Abstract
In oil and gas pipelines, the coexistence of native microorganisms with various solid compounds, such as corrosion products, scales, and reservoir sand, is well-established. However, research focused on understanding the collective impact of these components on carbon steel corrosion has been limited, with most studies concentrating on inert deposits like clay and sand. This study assessed the impact of electrically conductive deposits — magnetite (Fe3O4) and troilite (FeS) — on UDC and UDMC of carbon steel, employing silica (SiO2) as an inert control. A multifaceted approach, combining international corrosion standards, microscopy, and molecular microbiology techniques demonstrated that abiotic corrosion rates correlated with the electrical conductivity of the deposits. Magnetite, the deposit with the highest electrical conductivity, led to the highest uniform corrosion rate (0.110 mm/year). The troilite-containing reactor exhibited a corrosion rate of 0.017 mm/year, while the lowest rate was observed in the presence of sand (0.006 mm/year), the deposit with the lowest electrical conductivity. In biotic conditions, the highest average corrosion and pitting rates were also associated with magnetite, identifying UDC and UDMC in the presence of magnetite as the most corrosive scenario among the three deposits. Nevertheless, the average corrosion and pitting rates did not follow the same trend as in abiotic conditions, where electrical conductivity properties governed the behaviour. In biotic conditions, the heightened pitting corrosion observed in the presence of silica over troilite was attributed to higher metabolic activity and increased cell concentrations in the microbial consortium under this scenario. These findings underscore the complexity of UDMC, suggesting that an active microbial consortium does not solely drive severe corrosion but is influenced by multiple factors. This study offers insights into the role of different deposits and native microbial communities, paving the way for more targeted corrosion mitigation strategies to improve infrastructure longevity in oil and gas systems.
Similar content being viewed by others
Introduction
Corrosion represents a critical engineering problem that results in millions of dollars in losses to the oil and gas industry annually1. Metal deterioration can lead to pipe failures that, besides the financial consequences, cause substantial environmental damage and represent high health and safety risks for workers in oil and gas fields2. Some zones of pipelines transporting production fluids are readily covered by different types of deposits, such as scales, corrosion products, sand, and schmoo, leading to a corrosion phenomenon termed under-deposit corrosion (UDC)3,4. Several researchers have investigated UDC under different testing conditions and with different deposits and have proposed the formation of galvanic cells and the creation of mass transfer barriers as the main UDC mechanisms5,6,7,8. For instance, Huang et al.7 studied under-deposit CO2 corrosion in the presence of a silica deposit in an inhibited aqueous solution and found an acceleration of localised corrosion underneath each sand grain when compared against a bare specimen. The authors concluded that local damage was initiated by a galvanic effect between the corrosion inhibitor-covered areas (cathodes) and corrosion inhibitor-uncovered areas (anodes) found underneath each sand grain. Likewise, Tan et al.8 demonstrated the development of an oxygen differential aeration cell between bare and sand-covered steel when investigating the baseline of O2 corrosion through the wire beam electrode (WBE) method. Anodic currents were localised on sand-covered electrodes, while cathodic currents were dispersed on the bare electrodes. The oxygen reduction reaction occurred in the bare electrodes where the diffusion barrier effect of the sand to oxygen was absent.
The significance of galvanic cell formation as a UDC mechanism becomes particularly pronounced when considering the prevalence of conductive phases within oil and gas deposits in both CO2 and H2S environments. Among the semiconductive iron sulphides commonly encountered in sour pipelines are mackinawite (FeS1-x), troilite (FeS), pyrrhotite (Fe1-xS), and pyrite (FeS2)9,10. Penetration rates up to 14.6 mm/y were reported by Ning et al.11 in a 8-day corrosion experiment where an in situ formed iron sulphide deposit comprising mackinawite, greigite, and pyrite played a crucial role. In contrast, CO₂-dominated environments reveal the presence of the semiconductive mineral magnetite (Fe3O4) alongside the typically found iron carbonates12,13,14. Various authors have demonstrated the corrosion-inducing properties of magnetite15,16. A recent study by Owen et al.14 established that magnetite triggers both uniform and galvanic corrosion when coupled with carbon steel in CO2 environments, leading to pit depths ranging from 15 to 20 μm and corrosion rates of 0.5 mm/y during autoclave testing at 250 °C, under a pressure of approximately 45 bar. Collectively, researchers investigating UDC with electroconductive deposits attribute a cathode role to these phases. These conductive minerals can form a galvanic cell with a metal surface that behaves as an anode, thereby accelerating corrosion locally and uniformly.
Another type of corrosion faced by pipelines transporting production fluids that poses a significant economic challenge for the oil and gas industry is microbiologically influenced corrosion (MIC)17,18. MIC involves the deterioration of metals due to the activity of microorganisms near metal surfaces, accounting for approximately 40% of instances of internal corrosion. Despite its identification in 1963, MIC only garnered attention five decades later19, prompting extensive research into identifying implicated microorganisms and understanding their corrosion mechanisms. However, MIC remains complex, lacking a singular, generalised explanation tied to a specific biochemical reaction or microbial species. Moreover, several environmental factors have shown to impact MIC. For example, Salgar-Chaparro et al.20demonstrated the impact of temperature on MIC. The authors found that the temperature significantly influenced the composition of the microbial community within a system, thereby directly impacting the corrosion mechanisms and severity. In a subsequent study Salgar-Chaparro et al.21, studied the impact that the water chemistry has on the risk of MIC. The authors found that the presence of sulphate and thiosulphate increased up to 7 times the uniform corrosion rates and up to 4 times the pitting rates caused by the multispecies consortium compared to systems where sulphur compounds were absent. Similarly, Suarez et al.22 underscored the impact of deposits on MIC severity. Their research demonstrated a 1-fold decrease in uniform corrosion rates caused by a thermophilic microbial consortium in the presence of sand compared to its absence. The authors attributed these findings to the sand acting as a physical barrier, hindering microbial cells from reaching the metal surface and initiating corrosion.
Building upon the information above, it is plausible to contend that the presence of conductive deposits constitutes an environmental variable with the potential to impact MIC. Previous studies have documented an increase in MIC rates in the presence of conductive mineral phases on corroding metals23,24. For instance, Enning et al.23demonstrated that a biogenic electroconductive black corrosion crust facilitated the direct uptake of electrons by microbial sulphate-reducing bacteria (SRB) from the metal surface, resulting in corrosion rates of up to 0.7 mm/y. This corrosion mechanism, termed Electrical MIC (EMIC), occurs when microorganisms directly adsorb electrons from elemental metallic iron, utilizing it as their electron donor25.
Despite the wealth of literature on UDC, conclusive insights into the specific roles played by various electro-conductive deposits in influencing the severity of UDC remain elusive. Furthermore, considering the widespread coexistence of deposits and native microorganisms in field conditions, the quantification of the risk of MIC under electroconductive deposits assumes paramount significance. This study aimed to gain insights into three different but related phenomena, the influence of major mineral components (Fe3O4, FeS, and SiO2) commonly found in deposits within oil and gas pipelines on MIC, the impact that the presence of microorganisms has in UDC (a phenomenon termed as under deposit microbial corrosion (UDMC), and the relation that exists between the electrical conductivity of deposits and the corrosion of metals.
Materials and methods
Mineral deposit characterisation and sterilisation
To replicate the primary constituents of deposits typically encountered in oil and gas pipelines, three commonly found inorganic minerals (iron oxide, silicon dioxide, and iron sulphide) of analytical reagent grade quality were procured from Sigma-Aldrich (St. Louis, USA). These minerals were employed as the representative deposits in the study. The particle size distribution of these deposits was determined through laser diffraction analysis and assessed their specific surface area using the Brunauer, Emmett, and Teller (BET) method; results are presented in Table 1. Furthermore, the mineral phases of the three deposits were examined via powder X-ray diffraction (XRD) using a Bruker D8A diffractometer with a Cu Kα source (λ = 0.15405 nm) over a 5 to 100° range with a 0.02° step size—the DIFFRAC.EVA software (Version 5.2)26and the PDF-4 + 2020 database were used to analyse the XRD spectra. To ensure the sterility of the representative deposits, both the iron oxide and silicon dioxide underwent autoclaving at 134 °C for three minutes at 208 kPa before the experimental setup. Iron sulphide was subjected to gamma-ray radiation (50 kGy) for 56 h to achieve sterilisation. The choice of gamma irradiation, rather than autoclaving, for sterilising iron sulphide was made due to its susceptibility to surface oxidation under elevated temperatures, pressure, and humidity11,27,28.
