Abstract
Infection caused by neuroinvasive Borrelia often manifests long-term CNS disorders and is difficult to treat as most antibiotics fail to attain an effective concentration within the brain or cannot kill the persister forms of Borrelia (cysts and round bodies). Thus, this study focused on developing antimicrobial cyclic peptides (AMPs) from a combinatorial phage display library that target phosphatidylcholine of the borrelial cell membrane. Isolated cyclic peptides with anti-Borrelia properties were then fused with the CNS homing peptide developed in this study (designated as O-BBB) to facilitate AMP transport across the blood-brain barrier. Among all O-BBB fused AMPs, Bor-18 had half maximal effective concentration (EC50) 0.83 µM when tested against spirochetal Borrelia. Bor-16, Bor-18, and Bor-26 inhibited the cystic form with EC50 0.83 µM, while Bor-11 had EC50 0.41 µM. Within an hour, all four peptides caused a permeability breach in the borrelial cell membrane, causing depolarization of the membrane. Bor peptides did not inhibit eukaryotic cell metabolism or proliferation, nor did they cause erythrocyte lysis. Peptides were stable in serum, could cross the BBB in-vitro, and remained effective against Borrelia. Cyclic AMPs fused with a CNS homing moiety, the Bor peptides, deserve further investigation for their potential use in neuroborreliosis therapy.
Similar content being viewed by others
Introduction
Lyme borreliosis (LB) is the most common vector-borne disease prevalent in Europe and the United States, with an annual rising incidence1,2. Early-stage LB symptoms include fatigue, headache, fever, and, in some cases, erythema migrans3, whereas pathological conditions in other organs such as joints, eyes, the heart, and the central nervous system can be observed in later stages, depending on the Borrelia genospecies involved4. Borrelia garinii and B. bavariensis can cross the blood-brain barrier (BBB) and invade the immune-privileged central nervous system causing Lyme neuroborreliosis5. Neuroborreliosis has a favorable prognosis if treated early, however the long-term administration of antibiotics over many weeks for chronic neuroborreliosis carries the risk of serious adverse effects6. Furthermore, in chronic neuroborreliosis, a long latent period and persistence of Borrelia infection even after repeated antibiotic therapy can be noted, which may be accompanied by the presence of drug resistant atypical extracellular and intracellular pleomorphic and cystic forms in the brain7.
Several alternative therapeutic agents have been developed and tested to address the antibiotic resistance. Antimicrobial peptides (AMPs), particularly short cationic peptides, stand out as alternative therapeutic agents because they can have both antimicrobial and immunomodulatory properties. The mode of antimicrobial action of these peptides is related to either cell membrane disruption or interaction with intracellular components8, resulting in inhibition of cell wall, nucleic acid, and protein synthesis or inhibition of enzymatic activity9. Antimicrobial peptides have the advantage of being nonspecific defense weapons, making it difficult for microbial pathogens to develop resistant mutants. Previous studies report testing of naturally occurring antimicrobial peptides or their derivates against Borrelia. In particular, cathelicidin-derived synthetic peptides SMAP-29, LL-37, PG-1, CRAMP, and BMAP-28 had minimum inhibitory concentrations (MIC) ranging from 307 to 449.4 mg/l10. Anti-Borrelia activity of defensin, melittin and peptides from bee venom was also tested and compared with commonly used antibiotics11. The bee venom and melittin had a significant effect on the spirochetal form of Borrelia as well as biofilm, as compared to antibiotics, which had limited effects on biofilm12. It implies that AMPs might be intriguing therapeutic candidates; nevertheless, large-scale production of naturally occurring AMPs is difficult. No synthetic AMPs have been developed so far against Borrelia, despite the fact that synthetic peptides show promise as a less costly large-scale production option. A PubMed search for synthetic peptides developed against Borrelia produced no relevant results, demonstrating the complexity in development of AMPs against Borrelia and its various forms. The only credible report demonstrating the borreliacidal effect of synthetic peptides is a patent application in which peptides containing epsilon lysine residues were tested13. The development of antimicrobial drugs that target outer membrane proteins or the membrane itself is challenging since Borrelia frequently alters the expression of surface proteins14. To this point, membrane phospholipids seems to be attractive target for development of AMPs as borrelial membrane contains two major membrane phospholipids: phosphatidylglycerol and phosphatidylcholine. Using a murine model, it was shown that Borrelia infection causes the production of antibodies against phosphatidylcholine15. Therefore, our goal was to target phospholipid of the borrelial cell membrane for development of AMPs selected from combinatorial phage display library.
