the logistic regression model, recovery of dsRNA served as the response variable, time, seedling length, and RCD were considered continuous variables, and replicate and tissue type were treated as categorical. The logistic regression analysis was conducted using R-4.2.2.
Sanger sequencing
A subset of PCR samples (n = 5) representing the different tissue types and time points was chosen randomly and Sanger sequenced to confirm the presence of exogenously applied dsRNA within the plant material, resulting in 10 reads (five forward and five reverse). The online tool Benchling [Biology Software] was used to generate the consensus reading and Emboss Stretcher40 was used to create a pairwise alignment between the resulting consensus sequence and EAB hsp annotated sequence to assess the sequence similarities.
Data availability
RNA sequence data generated for this study are published on the NCBI Sequence Read Archive (SRA) online sequence depository under the accession number PRJNA1130544.
References
Siegert, N. W., McCullough, D. G., Liebhold, A. M. & Telewski, F. W. Dendrochronological reconstruction of the epicentre and early spread of emerald ash borer in North America. Divers. Distrib. 20, 847–858 (2014).
Emerald Ash Borer | Animal and Plant Health Inspection Service. https://www.aphis.usda.gov/plant-pests-diseases/eab.
Aukema, J. E. et al. Economic impacts of non-native forest insects in the continental United States. PloS One 6, e24587 (2011).
Ward, S. F., Liebhold, A. M., Morin, R. S. & Fei, S. Population dynamics of ash across the eastern USA following invasion by emerald ash borer. For. Ecol. Manag. 479, 118574 (2021).
Gandhi, K. J. K. & Herms, D. A. North American arthropods at risk due to widespread Fraxinus mortality caused by the Alien Emerald ash borer. Biol. Invasions 12, 1839–1846 (2010).
Herms, D. A. & McCullough, D. G. Emerald Ash Borer Invasion of North America: History, Biology, Ecology, Impacts, and Management. Annu. Rev. Entomol. 59, 13–30 (2014).
Hudgins, E. J., Koch, F. H., Ambrose, M. J. & Leung, B. Hotspots of pest-induced US urban tree death, 2020–2050. J. Appl. Ecol. 59, 1302–1312 (2022).
Haack, R. A. et al. The emerald ash borer: a new exotic pest in North America. Newsl. Mich. Entomol. Soc. 47(p1–5), 1 (2002).
Sadof, C., Hughes, G., Witte, A., Peterson, D. & Ginzel, M. Tools for staging and managing emerald Ash Borer in the Urban Forest. Arboric. Urban For. https://doi.org/10.48044/jauf.2017.002 (2017).
Gould, J. S., Booth, T., Sawallich, N., Petrice, T. Emerald Ash Borer Biological Control Release and Recovery Guidelines. US Dep. Agric. Anim. Plant Health Insp. Serv. For. Serv. North. Res. Stn. Agric. Res. Serv. 75 P (2024).
Sun, J., Koski, T.-M., Wickham, J. D., Baranchikov, Y. N. & Bushley, K. E. Emerald ash borer management and research: Decades of damage and still expanding. Annu. Rev. Entomol. 69, 239–258 (2024).
Bauer, L. S., Duan, J. J., Gould, J. R. & Driesche, R. V. Progress in the classical biological control of Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) in North America. Can. Entomol. 147, 300–317 (2015).
Duan, J. J., Bauer, L. S., Van Driesche, R. G. & Gould, J. R. Progress and challenges of protecting North American Ash trees from the emerald ash borer using biological control. Forests 9, 142 (2018).
Kashian, D. M., Bauer, L. S., Spei, B. A., Duan, J. J. & Gould, J. R. Potential impacts of emerald ash borer biocontrol on ash health and recovery in Southern Michigan. Forests 9, 296 (2018).
Smitley, D. R., Herms, D. A. & Davis, T. W. Efficacy of soil-applied neonicotinoid insecticides for long-term protection against emerald ash borer (Coleoptera: Buprestidae). J. Econ. Entomol. 108, 2344–2353 (2015).
Sadof, C. S., McCullough, D. G. & Ginzel, M. D. Urban ash management and emerald ash borer (Coleoptera: Buprestidae): facts, myths, and an operational synthesis. J. Integr. Pest Manag. 14, 14 (2023).
He, L., Huang, Y. & Tang, X. RNAi-based pest control: Production, application and the fate of dsRNA. Front. Bioeng. Biotechnol. 10, 1080576 (2022).
Christiaens, O., Whyard, S., Vélez, A. M. & Smagghe, G. Double-Stranded RNA technology to control insect pests: Current status and challenges. Front. Plant. Sci. https://doi.org/10.3389/fpls.2020.00451 (2020).
Huvenne, H. & Smagghe, G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: A review. J. Insect Physiol. 56, 227–235 (2010).
Bachman, P. M. et al. Ecological risk assessment for DvSnf7 RNA: A plant-incorporated protectant with targeted activity against western corn rootworm. Regul. Toxicol. Pharmacol. 81, 77–88 (2016).
Zhao, C., Gonzales, M. A. A., Poland, T. M. & Mittapalli, O. Core RNAi machinery and gene knockdown in the emerald ash borer (Agrilus planipennis). J. Insect. Physiol. 72, 70–78 (2015).