Electrical conductivity measurement
The electrical resistivity of the mineral samples was determined following the Wenner four-electrode method29,30. In this regard, an acrylic resistivity box equipped with copper plates as outer electrodes and fine copper rods as inner electrodes was used (Figure S1). The dimensions of the box were kept as 100 mm in length, 50 mm in width, and 50 mm in height, with a fixed separation distance of 50 mm between the inner electrodes.
A multi-meter was employed to measure the AC current applied to the mineral specimens through the outer plate electrodes, and the potential drop across the inner electrodes was determined. These measurements allowed us to calculate each specimen’s electrical resistance (R). Subsequently, each mineral’s resistivity (ρ) value was determined by performing triplicate calculations employing Eq. (1).
Where R is the electrical resistance [Ω], A is the cross-sectional area of the material [m2], and L is the length of the material [m]. In order to achieve precise measurements of the electrical resistivity of the three mineral powders, each of them was fully packed into the acrylic box described in the preceding paragraph.
Carbon steel sample preparation
Carbon steel 1030 grade used in the corrosion experiments had the following chemical composition (weight%): C (0.30), Mn (0.69), Si (0.24), S (0.030), P (0.010), Cr (0.022), Ni (0.001), Mo (0.001), Cu (0.005), and Fe (balance). For experimental purposes, the steel coupons were laser-cut into rectangular coupons measuring 12 mm x 22 mm x 9 mm. Coupons were subsequently electro-coated with inert epoxy resin (Powercron®, 6000CX) to confine the working surface to one side per sample (2.6 cm2). The working surface underwent wet-grinding using silicon carbide paper to achieve a 600-grit finish. Following this, all samples were degreased with acetone, washed with ethanol, and dried with nitrogen gas.
Before the corrosion test, the metal coupons were weighed and subjected to a 30-minute UV sterilisation process. Next, these samples were carefully positioned within custom 3D-printed epoxy holders specially designed to ensure precise control over the simulated deposits’ diameter and height across all experimental conditions. In the case of iron oxide-covered samples, 3.8 ± 0.2 g of the deposit were used. For silicon dioxide-covered samples, 3.6 ± 0.2 g were used, and for iron sulphide-covered samples, 10.6 ± 0.2 g were used to achieve a deposit height of 10 mm.
Oilfield microbial consortium
The microbial consortium used in this study was sourced from a sand deposit collected from a high-pressure (HP) separator at an Australian oil production facility experiencing microbiologically influenced corrosion (MIC), as previously described31. In brief, one gram of field sand was inoculated into synthetic produced water in Hungate tubes under a 99.9% N₂ gas atmosphere and incubated at 40 °C for 20 days. After incubation, an aliquot from the inoculated solution was transferred to fresh synthetic produced water prior to the experiment, establishing the microbial consortium for this study.
UDMC test
Corrosion experiments were conducted in custom-made 2 L glass bioreactors. Six different conditions, described in Table 2, were replicated in the bioreactors to evaluate the effect of deposit electrical conductivity on UDMC.
The test solution employed in these experiments consisted of synthetic produced water supplemented with nutrients to support microbial growth. This synthetic produced water had the following composition: 212.5 mM NaCl, 7.43 mM CaCl2·2H2O, 5 mM KCl, 27.4 mM MgCl2·6 H2O, 0.04 mM SrCl·6H2O, 10 mM D-glucose, 13.8 mM Na2SO4·5H2O, 11.3 mM Na2S2O3·5H2O, 1.54 mM NaHCO3, 26 mM Na-formate, 26 mM Na-lactate, 20 mM Na-acetate, 12.4 mM NH4NO3, 1.3 g L−1 casamino acids (Bacto™), and, 1 L of ultrapure water (Milli-Q system). The pH of the test solution was adjusted to 7.2 ± 0.2 using sodium hydroxide.
Solution stirring at 180 rpm and a constant temperature of 40 ºC were maintained within the reactors by a stirring IKA RTC digital hotplate with temperature control. The corrosion experiments were conducted under an N2 atmosphere maintained by continuing sparging during the 20 days of testing. Biotic reactors (reactors including microorganisms) were inoculated with the recovered microbial consortium in a concentration of 2.6 × 106 cell/mL, and microbial activity was sustained by daily test solution replenishment (40% of the reactor’s total volume) over the experimental duration by using a peristaltic pump (Thermo Fisher Scientific, FH100M).
Reactors monitoring
Microbial metabolism was monitored throughout the immersion period. Secondary metabolites (nitrates and sulphide) present in the test solution were measured every five days using spectrophotometry (Hach™, DR3900). The Methylene Blue Method and the Ferrous Sulphate Method were used for the determination of sulphide and nitrites, respectively. The test solution pH was measured using a portable pH meter (Thermo Scientific™, Star A221).
Electrochemical measurements
To investigate the influence of the three different deposits on the electron dynamics of carbon steel, galvanic currents were measured between a deposit-covered carbon steel coupon (designated as working electrode 1, WE1) with a bare carbon steel coupon (working electrode 2, WE2). These measurements were conducted in a split-chamber configuration under the six distinct conditions detailed in Table 2. The experimental parameters, including test solution composition, carbon steel specimens, temperature, and gas atmosphere, were identical to those employed in the UDMC experiments. In biotic experiments, WE1’s chamber was inoculated with a microbial consortium at an initial concentration of 2.6 × 10⁶ cells/mL.
A Nafion™ 117 membrane was prepared following the manufacturer’s guidelines and aseptically placed between the two chambers to maintain separation of the biotic chamber (containing the deposit-covered carbon steel coupon) from the abiotic chamber (containing the bare carbon steel coupon). It was confirmed that the membrane had no effect on the conductivity of the system. The galvanic current between the electrodes was recorded every second for 160 h using a VMP3 multichannel potentiostat (Biologic) operating in galvanic current mode.
Analytical methods
Corrosion measurements
To assess the extent of corrosion following a 20-day testing period, triplicate coupons from each tested condition were cleaned. Initially, the simulated deposits were gently removed with ultrapure deionized water. Subsequently, corrosion products strongly attached to metal surfaces were effectively eliminated via repeated ultrasonic cleaning cycles in Clarke’s solution, following the ASTM G1-03 Standard32. The weight of the cleaned metal samples was then measured using a high-precision mass balance (Mettler Toledo, ME204), and general corrosion rates were calculated using the equation described in NACE SP-0775 standard practice33:
Where CR = corrosion rate, mm/y; k = constant, 8.76 × 104; W = weight loss, grams; D = density, g/cm3; A = area in cm2; T = time of exposure in hours.
Furthermore, the exposed surfaces of the triplicate coupons were analysed using a 3D surface profilometer (SolariusTM, SolarScan) with a spot size of 10–100 μm and a resolution of 0.2 μm, in accordance with the ASTM Standard Guide for Examination and Evaluation of Pitting Corrosion (G46–21)34. Profilometry analysis was employed to identify the maximum intrusion depth on each coupon. The pitting rates (mm/y) were calculated by dividing each condition’s deepest point (in millimetres) by the exposure time in days35, using the following formula:
It is important to highlight that the pitting rate is a linear projection assuming pit growth over time.