Antibiotics and antimicrobial peptides have limited biodistribution in the CNS, making neuroinfections difficult to treat. The absence of fenestrations and the nearly impermeable cell-cell tight junction between endothelial cells of brain microvasculature prevent paracellular transport of drugs across the BBB16. Glucose and oxygen can cross the BBB via paracellular and transcellular transport. However, larger molecules require receptor-mediated endocytosis (RME)17. The CNS homing peptides, such as angiopep-2, TGN and TfrL are developed based on RME18. Several pathogens including Borrelia exploit RME to cross the BBB19. We have shown that borrelial OspA-CD40 interaction initiates translocation of the spirochete through the endothelial barrier20, and the interaction is mediated through endothelial-cell binding domain of OspA21. Our second goal was to use endothelial-cell binding domain of OspA or its truncated fragments as CNS homing peptide and fuse it with AMPs to promote AMP transport across BBB model.
In this study, we aimed to isolate Cx7C peptides from combinatorial phage library that bind to the phosphatidylcholine of borrelial cell membrane. We confirmed binding of individual phage clones (displaying Cx7C peptides) to different forms of Borrelia (spirochetes, cystic form and Borrelia devoid of outer membrane proteins). Simultaneously, we aimed to test ability of the endothelial-cell binding domain of OspA and its truncated fragments to traverse the BBB model. Then we fused the N-terminal fragment of endothelial-cell binding domain (which showed significant BBB crossing) with Cx7C peptides. Finally, we performed a series of tests to check concentration-dependent borreliacidal activity of fusion peptides, ability to cause membrane depolarization, non-toxicity to mammalian cells, hemocompatibility, etc. We were able to develop four fusion peptides, Bor-11, Bor-16, Bor-18, and Bor-26, that demonstrated cidal activity against both spirochetal and cystic forms of Borrelia. All four peptides altered the membrane polarity of Borrelia, their activity remained stable in the normal human serum. Bor peptides were able to cross the BBB model in-vitro and exhibit borreliacidal activity. The anti-Borrelia peptides developed in this study deserve further investigation in order to be translated into functional therapeutics against neuroborreliosis.
Results
Cx7C peptides against borrelial cell membrane (isolated from combinatorial phage display peptide library)
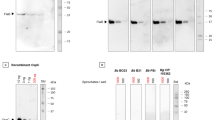
Small-sized seven-mer peptides flanked by cysteine residues (CxxxxxxxC, here x is any amino acid, Cx7C) that bind to the phospholipid of borrelial cell membrane were isolated from a combinatorial phage display peptide library (Fig. 1, panel A). The binding of isolated phage clones to borrelial phospholipid was tested using a dot blot, in which phages were incubated with various forms of Borrelia (spiral, cystic, and trypsin-shaved forms are depicted in Fig. 1, panel B-D, supplementary information 1). The binding of phages to Borrelia, as shown in Fig. 1 (panel E), demonstrated that phages enriched by pannings are against phospholipids rather than outer membrane proteins, as they demonstrated binding affinity to shaved Borrelia, devoid of outer membrane proteins.
The DNA from individual phage clones, separated from the elution of the 3rd panning, was amplified (amplicons depicted in supplementary information 2), sequenced and aligned to make clusters based on amino acid sequence homology in the Cx7C motif. A total of 19 clusters were formed (Fig. 1, panel F), which were screened through in silico predictors for various parameters such as (1) antimicrobial potential, (2) net charge, (3) water solubility, and (4) probable cell toxicity. Fourteen clones appear to possess antimicrobial potential (Supplementary Table 1). The majority of the predicted antimicrobial peptides were cationic and eight of them were predicted to be poorly soluble in water. All peptides except ACTNANHYFCGGGS (clone Ph.Bor-15) were predicted to be non-toxic (Supplementary Table 1). None of the peptides from 19 clusters had a match in Peptide search tool of UniProt database. Five peptides (namely Ph.Bor-4, Ph.Bor-7, Ph.Bor-9, Ph.Bor-20 and Ph.Bor-24) were predicted as non-antimicrobial peptide (non-AMP). All phage clones, when assessed for their affinity to borrelial membrane using dot blot, most of the clones showed good binding ability to spirochetes fixed on membrane (Fig. 1, panel G).
To select the clones for further testing (production in recombinant form), ten best binders from dot blot (Ph.Bor-1, 8, 9, 11, 16, 18, 23, 24, 26, 29) were shortlisted. Among those, Ph.Bor-9 and Ph.Bor-24 were predicted as non-AMP, thus clone 24 was excluded from further study, however owing to binding affinity of clone 9, it was retained.