Rodrigues, T. B. et al. Development of RNAi method for screening candidate genes to control emerald ash borer Agrilus planipennis. Sci. Rep. 7, 7379 (2017).
Pampolini, F. & Rieske, L. K. Emerald Ash Borer Specific Gene Silencing Has No Effect on Non-target Organisms. Front. Agron. https://doi.org/10.3389/fagro.2020.608827 (2020).
Pampolini, F. & Rieske, L. K. Foliar Application of dsRNA to Induce Gene Silencing in Emerald Ash Borer: Systemic Distribution, Persistence, and Bioactivity. Forests 14, 1853 (2023).
Cagliari, D. et al. Management of Pest Insects and Plant Diseases by Non-Transformative RNAi. Front. Plant Sci. https://doi.org/10.3389/fpls.2019.01319 (2019).
Pampolini, F., Rodrigues, T. B., Leelesh, R. S., Kawashima, T. & Rieske, L. K. Confocal microscopy provides visual evidence and confirms the feasibility of dsRNA delivery to emerald ash borer through plant tissues. J. Pest Sci. 93, 1143–1153 (2020).
Bragg, Z. & Rieske, L. K. Feasibility of Systemically Applied dsRNAs for Pest-Specific RNAi-Induced Gene Silencing in White Oak. Front. Plant Sci. https://doi.org/10.3389/fpls.2022.830226 (2022).
Hunter, W. B., Glick, E., Paldi, N. & Bextine, B. R. Advances in RNA interference: dsRNA Treatment in Trees and Grapevines for Insect Pest Suppression. Southwest. Entomol. 37, 85–87 (2012).
Dalakouras, A. et al. Delivery of Hairpin RNAs and Small RNAs Into Woody and Herbaceous Plants by Trunk Injection and Petiole Absorption. Front. Plant Sci. https://doi.org/10.3389/fpls.2018.01253 (2018).
Ghosh, S. K. B., Hunter, W. B., Park, A. L. & Gundersen-Rindal, D. E. Double-stranded RNA Oral Delivery Methods to Induce RNA Interference in Phloem and Plant-sap-feeding Hemipteran Insects. J. Vis. Exp. JoVE https://doi.org/10.3791/57390 (2018).
Bragg, Z. & Rieske, L. K. Spatial Distribution and Retention in Loblolly Pine Seedlings of Exogenous dsRNAs Applied through Roots. Int. J. Mol. Sci. 23, 9167 (2022).
Doering-Saad, C., Newbury, H. J., Bale, J. S. & Pritchard, J. Use of aphid stylectomy and RT-PCR for the detection of transporter mRNAs in sieve elements. J. Exp. Bot. 53, 631–637 (2002).
Taning, C. N. T., Andrade, E. C., Hunter, W. B., Christiaens, O. & Smagghe, G. Asian Citrus Psyllid RNAi Pathway – RNAi evidence. Sci. Rep. 6, 38082 (2016).
Dubelman, S. et al. Environmental Fate of Double-Stranded RNA in Agricultural Soils. PLOS ONE 9, e93155 (2014).
Joaquim, M. E. S. et al. Dissipation of DvSnf7 Double-Stranded RNA in Brazilian Soils. Agric. Environ. Lett. 4, 190016 (2019).
Fletcher, S. J., Reeves, P. T., Hoang, B. T. & Mitter, N. A Perspective on RNAi-Based Biopesticides. Front. Plant Sci. https://doi.org/10.3389/fpls.2020.00051 (2020).
Luo, X. et al. Risk assessment of RNAi-based biopesticides. New Crops 1, 100019 (2024).
US EPA, O. EPA Registers Novel Pesticide Technology for Potato Crops. https://www.epa.gov/pesticides/epa-registers-novel-pesticide-technology-potato-crops (2023).
Rivera-Vega, L., Mamidala, P., Koch, J. L., Mason, M. E. & Mittapalli, O. Evaluation of Reference Genes for Expression Studies in Ash (Fraxinus spp.). Plant Mol. Biol. Report. 30, 242–245 (2012).
Madeira, F. et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 47, W636–W641 (2019).
Acknowledgements
We thank Bruce Downie and Lynnette M.A. Dirk for fundamental technical assistance, Zachary Bragg for statistical assistance, and Kylie Bickler and Anna Hawse (University of Kentucky) for laboratory assistance.
Funding
This publication is from the Kentucky Agricultural Experiment Station and is published with the approval of the director. This work was supported by the USDA Forest Service Forest Health Research and Education Center and through funds provided by USDA APHIS AP20PPQS&T00C0032, the University of Kentucky, and the Kentucky Agricultural Experiment Station under McIntire-Stennis 2351197000.
Author information
Authors and Affiliations
Contributions
F.P. and L.K.R. conceived the experiments; F.P. conducted the experiments; F.P. and L.K.R. analyzed the results; F.P. and L.K.R. prepared the manuscript. All authors approve the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pampolini, F., Rieske, L.K. Root uptake, translocation and persistence of EAB-specific dsRNA in ash seedlings. Sci Rep 15, 6378 (2025). https://doi.org/10.1038/s41598-025-90266-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-90266-y