Microbial concentration
To estimate the concentration of sessile microorganisms within the three different deposits, three coupons were removed from each biotic reactor at the end of the exposure. The steel coupons were gently washed with sterile phosphate-buffered saline (PBS) solution to remove planktonic cells and immersed in falcon tubes containing 20 mL of PBS solution with Tween 20 (0.1% w/v final concentration). As outlined in previous studies, the detachment of sessile bacteria from the carbon steel and bulk deposits was carried out through a sonication method21. The falcon tubes containing the samples were sonicated for 10 s, followed by 15 s on ice, repeating for five cycles to detach sessile microorganisms from the deposits. After sonication, the falcon tubes were left undisturbed for 2 min to facilitate the sedimentation of the deposits at the tube’s base. Subsequently, the liquid phase, housing the detached cells, was carefully transferred to a new falcon tube.
Microbial concentration within the different deposits was measured using the serial dilution method described in the standard testing method NACE TM019435. Specifically, 1 mL of the PBS solution containing microbial cells was inoculated into 9 mL of fresh test solution (see Sect. 2.5) and serially diluted 10-fold in triplicate for MPN estimation. Inoculated vials were incubated at a temperature of 40 °C for 20 days, and the confirmation of bacterial growth was achieved through the observation of alterations in both the colour and turbidity of the culture medium. The concentration of microorganisms within each sample was determined by the MPN 3-tube standard table36. Sessile bacteria counts were expressed as cell per gram of deposit (cell/g). The remaining 19 mL of PBS solution containing detached cells was used for further microbiological analyses.
Microbial activity
The concentrations of Adenosine triphosphate (ATP), Adenosine diphosphate (ADP), and Adenosine monophosphate (AMP) of sessile microorganisms developed within the three deposits were measured by using the AXP assay and the Quench-Gone Organic Modified (QGO–M) test kit (LuminUltraTM), following manufacturer’s instructions. Cells that are actively growing tend to have higher ATP levels compared to those under stress. For this reason, ATP concentration is used as a field test method for estimating viable biomass in oil and gas systems35. However, when it comes to understanding microorganisms’ physiological and nutritional status, the relative ratio of ATP, ADP, and AMP concentrations holds more significance than the absolute ATP concentration37. This ratio is known as the adenylate energy charge (AEC), a term coined by Atkinson and Walton in 196738. In general, AEC values exceeding 0.8 indicate actively growing microorganisms, reflecting minimal stress. Values ranging between 0.5 and 0.8 suggest microorganisms in a stationary growth phase, indicating a moderate stress level. Conversely, values below 0.5 are associated with senescent or dormant microorganisms, signifying significant stress or stress-induced dormancy.
The microbial activity levels and physiological state of the sessile communities at the end of the 20-day immersion test were determined by measuring the luminescence of 5 mL of the remanent PBS solution containing the detached cells with a luminometer (LuminUltraTM, PhotonMaster). The AEC ratio was calculated according to the AXP test kit (LuminUltraTM) formula:
Microbial community taxonomic profiles
The total DNA and RNA of sessile microorganisms developed within the three deposits were extracted from the remanent PBS solution containing the detached cells using the Norgen DNA/RNA/Protein kit as directed by the manufacturer (Norgen Biotek Corp). Nucleic acids concentration was quantified using a Nanodrop 182 spectrophotometer (NanoDrop™ Lite Spectrophotometer). Following extraction, RNA was isolated from extracted RNA samples using the Turbo DNA-free kit (Invitrogen) according to the manufacturer’s instructions. Afterwards, RNA was purified by using an RNeasy MinElute cleanup kit (Qiagen) and transcribed into complementary DNA (cDNA) by the SuperScript IV first-strand synthesis system (Invitrogen), as described elsewhere21.
The amplicon library preparation followed a standard procedure for the Illumina MiSeq platform (Illumina, San Diego, CA)39. The V3-V4 hypervariable region of the bacteria and archaea 16S rRNA gene was amplified using the Forward Primer = 5’ TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG, and Reverse Primer = 5’ GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC40. Sequencing was performed by the Marshall Centre at the University of Western Australia (UWA) using paired-end sequencing on an Illumina MiSeq device.
A bioinformatics analysis of the metabarcoding samples was performed with the Quantitative Insights Into Microbial Ecology Software (QIIME 2)41. Data underwent trimming and quality filtering through the “dada2 denoise-paired” plugin. Forward and reverse sequences read lengths were truncated at 280 and 220 positions, respectively, with the plugin “- -p-trunc- len-f 280” and “- -p-trunc-len-r 220”. Taxonomy was assigned to each amplicon sequence variant (AVS) by employing BLAST (“feature-classifier classify-blast”) against the pre-trained SILVA reference database (v138)42. The ASV tables generated by QIIME2 were exported into R (v4.2.1) for taxonomic composition analysis. The reported relative abundances in the active and total communities represent the triplicate samples’ average.
Statistical analysis
The data presented in this study represents the average values obtained from three distinct experimental replicates, as previously described43,44,45. Statistical differences in the average uniform corrosion and pitting rates across the different experimental scenarios were analysed by using PAST (Version 4.10). The normality of each variable within the dataset was assessed using the Shapiro-Wilk method. A one-way analysis of variance (ANOVA) was performed in conjunction with Tukey’s post-hoc means separation test to determine whether statistically significant distinctions existed among normally distributed variables. It is important to note that the statistical tests were considered significant when the p-value was equal to or below 0.05.
Results
Mineral deposit characterisation
Figure 1 shows XRD patterns of the three procured minerals. Silicon dioxide exhibited an XRD pattern aligned with the crystallographic card PDF#04–008-7812, indicating its composition as silica (cristobalite). The XRD analysis for iron oxide (Fe3O4) revealed peak positions and relative intensities consistent with those found in the crystallographic card PDF#04–007-1061, confirming its identity as magnetite. In the case of iron sulphide, the XRD pattern agreed with the crystallographic card PDF#01–071-4468 signifying its presence as troilite. Nevertheless, while the analysis revealed that 77% of the sample comprised troilite, it also indicated a composition of 8.7% corresponding to pyrrhotite and 19.0% to an amorphous component.
Electrical resistivity of mineral deposits
Table 3 presents the results of soil resistivity measurements on the three mineral deposits by the Wenner method. Since electrical resistivity (ρ) is inversely proportional to electrical conductivity (σ), it was evidenced that among the three procured minerals, magnetite possesses the higher ability to conduct electrical current (1.0 × 10−3 S/m), followed by troilite (3.4 × 10−4 S/m). In contrast, silica has the highest ability to resist the flow of electrical current (4.3 × 10−5 S/m).
Changes in water chemistry throughout exposure
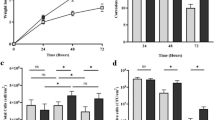
Figure 2 shows the pH and concentrations of total sulphides (H2S, HS–) and nitrites (NO2–) in the test solution for the different experimental scenarios and throughout the exposure period. Figure 2A, B and C demonstrate that the SB and MB reactors exhibited similar pH values and concentrations of nitrites and sulfides during the immersion period, while the IB reactor followed a distinct trend. From day five (5) onward, the IB reactor showed a more rapid decrease in nitrites concentration compared to the slight decrease evidenced in the MB and SB reactors. Moreover, by day 10, the IB reactor reached a pH of 6.7 and a sulphide concentration of 6 mg/L — the lowest pH and highest sulphide concentration measured across all experimental conditions and sampling days.