Isolation of phages from Cx7C library that bind to phospatidylcholine of borrelial membrane. (A) Borrelial cell wall depicted along with elution strategy adopted in panning to elute phages bound to cell membrane. OM - outer membrane, PS - periplasmic space, CM - cytoplasmic membrane, LP - lipoproteins such as OspA, OspC, etc. Phospholipase (PL) was used to elute phages binding to phospatidylcholine. (B) spirochetal form of Borrelia expressing GFP. (C) bleb or cystic form of the Borrelia. (D) Borrelia treated with trypsin (shaving). (E) Dot blotting performed to check if the phages from 3rd round of panning possess binding affinity to Borrelia. Either Borrelia in early log phase (Bb*) or cystic form (BbC) or trypsin-shaved (BbT) or phages eluted from 3rd panning (Ph*) were immobilized on membrane. Borrelia were incubated with phages and interaction was detected by anti-M13 antibody (Ab). For negative control phages were omitted (Bb - Ab). For input control Ph* were directly detected by Ab. (F). Phages clones eluted from the 3rd round of panning were subjected for sequencing and aligned to make clusters based on sequence homology. Sequence of representative clone from each cluster is presented. (G) Binding of the phage clone assessed by dot blot, wherein Borrelia were fixed on the membrane, incubated with each phage clone and the interaction was detected by anti-M13 antibody. Blot ‘a’ - phages were omitted. ‘b’ and ‘c’ input controls – phages and primary antibody, respectively, were immobilized on membrane and detected. Underlined clones showed strong signal, indicating good binding ability to Borrelia.
BBB homing peptide derived from OspA and its fusion with Cx7C
The N-terminal fragment of endothelial cell binding domain of OspA (the O-BBB) showed significantly higher crossing than the other two forms (full length and C-terminal fragment of endothelium binding domain, t-test p < 0.05). A significant difference in angiopep-2 and O-BBB crossing was observed at the 1st hr (P = 0.0175). At the 2nd hr, O-BBB crossing was comparable to that of angiopep-2, but at the 3rd hr, O-BBB crossing was significantly higher than that of angiopep-2 (Fig. 2, panel B). Thus, the O-BBB, which contains antiparallel beta sheets (Fig. 2, panel C), was used in the current study to fuse with Cx7C. The fusion constructs consist of O-BBB at N-terminus of the Cx7C, flanked by GGGS at both ends (Fig. 2, panel D). DNA fragment coding Cx7C, amplified from shortlisted phage clones (Fig. 1, panel G; amplicons are in Fig. 3, panel A) were ligated in pQE-30 N-tag-II vector22, and overexpressed in E. coli. Purified peptides had the expected molecular weights, as seen in LDS-PAGE and MALDI-TOF (Fig. 3, panels B-C, Table 1). Fusion O-BBB-Cx7C peptides were designated as Bor peptides (Bor-1, Bor-8, and so on).
BBB homing peptide derived from OspA of Borrelia and its fusion with Cx7C. (A) Organization of domains in OspA monomer. Tick gut epithelial cell binding domain 1 (TGE1) - green, TGE2 - orange, TGE3 – red, endothelial cell binding domain – blue and magenta. N-terminal fragment of the endothelial cell binding domain is in blue (comprised of two beta sheets 10 and 11), while C terminal part in magenta is comprised on 5 beta sheets (12 to 16). (B) Time dependent translocation of OspA derived peptides through BBB model.  N terminal fragment of endothelial cell binding domain (O-BBB),
N terminal fragment of endothelial cell binding domain (O-BBB),  C terminal fragment,
C terminal fragment,  full endothelial cell binding domain,
full endothelial cell binding domain,  angiopep-2-Cy5.5,
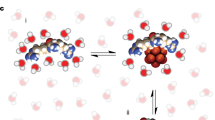
angiopep-2-Cy5.5,  dextran-CF770. ***Significantly lower crossing (two tailed t-test P < 0.05) of designated molecules than O-BBB and angiopep-2. Significant difference between crossing of angiopep-2 and O-BBB was observed (P = 0.0175) at 1st hr. At 2nd hr crossing of O-BBB was matched with angiopep-2, while at 3rd hr significantly higher crossing of O-BBB was evident. Thus, for the fusion of CX7C, O-BBB was preferred in the study. Error bars represent the standard deviation of triplicate measurements. (C) A graphical representation of the Cx7C fused O-BBB. Beta sheets forming O-BBB are colored blue, Cx7C (red loop) is separated from O-BBB by a linker. Yellow dots represent cysteine residues that help to form a loop-like structure. (D) Schematic representation of the construct to produce anti-Borrelia Cx7C peptide fused with O-BBB. Construct contains 6x histidine tag, O-BBB, GGGS linker, sequence digested by enterokinase protease (Ent), ACxxxxxxxC – 7-mer peptides which showed affinity to Borrelia cell membrane (peptide is are flanked by cysteine residues and alanine at N-terminus), three glycine residues followed by serine and then stop codon.