Corrosion measurements
Average uniform corrosion rates, determined by analysing coupon mass loss after exposure to the different conditions, are presented in Fig. 3A. A consistent trend in uniform corrosion rates emerges in both biotic and abiotic conditions, i.e., in the presence and absence of microorganisms, respectively, across the range of mineral deposits. Conditions including magnetite exhibited the highest corrosion rates, followed by conditions with troilite, while the lowest corrosion rates were observed in conditions containing silica. Statistical analysis further reinforces these observations, highlighting the significant differences between the corrosion rates for MA in comparison to silica (17 times higher) and troilite (6 times higher) (Table S1). Moreover, results evidenced that the presence of microorganisms caused a significant increase in the corrosion rates across all three deposits (Table S1). Specifically, the average uniform corrosion rate of MA (0.110 mm/y) increased five times in the presence of microorganisms, resulting in an average uniform corrosion rate of 0.611 mm/y for MB. Similarly, the average uniform corrosion rate of SA (0.006 mm/y) increased 16 times in the presence of microorganisms, leading to an average uniform corrosion rate of 0.099 mm/y (SB). The average uniform corrosion rate of TA (0.017 mm/y) increased 16 times in the presence of microorganisms, resulting in an average uniform corrosion rate of 0.278 mm/y (TB). In summary, these findings emphasise that magnetite poses a significantly higher corrosion risk to carbon steel in comparison to silica and troilite. Additionally, these results underscore that the occurrence of UDMC leads to more severe corrosion scenarios when compared to UDC alone.
Average uniform corrosion and pitting rates were calculated for the six conditions evaluated: abiotic silica (SA), abiotic magnetite (MA), abiotic troilite (TA), biotic silica (SB), biotic magnetite (MB), biotic troilite (TB). (A) Average uniform corrosion rates are calculated based on weight loss measurements. (B) Pitting rates are calculated based on the deepest pit on the working surface. Error bars represent standard deviations of the average uniform corrosion and pitting rates calculated in three independent replicates.
Pitting rates, derived from the average maximum observed pit depth in each condition, are presented in Fig. 3B. The pitting rates in abiotic conditions exhibited a parallel trend to that observed in average uniform corrosion rates (Fig. 3A), where the highest pitting was observed in conditions containing magnetite (0.63 mm/y), followed by conditions with troilite (0.29 mm/y) and the lowest pitting rates were observed in conditions with silica (0.20 mm/y). Conversely, the pitting rates in biotic conditions exhibited a different trend; the lowest pitting rates did not correspond to the silica (1.56 mm/y) but to troilite (1.24 mm/y). In biotic conditions, MB pitting rates were significantly higher than those caused by SB and TB. However, no significant differences were evidenced between SB and TB (Table S2). These results indicate that magnetite not only poses a higher uniform corrosion risk to carbon steel but also a higher pitting risk in comparison to silica and troilite.
Moreover, pitting rates revealed a significant increase due to the presence of microorganisms across all three deposits. The pitting rates of SA experience an 8-fold increase in the presence of microorganisms, resulting in an average pitting rate of 1.55 mm/y for SB. Similarly, the average pitting rates of MA increased 5-fold in the presence of microorganisms, leading to an average pitting rate of 3.04 mm/y, and the average pitting rates of TA increased 4-fold in the presence of microorganisms, resulting in an average pitting rate of 1.24 mm/y. These findings indicate that localised corrosion resulting from UDMC, represents a more severe corrosion scenario than localised corrosion caused by UDC alone.
Surface profilometry was conducted to assess the depth of pits formed on the exposed surfaces of the carbon steel coupons (Fig. 4, Figure S2). Since profilometry images evidenced different surface roughness in the metal samples exposed to the different deposits in abiotic conditions, surface measurements were performed (Table S3). The surfaces of carbon steel samples exposed to silica (Fig. 4A and B) exhibited an average roughness of 1.80 and 0.66 μm under biotic and abiotic conditions, respectively. When exposed to magnetite (Fig. 4C and D), the surfaces displayed an average roughness of 7.05 and 1.04 μm under biotic and abiotic conditions, respectively. Finally, samples exposed to troilite (Fig. 4E and F) resulted in an average surface roughness of 2.85 and 0.78 μm under biotic and abiotic conditions, respectively. Notably, pit depths as deep as 42.7 μm were found on the surface of MA, while in TA, the maximum pit depth was 17.6 μm.
Across all scenarios, deeper pits were observed in the presence of microorganisms (UDMC) compared to their absence (UDC) for all three evaluated deposits. Carbon steel exposed to the magnetite deposit in conjunction with the microbial consortium exhibited the most severe pitting, with a maximum pit depth of 201.3 μm. Furthermore, different corrosion patterns were observed across the different biotic scenarios: Fig. 4B shows that the steel exposed to silica and the microbial consortium suffered severe localised corrosion, characterised by the presence of narrow-mouth pits dispersed across the sample surface. Figure 4D shows a distinct corrosion pattern on the metal coupons exposed to magnetite and the microbial consortium, with the formation of large, wide, and deep cavities. Lastly, Fig. 4F highlights a corrosion pattern similar to scenario SB; however, the pits appear smaller and less profound on the steel exposed to troilite and the microbial consortium.
Microbial cell concentration
The concentrations of microbial cells within the three evaluated deposits at the end of the immersion period are shown in Fig. 5. Higher cell numbers were observed in the reactor with silica deposit (11 × 1010 cell/g), representing a five-order magnitude increase compared to the reactors with magnetite (4.6 × 105 cell/g) and a seven-order magnitude increase compared to the reactors with troilite (4.1 × 103 cell/g). These results suggest that the presence of silica substantially impacted the growth of the microbial community.
Microbial activity
In Fig. 6, the ATP, ADP, and AMP concentrations are presented, along with the corresponding calculated energy charge (AEC) within the sessile microbial communities. The results reveal distinct trends among the microbial communities exposed to the three different deposits. Specifically, the microbial community exposed to silica exhibited higher concentrations of ATP (67.6 µg/cm2) and ADP (21.27 µg/cm2) when compared to the communities exposed to magnetite and troilite, which exhibited ATP concentrations of 30.54 and 0.03 µg/cm2, and ADP concentrations of 9.06 and 0.84 µg/cm2, respectively. This observation is consistent with the higher AEC (1) measured in the silica-exposed microbial community, suggesting that these microorganisms were in an actively growing phase. In contrast, the AEC for the magnetite-exposed microbial community (0.8) indicates that these microorganisms were under stress and in a stationary growth phase. Finally, the troilite-exposed microbial community (TB) showed the lowest AEC (0.2) among the three scenarios, implying that the microbial cells within the troilite were under significant stress and in a state of senescence. This finding is supported by the fact that the concentration of AMP was higher than ATP in this microbial community.
Microbial activity level measurements align with the findings from microbial cell concentrations (Sect. 3.4). Higher cell numbers were observed in the silica-exposed scenario compared to magnetite and troilite, reflecting the absence of stress and active growth. In contrast, the lower cell concentrations found in the troilite-exposed microbial community are associated with high stress and a senescent state within the community.
Microbial community taxonomic profiles
This study aimed to identify the total and active microbial communities within biofilms that developed on various deposits using DNA and RNA-based amplicon sequencing. However, during the process of quality filtering the raw reads, challenges related to RNA sample quality in the troilite sample were encountered, which led to the successful generation of high-quality sequences for the three samples only from DNA-based samples. The active microbial community from RNA-based samples was only determined for magnetite and silica samples.
The taxonomic classification of the active microbial community uncovered the presence of three distinct microbial genera. The relative abundances of these genera in each experimental scenario are visually presented in Fig. 7. Molecular identification further revealed variations in the relative abundances of these microbial genera among the different deposits. Notably, Tepidibacillus emerged as the dominant genus across all scenarios, with a relative abundance of 72% in silica, 50% in magnetite, and 44% in troilite. For the magnetite scenario (MB), Pseudomonas shared dominance with Tepidibacillus, representing 48% of the microbial community, while in silica, it constituted 27% of the total community, and in troilite, 16%. Bacillus exhibited lower relative abundances within the microbial communities of silica (0.8%) and magnetite (1%) but accounted for 31% of the microbial community in the troilite scenario.
The comparison of the taxonomic classification of the DNA and RNA profiles did not show significant differences in the relative abundances of total and active populations in the reactors with silica and magnetite. While Pseudomonas dominated the active community in the reactor with magnetite, with a relative abundance of 52%, in the active community, this genus accounted for 48%. Similarly, while Tepidibacillus dominated the total community in the reactor with magnetite, in the active community, this genus accounted for 47%. In the reactor with silica, the active community was dominated by Tepidibacillus, with a relative abundance of 59%, while in the total community, this genus accounted for 72%. Pseudomonas accounted for 40% of the active community in the reactor with silica, while in the total community, this genus accounted for 27%. This diversity profiling analysis highlighted variations in sessile bacterial community composition in response to environmental conditions, including the presence of different deposits.