dextran-CF770. ***Significantly lower crossing (two tailed t-test P < 0.05) of designated molecules than O-BBB and angiopep-2. Significant difference between crossing of angiopep-2 and O-BBB was observed (P = 0.0175) at 1st hr. At 2nd hr crossing of O-BBB was matched with angiopep-2, while at 3rd hr significantly higher crossing of O-BBB was evident. Thus, for the fusion of CX7C, O-BBB was preferred in the study. Error bars represent the standard deviation of triplicate measurements. (C) A graphical representation of the Cx7C fused O-BBB. Beta sheets forming O-BBB are colored blue, Cx7C (red loop) is separated from O-BBB by a linker. Yellow dots represent cysteine residues that help to form a loop-like structure. (D) Schematic representation of the construct to produce anti-Borrelia Cx7C peptide fused with O-BBB. Construct contains 6x histidine tag, O-BBB, GGGS linker, sequence digested by enterokinase protease (Ent), ACxxxxxxxC – 7-mer peptides which showed affinity to Borrelia cell membrane (peptide is are flanked by cysteine residues and alanine at N-terminus), three glycine residues followed by serine and then stop codon.
Functional analysis of O-BBB-Cx7C fusion peptides (bor peptides)
The binding of Bor peptides to Borrelia was verified by ELISA, in which all peptides, except Bor-1, showed affinity to Borrelia and no non-specific interaction with empty wells was observed. O-BBB alone showed no affinity for Borrelia (Fig. 3, panel D). The Bor peptides were tested further for cidal activity against the spirochetal form of Borrelia. Bor-11, 16, 18, and 26 demonstrated significant cidal activity even at 1.67 µM concentration (viability reduced < 50%, Fig. 4 panel A). At 6.67 µM, all other peptides possessed borreliacidal activity, which was mostly lost after further dilution (3.3 µM). The O-BBB exhibited no borreliacidal effect even at 6.67 µM. Bor-11, 16, 18, and 26 were then tested to determine the MIC (EC50). Apart from Bor-16 and Bor-26, the other two peptides inhibited the spiral form of Borrelia by more than 50% at 0.41 µM (Fig. 4, panel B).
Production of Cx7C peptides fused with O-BBB and assessment of their binding to Borrelia. (A) Amplicons encompassing Cx7C. (B) Purified Cx7C peptides fused with O-BBB (designated as Bor-1 to Bor-29) separated on SDS-PAGE and stained with Coomassie blue. (C) Molecular mass of the purified peptides measured by MALDI-MS. Full spectra are available in supplementary information 4. (D) Binding of the selected peptides on Borrelia assessed by ELISA. In assay wells were coated either Borrelia (Bb) or coating buffer (-), incubated with each peptide (Bor-1 to Bor-29) or phosphate buffer saline (PBS, negative control) or with O-BBB only (negative control). Error bars represent the standard deviation of triplicate measurements.
Since many antibiotics fail to kill the cystic form of Borrelia23,24, we wanted to see if Bor-11, 16, 18, and 26 would be effective against it. The doxycycline which is effective against spirochetal form but unable to kill round bodies25, was used as control. Exposing cystic Borrelia to peptides (1.66 µM) for 16 h significantly reduced their recovery and transformation into the spirochetal form (Fig. 4, panel C). Bor-11 was most effective against cystic form, inhibiting growth significantly (t-test p < 0.05) even at 0.41 µM. Incubation of cysts with 0.05 µM of the doxycycline for 16 h did not inhibit the transformation and recovery into the spirochetal form.
Inhibitory effect of Bor peptides against spirochetal and cystic forms of Borrelia. In all graphs numbers beneath X axis show concentration in µM. Error bars represent the standard deviation of triplicate measurements in all panels. (A) Borreliacidal effect of the Bor peptides tested at various concentrations. As a live control, peptides were replaced by PBS in assay and percent viability was calculated taking into account live control. Doxycycline was used as positive control. Red bracket show peptides with significant borreliacidal activity even at 1.67 µM (viability < 50%, ***t test P < 0.05). (B) Determination of minimum inhibition concentration of Bor-11, Bor-16, Bor-18 and Bor-26 against spiral form Borrelia. (C) Determination of minimum inhibition concentration of Bor-11, Bor-16, Bor-18 and Bor-26 against cystic form Borrelia. Percent viability of Borrelia in B and C was calculated by taking into account the number of Borrelia in medium containing 0 µM of peptide. In panel C *** present significant difference between the borreliacidal effect observed by other Bor peptides and Bor-11 (t-test P < 0.05). Ξ indicates % inhibition observed in case of 0.05 µM doxycycline (this concentration was selected for control as it showed inhibitory effect against spirochetal form (see panel A).