Split-chamber configuration
The effects of different deposits on the net galvanic current between a deposit-covered carbon steel electrode and a bare carbon steel electrode under abiotic and biotic conditions are shown in Fig. 8. Overall, the net galvanic currents between the two electrodes in the presence of sand (Fig. 8A) and troilite (Fig. 8C), in both biotic and abiotic conditions, were negligible throughout the 6-day incubation period, indicating minimal net electron transfer between the electrodes. In the presence of troilite (Fig. 8C), the current in abiotic conditions shifted slightly from − 0.5 µA to 9 µA, while in biotic conditions it shifted from − 10 µA to 11 µA. Conversely, the current recorded in the presence of silica remained stable at approximately 0.05 µA under abiotic conditions but shifted from − 5 µA to 1 µA under biotic conditions.
In contrast, the net galvanic currents in the presence of magnetite were consistently negative, ranging from − 15 to −55 µA, indicating that this deposit promoted redox processes between the electrodes. These results align with and support the uniform corrosion rates calculated from weight loss measurements.
Current fluctuations observed under biotic conditions for all three deposits were absent in the abiotic controls. This suggests changes in the microenvironment due to biofilm formation or alterations in solution chemistry as a result of microbial metabolism.
Discussion
Carbon steel under-deposit corrosion in abiotic conditions
This section will discuss the influence of major mineral components (Fe3O4, FeS, and SiO2) commonly found in deposits within oil and gas pipelines on carbon steel’s abiotic corrosion under anaerobic conditions. The experimental findings evidenced that the nature of these deposits significantly affects the occurrence of localised and uniform corrosion of carbon steel. These results align with previous studies in this field. For instance, Pandarinathan et al.46, studied carbon steel corrosion under three mineral deposits (analytical reagent grade) and found that alumina promotes a more severe uniform and localised corrosion than calcite and silica. The authors determined that the acidity generated through alumina hydrolysis likely contributed to the increase in the surface roughness and acceleration of metal corrosion by affecting the microenvironment beneath the deposit. Similarly, Jeannin et al.47 examined the corrosion of carbon steel under clay deposits, including montmorillonite, kaolinite, and chlorite, and found that the three deposits caused similar uniform corrosion but only montmorillonite-induced pitting corrosion. This effect was attributed to the partial dissolution of montmorillonite in acidic environments, resulting in some areas of carbon steel being exposed to corrosive species in the bulk solution.
In this work, the experimental matrix incorporated three mineral deposits with distinct electrical conductivities, as measured experimentally (Table 1) and reported in the literature48,49,50,51,52. The presence of electrical conductive phases within deposits has been linked to carbon steel corrosion due to the potential for galvanic coupling between the deposit and the steel when exposed to a corrosive electrolyte11,53. Results of this study revealed a direct relationship between the deposit’s electrical conductivity and the severity of carbon steel corrosion. Specifically, the presence of a magnetite deposit, with an electrical conductivity of 1.0 × 10 –3 S/m, increased the average uniform corrosion rates on carbon steel by a factor of 6 compared to troilite and 17 compared to silica (Fig. 3A). Moreover, pitting corrosion rates under magnetite were twice as high as under troilite, with an electrical conductivity of 3.4 × 10 –4 S/m, and 3 times as high as under silica (Fig. 3B), with an electrical conductivity of 4.3 × 10 –5 S/m. Notably, magnetite was responsible for the highest average uniform and pitting corrosion on carbon steel.
Both magnetite and troilite are classified as p-type semiconductors49,50,54,55. Therefore, they can accelerate carbon steel corrosion via galvanic coupling13,56,57. Previous studies have demonstrated the substantial impact of these minerals on carbon steel corrosion under specific conditions. Magnetite, for instance, has been observed to result in corrosion rates as high as 5 mm/y on carbon steel due to galvanic coupling in CO2environments16. In contrast, troilite/pyrrhotite has been associated with carbon steel corrosion rates ranging from 2 mm/y to 3 mm/y due to galvanic coupling when exposed to aqueous CO2solutions58. However, it is noteworthy that this study represents a significant departure from previous research, as it is the first, to the best of the authors knowledge, to comprehensively investigate the risk of both uniform corrosion and pitting for both magnetite and troilite under identical conditions. The observed trend in uniform corrosion rates—magnetite-covered > troilite-covered > silica-covered—was directly associated with the trend in electrical conductivity of the deposits (magnetite > troilite > silica). This finding demonstrates that the electrical conductivity properties of deposits play a direct role in influencing uniform corrosion. Furthermore, split-cell incubations revealed that the mechanism through which deposit conductivity influences carbon steel corrosion is by promoting anodic and cathodic reactions between the deposit-covered areas and the bare metal surface (Fig. 8).
Carbon steel under-deposit microbial corrosion
The comparative analysis of both corrosion rates and pitting rates induced by the microbial consortium in the presence of magnetite, silica, and troilite, in contrast to the abiotic corrosion rates occurring under the same conditions, clearly demonstrates the consortium’s role in accelerating the kinetics of UDC. This acceleration is attributed to the corrosive metabolites that the microorganisms produce during their metabolic activities. The microbial community in all three conditions comprises species from the genera Pseudomonas, Tepidibacillus, and Bacillus (Fig. 7). While previous corrosion studies have extensively associated Pseudomonas and Bacillus with corrosion of various metals, there is a noticeable gap in the literature regarding the contribution of Tepidibacillus to any corrosion process. The genus Pseudomonascan engage in the corrosion of metals through multiple mechanisms, including nitrate reduction59,60, acid production61, iron oxidation62,63, and the formation of oxygen concentration cells in aerobic environments64. Similarly, members of the Bacillusgenus exhibit a wide range of metabolic capabilities, which could potentially accelerate corrosion processes. These capabilities encompass nitrate reduction coupled to iron oxidation65,66,67, iron/manganese oxidation68, and organic acid production69. Finally, the few species within the Tepidibacillusgenus have shown capable of utilizing various terminal electron acceptors such as iron, iron oxides and other transition metals, nitrate, sulphur, and thiosulphate, while using different organic and inorganic substrates as the electron donors70,71,72. Consequently, it is plausible that Tepidibacillus may engage in corrosion mechanisms similar to those observed in Pseudomonas and Bacillus, including potential involvement in sulphur/thiosulphate reduction.
To get insights into the metabolic activities carried out by the microbial consortium, the chemistry of the test solution was monitored throughout the immersion period by measuring pH, total sulphides, and nitrites (Fig. 2). Results indicated that the microbial consortium was engaged in sulphate/thiosulphate reduction and nitrate reduction. Although nitrate reduction coupled to iron oxidation could not be conclusively demonstrated with the used methods, this mechanism cannot be entirely disregarded in this study. During sulphate/thiosulphate reduction, HS− is produced, which reacts with H+ present in the bulk liquid form H2S, a potent cathodic and anodic reactant73. Similarly, nitrites are produced during nitrate reduction, and the accumulation of high concentrations of nitrites on the metal surface can cause a decrease in the corrosion potential and induce the formation of pits74,75. Considering these MIC potential mechanisms, the highest average uniform corrosion rates and pitting in biotic conditions were expected to be found with magnetite, as the baseline UDC corrosion in MA was the highest among the three deposits. Results confirmed that UDMC was more severe in the presence of magnetite. This could be attributed to the introduction of corrosive microbial metabolites influencing redox reactions at the metal-deposit interface and the baseline water reduction occurring in magnetite. This interpretation is supported by the ZRA incubation results, which display current fluctuations under biotic conditions (Fig. 8), with the hisghest fluctuation evidenced in MB. Previous studies have attributed the corrosion effect caused by microorganisms and deposits to the accumulation of corrosive metabolites under the deposit22,76,77.