Bor-11, 16, 18, and 26 were examined for concentration-dependent binding to Borrelia. Bor-18 binding was significantly higher even at 0.16 µM than other peptides at the same concentration, but decreased significantly at 0.08 µM (Absorbance450 0.8). Other peptides exhibited concentration-dependent linear decreases in absorbance (Fig. 5, panel A). The ELISA was also performed to check the binding ability of peptides to cystic form and Borrelia devoid of outer membrane proteins (spirochetes shaved with trypsin). All four peptides showed affinity for all three forms (Fig. 5, panel B), indicating that peptides interact with the borrelial membrane (most probably with phosphatidylcholine), and that the binding is not mediated by outer membrane proteins.
Assessment of affinity of Bor peptides to different forms of Borrelia, their ability to depolarize the membrane and effect on borrelial viability. In panels A and B, Ab 450 nm at Y axis indicates absorbance measured at 450 nm in ELISA. Error bars in both panels represent the standard deviation of triplicate measurements. (A) Concentration dependent affinity of the peptides to Borrelia growing in early log phase. Values beneath X axis presents concentration in µM. The absorbance values presented are after subtraction of the absorbance of negative control (peptides omitted from assay). (B) Affinity of peptides to three different forms of Borrelia. Ab – peptides were replaced by anti-OspA antibody in the assay. (C) Borrelial membrane depolarization caused by peptides. RFU – relative fluorescence unit. Concentration of all peptides used in assay was 6.67 µM. a – significant difference in the RFU values compared to negative control (PBS, t-test P < 0.05), however no statistically significant difference among the treatments at 2, 3 and 4 h was observed (t-test P = ranged between 0.09–0.178). (D) Effect of peptides on the borrelial membrane was also determined by measuring the permeability to propidium iodide. Triton X – (0.1%) non-ionic detergent was used as control, which causes immediate destabilization of the borrelial membrane. Doxy – doxycycline was also used as control.
Since Bor peptides showed affinity for borrelial cell membrane, we sought to investigate if they cause a smaller breach of the permeability barrier of the outer and cytoplasmic membranes of Borrelia. In membrane depolarization assay, we clearly observed that Bor peptides caused a permeability breach, resulting membrane depolarization and a significant increase in fluorescence when compared to the negative control (Fig. 5, panel C). The breach in cytoplasmic membrane that may cause borreliacidal effect was also monitored using propidium iodide (live/dead staining). This test may also reveal how long it takes for the peptide to exert borreliacidal effect (time to kill). Bor peptides caused an increase in fluorescence after 1 h of incubation, indicating that borreliacidal activity began at this timepoint, which was approximately 90 min. for doxycycline (Fig. 5, panel D).
Toxicity and hemocompatibility of peptides
Bor-11, 16, 18, and 26 were tested for cytotoxicity and if they cause lysis of red blood cells. At 3.33 µM, Bor peptides and O-BBB had no adverse effects on cell metabolic activity (Fig. 6, panel A). None of the peptides significantly inhibited cell proliferation (at 1.67 µM and 3.3 µM), indicating their safety (Fig. 6, panels B–E, Pearson correlation coefficient 0.98 to 0.99 between negative and treated cells).
All of the peptides appeared to be hemocompatible, with none causing hemolysis within 5 h, even at the highest concentration (300, 100, and 50 ng, Fig. 6, panel F).
Effect of Bor peptides on the eukaryotic cell metabolism, hemocompatibility and proliferation of eukaryotic cells. (A) Cell toxicity - effect of peptides on the cell metabolism tested by XTT. In control wells, the peptides were replaced either by PBS or Triton X (0.1%). Error bars represent the standard deviation of triplicate measurements. (B–E) Cell toxicity – effect of peptides on the proliferation of cells was assessed up to 120 h.  3.33 µM,
3.33 µM,  1.67 µM or
1.67 µM or  0 µM (only PBS) of peptides were used in assay. The Pearson correlation test coefficient (PCC) revealed no significant difference in cell proliferation in presence of PBS and peptides (r ranged from 0.98 to 0.99 for all peptides; P value < 0.005). (F) Hemocompatibility of the peptides tested for three different concentrations at three timepoints. Sheep erythrocytes were used to test any hemolysis caused by peptides. Erythrocytes incubated only with Triton X served as positive control.