At the same time, this study investigated the impact of three major mineral components (Fe3O4, FeS, and SiO2) commonly found in deposits within oil and gas pipelines on the UDMC of carbon steel. Average uniform corrosion measurements varied among the different deposits, and these variations were attributed to the electrical conductivity of the deposits. Firstly, because higher average uniform corrosion rates were observed in the deposit with the highest electrical conductivity (magnetite), followed by the mineral with the second-highest electrical conductivity (troilite), and the lowest average uniform corrosion rates were found in the deposit exhibiting the lowest electrical conductivity (silica). Secondly, the relative abundances of different taxa constituting the microbial populations in SB (inert) and MB (conductive) were very similar, suggesting that differences in the severity of corrosion were not associated with specific microbial species. Finally, higher numbers of cells and higher levels of metabolic activity were measured in the microbial consortium in SB (Figs. 4 and 5), indicating that a greater concentration of microorganisms was not correlated with higher corrosion rates. This finding suggests that not only is a highly active microbial consortium needed for severe MIC rates, and that it is instead a multifactorial phenomenon.
Pitting rates also varied among the different deposits, however, these variations were ascribed to the consortium microbial metabolic state in each condition and deposits’ inherent characteristics. Previous studies have shown an association between the microbial activity levels of microorganisms and the severity of MIC72,73,74,75,76,77,78,79,80. In this research, it was found that the microbial consortium in SB showed higher levels of metabolic activity than in TB, suggesting that the majority of cells were continuously growing, as evidenced by the higher cell concentrations in SB than in TB. Moreover, the microbial community structure changes support the proposed hypothesis to some extent. Pseudomonas and Tepidibacillus dominated the microbial community in the systems with the absence of stress or moderate stress levels. However, in an environment that posed severe stress to the bacteria, there was a shift in the relative abundances of the genera that dominated the microbial community, and the genus with the lowest abundance (Bacillus) thrived.
The microbial activity levels and cell concentrations observed in TB indicate that interactions with depotsit can impose significant stress on the microorganisms. The intense stress experienced by the microbial consortium in TB could be attributed to the dissolution of the pyrrhotite phase in the commercially purchased FeS (see Sect. 2.1). Pyrrhotite dissolution to troilite is known to occur in acidic conditions, resulting in the production of H2S81. The high H2S concentration (6.2 mg/L) and pH reduction (6.6) measured in the test solution on day 10 (Fig. 2) support the hypothesis. The combined effects of H2S resulting from microbial metabolism and that resulting from pyrrhotite oxidation likely resulted in toxic levels of H2S for the microorganisms82, as evidenced by a low AEC and cell concentration. In the presence of a heavily stressed microbial community, localised corrosion that may have occurred during the initial hours or days of the immersion period ceased as the microorganisms became senescent.
Conclusions
The influence of major mineral components (Fe3O4, FeS and SiO2) commonly found in deposits within oil and gas pipelines on the interplay of UDC and MIC on carbon steel was investigated. The main findings of the study are as follows:
In biotic and abiotic conditions, the highest average uniform corrosion and pitting rates were found in the presence of magnetite, establishing UDC and UDMC with magnetite as the most corrosive scenario across the three deposits. The main factor contributing to magnetite’s high corrosiveness is its electrical conductivity.
- ZRA measurements revealed that troilite and silica deposits generate negligible galvanic currents between deposit-covered carbon steel and bare carbon steel, indicating minimal electron transfer and, consequently, limited influence on corrosion. In contrast, magnetite deposits consistently generated significant negative currents throughout the incubation period, demonstrating that the corrosion acceleration caused by magnetite is due to its ability to promote electron transfer between deposit-covered and bare metal areas. This behavior highlights the role of deposit electrical conductivity in facilitating anodic and cathodic reactions, thereby enhancing corrosion processes.- Microorganisms accelerated both average uniform corrosion and pitting rates of deposited carbon steel regardless of the deposit type and its electrical conductivity. Notably, the microbial consortium demonstrated the highest acceleration of carbon steel uniform corrosion in the presence of silica, which was associated with a heightened microbial activity and cell concentration compared to magnetite and troilite.
- Despite troilite exhibiting higher electrical conductivity than silica, heightened pitting corrosion took place in the presence of silica over troilite. This phenomenon was attributed to the greater metabolic activity and cell concentrations observed in the microbial consortium under this scenario.
- The microbial community assembly identification based on DNA revealed that microbial populations shift under different deposit conditions and stress levels. While Pseudomonas and Tepidibacillus dominated environments with acceptable stress levels offered by silica, the prevalence of Bacillus increased under stressful conditions offered by troilite. These findings contribute to the understanding of how microbial communities’ adaptation to an environment impacts corrosion processes.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material. Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Koch, G. et al. International measures of prevention, application, and economics of corrosion technologies study. CORROSION 2016 CONFERENCE & EXPO, Houston, TX. (2016).
Beech, I., Sztyler, M., Gaylarde, C., Smith, L. & Sunner, J. Biofilms and biocorrosion. In: (eds Liengen, T., Féron, D., Basséguy, R. & Beech, I. B.) Understanding Biocorrosion: Fundamentals and Applications. European Federation of Corrosion Publications, Number 66. Elsevier, Cambridge, 33–56 (2014).
Obot, I. Under-Deposit Corrosion on Steel Pipeline surfaces: mechanism, Mitigation and Current challenges. J. Bio-and Tribo-Corrosion. 7 (2), 1–14 (2021).
Brown, B. & Moloney, J. Under-deposit corrosion. Trends in Oil and Gas Corrosion Research and Technologies. ed. A. M. El-Sherik (Sawston: Woodhead Publishing), 363–383. (2017).
Robineau, M. et al. Galvanic corrosion of carbon steel in anoxic conditions at 80° C associated with a heterogeneous magnetite (Fe3O4)/mackinawite (FeS) layer. Electrochim. Acta. 255, 274–285 (2017).
Xu, Y., Yang, L., He, L., Huang, Y. & Wang, X. The monitoring of galvanic corrosion behaviour caused by mineral deposit in pipeline working conditions using ring form electronic resistance sensor system. Corros. Eng. Sci. Technol. 51 (8), 606–620 (2016).
Huang, J., Brown, B., Nesic, S., Papavinasam, S. & Gould, D. Localized corrosion of mild steel under silica deposits in inhibited aqueous CO2 solutions. NACE CORROSION 2013. Orlando, FL. (2013).
Tan, Y., Fwu, Y. & Bhardwaj, K. Electrochemical evaluation of under-deposit corrosion and its inhibition using the wire beam electrode method. Corros. Sci. 53 (4), 1254–1261 (2011).
Wang, Z., Pang, L. & Zheng, Y. A review on under-deposit corrosion of pipelines in oil and gas fields: testing methods, corrosion mechanisms and mitigation strategies. Corros. Commun. 7, 70–81 (2022).
Wen, X., Bai, P., Luo, B., Zheng, S. & Chen, C. Review of recent progress in the study of corrosion products of steels in a hydrogen sulphide environment. Corros. Sci. 139, 124–140 (2018).
Ning, J., Zheng, Y., Brown, B., Young, D. & Nešić, S. The role of iron sulfide polymorphism in localized H2S corrosion of mild steel. Corrosion 73 (2), 155–168 (2017).
De Motte, R. et al. A study by electrochemical impedance spectroscopy and surface analysis of corrosion product layers formed during CO2 corrosion of low alloy steel. Corros. Sci. 172, 108666 (2020).
Hua, Y. et al. The formation of FeCO3 and Fe3O4 on carbon steel and their protective capabilities against CO2 corrosion at elevated temperature and pressure. Corros. Sci. 157, 392–405 (2019).
Owen, J. et al. Modified Magnetite Surface Layers on Carbon Steel in Aqueous CO2 Environments. AMPP Annual Conference + Expo 2023. Denver, CO. (2023).
Song, G. D., Jeon, S. H., Son, Y. H., Kim, J. G. & Hur, D. H. Galvanic effect of magnetite on the corrosion behavior of carbon steel in deaerated alkaline solutions under flowing conditions. Corros. Sci. 31, 71–80 (2018).
Chan, E. W. L. Magnetite and its galvanic effect on the corrosion of carbon steel under carbon dioxide environments. Doctoral dissertation, Curtin University. (2011).
Little, B. J. et al. Microbially influenced corrosion—any progress? Corros. Sci. 170, 108641 (2020).
Moura, V. et al. The influence of surface microbial diversity and succession on microbiologically influenced corrosion of steel in a simulated marine environment. Arch. Microbiol. 200 (10), 1447–1456 (2018).
Usher, K. M., Kaksonen, A. H., Cole, I. & Marney, D. Critical review: microbially influenced corrosion of buried carbon steel pipes. Int. Biodeterior. Biodegrad. 93, 84–106 (2014).
Salgar-Chaparro, S. J., Machuca, L. L., Lepkova, K., Pojtanabuntoeng, T. & Darwin, A. Investigating the effect of temperature in the community structure of an oilfield microbial consortium, and its impact on corrosion of carbon steel. NACE CORROSION 2019. Nashville, TN. (2019).
Salgar-Chaparro, S. J., Lepkova, K., Pojtanabuntoeng, T., Darwin, A. & Machuca, L. L. Microbiologically influenced corrosion as a function of environmental conditions: a laboratory study using oilfield multispecies biofilms. Corros. Sci. 169, 108595. https://doi.org/10.1016/j.corsci.2020.108595 (2020).
Suarez, E. M., Lepkova, K., Kinsella, B. & Machuca, L. L. Aggressive corrosion of steel by a thermophilic microbial consortium in the presence and absence of sand. Int. Biodeterior. Biodegrade. 137, 137–146 (2019).
Enning, D. et al. Marine sulfate-reducing bacteria cause serious corrosion of iron under electroconductive biogenic mineral crust. Environ. Microbiol. 14 (7), 1772–1787 (2012).
Venzlaff, H. et al. Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria. Corros. Sci. 66, 88–96 (2013).
Kato, S. Microbial extracellular electron transfer and its relevance to iron corrosion. Microb. Biotechnol. 9 (2), 141–148 (2016).
Bruker, A. X. S. DIFFRAC.EVA, Version 5.2 Software. URL: (2019). https://www.bruker.com/en/products-and-solutions/diffractometers-and-x-ray-microscopes/x-ray-diffractometers/diffrac-suite-software/diffrac-eva.html
Menendez, C. M., Jovancicevic, V., Ramachandran, S., Morton, M. & Stegmann, D. Assessment of Corrosion Under Iron Sulfide deposits and CO2 /H2S conditions. Corrosion 69 (2), 145–156 (2013).
Robineau, M. et al. Formation of iron sulfides on carbon steel in a specific cement grout designed for radioactive waste repository and associated corrosion mechanisms. Materials 14 (13), 3563 (2021).
Wenner, F. A method for measuring earth resistivity. Bull. Bureau Standards. 12 (4), 469–478 (1916).
Samouëlian, A., Cousin, I., Tabbagh, A., Bruand, A. & Richard, G. Electrical resistivity survey in soil science: a review. Soil Tillage. Res. 83 (2), 173–193 (2005).
Diaz-Mateus, M. A., Machuca, L. L., Farhat, H. & Salgar-Chaparro, S. J. Unveiling the effect of magnetite on the synergistic action of deposits and microorganisms on carbon steel corrosion. Corros. Sci. 231, 111940 (2024).
ASTM G1. Standard Practice for Preparing, Cleaning, and Evaluation Corrosion Test Specimens (ASTM International, 2003).
NACE SP0775. Standard Practice Preparation, Installation, Analysis, and Interpretation of Corrosion Coupons in Oilfield Operations (NACE International. Houston, TX, 2013).
ASTM G46. Standard Guide for Examination and Evaluation of Pitting Corrosion (ASTM International, 2021).
NACE TM0194. Standard test Method: Field Monitoring of Bacterial Growth in oil and gas Systems (NACE International. Houston, TX, 2014).
Da Silva, N. et al. Microbiological Examination Methods of food and Water: A Laboratory Manual (CRC, 2018).
Guimarães, P. M. & Londesborough, J. The adenylate energy charge and specific fermentation rate of brewer’s yeasts fermenting high-and very high‐gravity worts. Yeast 25 (1), 47–58 (2008).
Atkinson, D. E. & Walton, G. M. Adenosine triphosphate conservation in metabolic regulation: rat liver citrate cleavage enzyme. J. Biol. Chem. 242 (13), 3239–3241 (1967).
Illumina Preparing 16S ribosomal RNA gene amplicons for the illumina MiSeq system. Illumina technical note. (2011). Retrieved from: https://www.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf
Yu, Y., Lee, C., Kim, J. & Hwang, S. Group-specific primer and probe sets to detect methanogenic communities using quantitative real‐time polymerase chain reaction. Biotechnol. Bioeng. 89 (6), 670–679 (2005).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 13, 581–583. https://doi.org/10.1038/nmeth.3869 (2016).
Bokulich, N. A. et al. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6 (1), 1–17 (2018).
Huang, L. et al. Pyocyanin-modifying genes phzM and phzS regulated the extracellular electron transfer in microbiologically-influenced corrosion of X80 carbon steel by Pseudomonas aeruginosa. Corros. Sci. 164, 108355 (2020).
Liu, H. et al. Corrosion behavior of carbon steel in the presence of sulfate reducing bacteria and iron oxidizing bacteria cultured in oilfield produced water. Corros. Sci. 100, 484–495 (2015).
Sowards, J. W. & Mansfield, E. Corrosion of copper and steel alloys in a simulated underground storage-tank sump environment containing acid-producing bacteria. Corros. Sci. 87, 460–471 (2014).
Pandarinathan, V., Lepková, K., Bailey, S. I. & Gubner, R. Impact of Mineral deposits on CO2 corrosion of Carbon Steel. Proceedings of the NACE CORROSION 2013 Conference & Expo, Mar 17-21 2013, pp 5118-5132. Orlando, FL. (2013)
Jeannin, M., Calonnec, D., Sabot, R. & Refait, P. Role of a clay sediment deposit on the corrosion of carbon steel in 0.5molL – 1 NaCl solutions. Corros. Sci. 52 (6), 2026–2034 (2010).
Shuey, R. T. CHAPTER 22 - Magnetite - Fe3O4. In: Semiconducting Ore Minerals. Developments in Economic Geology. Elsevier. Vol 4, pp. 290–303. (1975).
Pearce, C. I., Pattrick, R. A. & Vaughan, D. J. Electrical and magnetic properties of sulfides. Rev. Mineral. Geochem. 61 (1), 127–180 (2006).
Cornell, R. M. & Schwertmann, U. The iron Oxides: Structure, Properties, Reactions, Occurrences, and usesVol. 664 (Wiley-vch Weinheim, 2003).
Schieck, R., Hartmann, A., Fiechter, S., Könenkamp, R. & Wetzel, H. Electrical properties of natural and synthetic pyrite (FeS2) crystals. J. Mater. Res. 5 (7), 1567–1572 (1990).
Wang, Y., Wang, L. & Chou, K. C. Estimating Electrical conductivities of CaO-MgO-Al2O3-SiO2 using Ion-Oxygen parameter. High. Temp. Mater. Process. 35 (3), 253–259 (2016).
Crolet, J. L., Thevenot, N. & Nesic, S. Role of conductive corrosion products in the protectiveness of corrosion layers. Corrosion 54 (3), 194–203 (1998).
Esmaeely, S. N. & Nešić, S. Localized corrosion of mild steel in H2S containing aqueous environments—case studies and common mechanisms. Corrosion 75 (8), 938–945 (2019).