0 µM (only PBS) of peptides were used in assay. The Pearson correlation test coefficient (PCC) revealed no significant difference in cell proliferation in presence of PBS and peptides (r ranged from 0.98 to 0.99 for all peptides; P value < 0.005). (F) Hemocompatibility of the peptides tested for three different concentrations at three timepoints. Sheep erythrocytes were used to test any hemolysis caused by peptides. Erythrocytes incubated only with Triton X served as positive control.
BBB crossing and borreliacidal activity of peptides
The primary mode of transport for peptides across the BBB is receptor-mediated endocytosis, thus protein attachment to endothelial cells is critical. Cell-ELISA used to test the binding of Bor peptides on brain microvascular endothelial cells (BMECs), showed that all four peptides (Bor-11, 16, 18, and 26) as well as O-BBB bind endothelial cells (Fig. 7, panel A). Peptides were also tested for their serum stability (for 1, 3 and 6 h). All peptides caused significant reduction (viability of Borrelia < 2% in all treatments) in the number of Borrelia even after 6 h of incubation in human serum, indicating that peptides maintained anti-Borrelia activity (Fig. 7, panel B).
Finally, the ability of peptides to cross the BBB model and exert a borreliacidal effect was assessed using BBB cultivated in cell-inserts. Bor peptide did not affected integrity of the BBB model as no significant difference in TEER values before and after the addition of peptides or doxycycline was observed. The crossing of dextran was observed between 4.0 and 5.3% (dextran crossing ≤ 6% indicates intactness of BBB). The abluminal content after 5 h, which contained Borrelia and possibly peptides (if they were able to cross the BBB), was transferred to a new tube and incubated for 5 days to allow borrelial growth. A significant reduction (t-test, P < 0.05, % survival ranged between 4.0 and 6.1) in growth of Borrelia was observed in case of Bor peptides, compared to a control in which only PBS was added in the luminal chamber (Fig. 7, panel C), indicating that the Bor peptides can cross the BBB and maintain their borrelicidal properties. Doxycycline, although was able to cross the BBB in-vitro, 24.9% of the Borrelia were able to survive in the abluminal content (Fig. 7, panel C).
Serum stability of Bor peptides, their affinity to endothelial cells and borreliacidal effect after crossing the BBB model. (A) Binding of Bor peptides to BMECs. O-BBB served as positive control. The absorbance values presented are after subtraction of the absorbance of negative control (peptides omitted from assay). (B) Serum stability of the Bor peptides at three time-points. Doxy - doxycycline serves as control. Borrelia without any treatment served as live bacteria control. Serum – serum control, Borrelia incubated in presence of inactivated normal human serum. (C) Bor peptides were tested for their ability to cross the BBB model while maintaining their borrelicidal properties (left side graph). One the right hand side pictorial representation of the BBB model and overall results are presented. Dextran and Bor peptides were added to upper chamber (luminal). Before addition of test molecules, the TEER was ~ 80 Ω/cm2 indicating proper BBB formation. 20,000 Borrelia were added in abluminal chamber, incubated for 1 h and then test molecules were added to luminal chamber. After 5 h of incubation, TEER (in red text) and % of dextran crossed from luminal to abluminal chamber (in blue text) were measured. Abluminal content was retrieved from each insert, incubated for 5 days to allow borrelial growth and number of Borrelia were measured with flow cytometry. SD - standard deviation. In the graph - panel C, ***Significant inhibition of borrelial growth when compared to doxycycline (t-test = 0.0013). In panels A and C, error bars represent the standard deviation of triplicate measurements.
Discussion
Therapeutic management of borreliosis relies on per oral doxycycline or intravenous ceftriaxone26. However, patients can show recurrence of residual symptoms such as pain, fatigue, or cognitive difficulty known as post-treatment Lyme disease syndrome 27,26. One possible explanation for this clinical observation is that Borrelia shields itself by generating cystic forms, biofilm, or biofilm-like protective structure that are resistant to antibiotics23,24,29,30,31 and can be transformed back into normal spirochetes32. Despite having a functional cell wall and intact peptidoglycan, β-lactam antibiotics are ineffective against cystic forms due to low metabolic activity23. The Cx7C-O-BBB fusion constructs developed by us, particularly Bor-11, 16, 18, and 26, killed not only the spirochetes, but also significantly inhibited the cystic form (Fig. 4). Doxycycline, on the other hand, was unable to inhibit cystic Borrelia in our study (Fig. 4, panel C), consistent with earlier findings25. We presume that the inhibition of cystic Borrelia was caused by membrane destabilisation, as evidenced in depolarisation and propidium iodide assays (Fig. 5, panels C,D). Membrane depolarisation and permeability breach is one of the key modes of action for AMPs (reviewed by Epand and Vogel33). Interestingly, the destabilisation of the borrelial cell membrane was within 1 h (Fig. 5, panel D), shorter than melittin (one of the widely studied natural AMPs), which destabilises the bacterial cell wall in roughly 75 min34.
AMPs have drawn attention as an alternative strategy to combat antibiotic-resistant bacteria, including cystic forms, as they can destroy dormant forms and bacteria rarely develop resistance to AMPs. Natural cationic antibacterial peptides show high affinity for the negatively charged surfaces of Gram-negative bacteria, however many of them are predicted to be toxic35,36. The Antimicrobial Peptide Database36 lists nearly 3200 AMPs that are not approved for therapeutic purpose due to their cell toxicity37,38,39. Previously it was shown that the antimicrobial activity of short cationic peptides is strongly dependent on the net charge, wherein a higher antibacterial activity was displayed by peptides with a net charge from + 2 to + 4, however at the same time peptides endowed with a greater positive charge were more toxic to human erythrocytes40. This clearly suggests that an AMP molecule needs proper balance between a net charge and antimicrobial activity. In addition to these features, the conformation of peptide, primarily their linear versus cyclic nature, should be taken into account. We used a combinatorial phage library that displays cyclic peptides because structurally constrained molecules have several advantages over linear molecules. The cyclic peptides are more resistant to proteolytic degradation under physiological conditions, particularly in serum41. The stability of our cyclic Bor peptide was demonstrated by the fact that the binding ability of all tested peptides remained constant even after 6 h of incubation in normal human serum. Another reason for exploiting cyclic peptides against Borrelia is that their binding to bacterial cells is governed not only by electrostatic interaction (a higher positive charge), but also by molecular interactions based on the peptides’ conformation. With a net charge of + 0.9 (well below the + 2 to + 4 range), all four Bor peptides (Bor-11, 16, 18, and 26) demonstrated strong binding affinities to both the spiral and cystic forms of Borrelia. In particular, Bor-18 showed binding affinity to Borrelia at concentration as low as 0.16 µM (Fig. 5, panel A). It has previously been demonstrated that cyclic peptides containing cysteine residues have a higher binding affinity for the target than their linear counterparts42. The third reason for exploiting the potential of cyclic peptides was their low cytotoxicity43. In line with previous finding, none of our peptides inhibited cell metabolism in the XTT assay, hindered cell proliferation, or caused hemolysis (Fig. 6).
Several synthetic and phage display library-derived AMPs have been generated so far, especially against antibiotic-resistant bacteria. One notable AMP, EC5, was able to permeabilise the outer membrane of E. coli and P. aeruginosa within 5 min of treatment44. EC5, like the Bor peptides produced by us, caused depolarisation of the cytoplasmic membrane, most likely through membrane-lipid interaction. Another AMP, comprised of 10 amino acids QEKIRVRLSA, was effective against several multidrug-resistant members of the Enterobacteriaceae family45. Many of the AMPs developed to date are from phage libraries displaying longer peptide, such as the 12-mer linear peptide library, which is available commercially (Ph.D.-12 from New England Biolabs). It was used to isolate AMPs against Listeria monocytogenes and E. coli46,47, while the 15-mer library was used to develop AMPs against Haemophilus influenzae and Campylobacter jejuni48,49. The combinatorial library used in our study was composed of only 7-mers flanked by cysteine residues. Several researchers prefer libraries displaying more than 10 amino acids in order to increase sequence diversity of the library and increase the likelihood of obtaining a better peptide candidate with the desired antibacterial capabilities.
Many therapeutic peptides and proteins discovered to date have proven ineffective in treating CNS infections. Poor transportation of the peptides across the BBB, as well as inadequate pharmacokinetics, are significant barriers to this progress. Thus, while developing the recombinant AMPs against Borrelia, we sought to improve their transport across the BBB. The presence of spirochetes and their cystic forms in the cerebral cortex of a patient with chronic neuroborreliosis7 emphasizes the need of AMPs that can cross the BBB. To date, several CNS homing peptides have been identified. Some are derived from human proteins that interact with brain microvascular endothelial cell receptors50,51,52, while others are derived from combinatorial libraries53,54,55,56,57, or pathogen-expressed proteins that help to evade the microvasculature58,59,60,61. The OspA protein of Borrelia is known to bind endothelial and epithelial cells via distinct domains (Fig. 2, panel A). In particular, brain microvascular endothelial cell binding domain of OspA of neuroinvasive Borrelia binds to the cell receptors and evokes borrelial translocation, mimicking the events that occur during RME20. We have also shown that blocking of this domain significantly reduces borrelial translocation across the BBB20. Based on this knowledge, we wanted to develop CNS homing peptide that could be fused with Cx7C for better transportation of antibacterial moiety through the BBB. The endothelial cell binding domain of OspA is composed of seven antiparallel beta sheets. Results from present study show that N-terminal region encompassing 10th and 11th beta sheets (designated as O-BBB) was able to cross the barrier readily than the C-terminal region that consists of 12th to 16th beta sheets21 (supplementary information 3).