Huang, J., Shinohara, T. & Tsujikawa, S. Effects of interfacial iron oxides on corrosion protection of carbon steel by TiO2 coating under illumination. Zairyo-to-kankyo 46 (10), 651–661 (1997).
Baboian, R. Galvanic and Pitting Corrosion-Field and Laboratory Studies (ASTM International. Cockeysville, 1976).
Esmaeely, S. N. & Nesic, S. Reduction reactions on iron sulfides in aqueous acidic solutions. J. ELECTROCHEM. SOC. 164 (12), C664 (2017).
Esmaeely, S. N., Bota, G., Brown, B. & Nešić, S. Influence of pyrrhotite on the corrosion of mild steel. Corrosion 74 (1), 37–49 (2018).
Salgar-Chaparro, S. J., Darwin, A., Kaksonen, A. H. & Machuca, L. L. Carbon steel corrosion by bacteria from failed seal rings at an offshore facility. Sci. Rep. 10 (1), 12287–12287 (2020).
Jia, R., Yang, D., Xu, D. & Gu, T. Electron transfer mediators accelerated the microbiologically influence corrosion against carbon steel by nitrate reducing Pseudomonas aeruginosa biofilm. Bioelectrochemistry.118: pp. 38–46. (2017).
Tang, J., Guo, R., Zhang, X. & Zhao, X. Effect of Pseudomonas aeruginosa on corrosion of X65 pipeline steel. Heliyon 8 (12), e12588 (2022).
Yuan, S. J. & Pehkonen, S. O. Microbiologically influenced corrosion of 304 stainless steel by aerobic Pseudomonas NCIMB 2021 bacteria: AFM and XPS study. Colloids Surf., B. 59 (1), 87–99 (2007).
Jia, R., Yang, D., Xu, J., Xu, D. & Gu, T. Microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing Pseudomonas aeruginosa biofilm under organic carbon starvation. Corros. Sci. 127, 1–9 (2017).
Yuan, S. J., Choong, A. M. & Pehkonen, S. O. The influence of the marine aerobic Pseudomonas strain on the corrosion of 70/30 Cu–Ni alloy. Corros. Sci. 49 (12), 4352–4385 (2007).
Li, J., Du, C., Liu, Z. & Li, X. Extracellular electron transfer routes in microbiologically influenced corrosion of X80 steel by Bacillus licheniformis. Bioelectrochemistry. 145: p. 108074. (2022).
Xu, D., Li, Y., Song, F. & Gu, T. Laboratory investigation of microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing bacterium Bacillus licheniformis. Corros. Sci. 77, 385–390 (2013).
Wan, H. et al. Corrosion effect of Bacillus cereus on X80 pipeline steel in a Beijing soil environment. Bioelectrochemistry 121, 18–26 (2018).
Parthipan, P., Babu, T. G., Anandkumar, B. & Rajasekar, A. Biocorrosion and its impact on carbon steel API 5LX by Bacillus subtilis A1 and Bacillus cereus A4 isolated from Indian crude oil reservoir. J. Bio-and Tribo-Corrosion. 3, 1–12 (2017).
Pusparizkita, Y. M. et al. Microbiologically influenced corrosion of the ST-37 carbon steel tank by Bacillus licheniformis present in biodiesel blends. Biomass Bioenerg. 168, 106653 (2023).
Dong, Y., Chang, Y. J., Sanford, R. A. & Fouke, B. W. Draft genome sequence of tepidibacillus decaturensis strain Z9, an anaerobic, moderately thermophilic, and heterotrophic bacterium from the deep subsurface of the Illinois Basin, USA. Genome Announc. 4 (2), 10–1128 (2016).
Slobodkina, G. B. et al. Tepidibacillus fermentans gen. nov., sp. nov.: a moderately thermophilic anaerobic and microaerophilic bacterium from an underground gas storage. Extremophiles 17, 833–839 (2013).
Podosokorskaya, O. A. et al. Tepidibacillus infernus sp. nov., a moderately thermophilic, selenate-and arsenate-respiring hydrolytic bacterium isolated from a gold mine, and emended description of the genus Tepidibacillus. Int. J. Syst. Evol. Microbiol. 66 (8), 3189–3194 (2016).
Enning, D. & Garrelfs, J. Corrosion of iron by sulfate-reducing bacteria: new views of an old problem. Appl. Environ. Microbiol. 80 (4), 1226–1236 (2014).
Lee, D. Y., Kim, W. C. & Kim, J. G. Effect of nitrite concentration on the corrosion behaviour of carbon steel pipelines in synthetic tap water. Corros. Sci. 64, 105–114 (2012).
Lahme, S. et al. Metabolites of an oil field Sulfide-Oxidizing, nitrate-reducing Sulfurimonas sp. Cause severe corrosion. Appl. Environ. Microbiol. 85 (3), e01891–e01818 (2019).
Yang, J., Wang, Z. B., Qiao, Y. X. & Zheng, Y. G. Synergistic effects of deposits and sulfate reducing bacteria on the corrosion of carbon steel. Corros. Sci. 199, 110210 (2022).
Liao, W. et al. Under-deposit microbial corrosion of X65 pipeline steel in the simulated shale gas production environment. Int. J. Electrochem. Sci. 18 (3), 100069 (2023).
Salgar-Chaparro, S. J., Lepkova, K., Pojtanabuntoeng, T., Darwin, A. & Machuca, L. L. Nutrient Level Determines Biofilm Characteristics and Subsequent Impact on Microbial Corrosion and Biocide Effectiveness. Appl. Environ. Microbiol. 86(7). (2020).
Diaz-Mateus, M. A., Salgar-Chaparro, S. J., Machuca, L. L. & Farhat, H. Effect of deposit chemistry on microbial community structure and activity: implications for under-deposit microbial corrosion. Front. Microbiol. 14. (2023).
Li, S. Y., Kim, Y. G., Jeon, K. S., Kho, Y. T. & Kang, T. Microbiologically influenced corrosion of carbon steel exposed to anaerobic soil. Corrosion 57 (9), 815–828 (2001).
Esmaeely, S. N., Bota, G., Brown, B. & Nesic, S. Effect of pyrrhotite on Mild Steel Corrosion in aqueous CO2 and H2S solutions. Proceedings of the NACE CORROSION 2018 Conference & Expo, April 15-19 2018 Phoenix, AZ. (2018)
Jia, R. et al. Effects of biogenic H2S on the microbiologically influenced corrosion of C1018 carbon steel by sulfate reducing Desulfovibrio vulgaris biofilm. Corros. Sci. 130, 1–11 (2018).
Acknowledgements
The authors acknowledge the financial support from the Qatar Environment and Energy Research Institute (QEERI) for the QEERI - Curtin University Corrosion Center Research Alliance and the Microbiologically Influenced Corrosion (MIC) - Joint Industry Project (JIP) “Preventing and Managing Microbiologically Influenced Corrosion in the Oil and Gas Industry.“. Additionally, the authors acknowledge the use of the Microscopy & Microanalysis Facility in the John de Laeter Centre (JDLC) at Curtin University, whose instrumentation has been partially funded by the University, State, and Commonwealth Governments.
Funding
The authors declare that Qatar Environment and Energy Research Institute (QEERI) contributed financial resources to assist this work via a postgraduate scholarship. The study sponsor has reviewed and approved the submission of the manuscript for publication.
Author information
Authors and Affiliations
Contributions
MD-M and SS-C contributed to the conception and design of the study. MD-M executed the experiments and carried out the microbial, statistical and corrosion analysis with support of JT and SS-C. MD-M prepared the manuscript with the contribution of LM, KL, SS-C, and HF.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Diaz-Mateus, M.A., Machuca, L.L., Lepkova, K. et al. Exploring the interplay of under-deposit corrosion and microbiologically influenced corrosion in the presence of deposits with varied electrical conductivities. Sci Rep 15, 7455 (2025). https://doi.org/10.1038/s41598-025-90303-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-90303-w