There are several advantages for selecting O-BBB as a shuttle molecule, such as: (1) It contains no cysteine residue, which ensures no interference in the correct cyclisation of AMP mediated by the C–C disulphide bond; (2) It contains 85.79% hydrophilic amino acids (PEPTIDE 2.0 server), which could facilitate better solubilization of the final fusion construct as well as better production in the E.coli expression system; (3) The net charge of O-BBB is predicted to be -3.4 at pH 7.0, which may help in reducing the nonspecific electrostatic interaction to the negatively charged domains of eukaryotic and borrelial cell membrane and thus maintaining the specific interaction of Cx7C with borrelial phosphatidylcholine; and (4) Reducing the net charge may also help to reduce toxicity. We also assumed that angiopep-2 (TFFYGGSRGKRNNFKTEEY), despite being much smaller than O-BBB, has a net charge of + 2 at pH 7, which could result in non-specific electrostatic interaction of the fusion peptide with endothelial and borrelial cell membrane. With all these advantages, Cx7C fused with O-BBB exhibited ability to cross the BBB model significantly higher than the doxycycline (Fig. 7 panel C).
Conclusion
Using a combinatorial phage display library and phospholipase D for specific elution of peptides that bind to the borrelial membrane, we isolated and idenfied new anti-Borrelia peptides. Combining the potential of AMPs with a CNS homing peptide, we produced recombinant molecules that can cause destabilisation of the borrelial cell membrane within a1 hr, which results in a borreliacidal effect. Bor-11, Bor-16, Bor-18, and Bor-26 exhibited cidal activity against both spirochetal and cystic forms. Four peptides had no deleterious effect on eukaryotic cell metabolic activity or cell proliferation, and caused no lysis of sheep red blood cell. Bor peptides’ serum stability and ability to retain borreliacidal activity even after crossing the BBB model make them attractive molecules for the developing novel borrelicidal drug, especially against neuroborreliosis.
Materials and methods
Borrelia garinii culture and Borrelia with GFP epifluorescence
Neuroinvasive B. garinii (strain SKT-7.1, Serotype 4, GenBank accession number - GU906888.1) was cultured in complete BSK-II medium (Sigma Aldrich, USA) enriched with 6% rabbit serum at 33 °C for approximately 2 weeks to achieve early log phase. Cultures were examined under dark field microscopy (40x magnification) to assess the shape and motility of Borrelia. Early log phase was defined when borrelial count was approximately 4 × 106/ml and 99% Borrelia were seen as individual bacteria (no aggregations and cysts) under the dark field microscopy.
SKT-7.1 expressing GFP was prepared as described before62. Shortly, pTM61 plasmid harboring constitutively expressing green fluorescent protein (GFP) under the control of the B. burgdorferi flaB promoter (PflaB), determinants for replication in both E. coli and B. burgdorferi, and gentamycin resistance (aacC1) cassette was electroporated in electrocompetent SKT7.1 exactly as described previously63. SKT7.1 expressing GFP was selected by adding 100 µg/ml of gentamycin. The presence of GFP was checked with epifluorescence microscopy.
To obtain the cystic form, culture in early log phase was diluted in fresh BSK-II medium without serum (1:1 V: V) and incubated for 2 weeks at 15 °C. Formation of cysts and absence of spiral form was checked with epifluorescence microscopy (Supplementary information 1).
Counting of Borrelia using flow cytometry
50 µl of culture was mixed with 150 µl of PBS (containing 1x SYBR Green I; Molecular probes, USA) and used to enumerate bacteria using CytoFLEX flow cytometer (Beckman Coulter, USA). For Borrelia with GFP fluorescence, the SYBR green was omitted. Conditions for acquisition of data were: Volume measurement – 15 µl, Flow rate – medium (30 µl/min). Bacteria were differentiated from debris by adjusting the threshold (FSC 10 000/SSC 10 000) and polygonal gate (SSC vs. FITC) as shown in supplementary information 1.
To count cysts, all conditions were similar as described above; however different gates were applied to differentiate cystic form from the spirochetal Borrelia as shown in supplementary information 1.
Shaving of Borrelia with trypsin: Borrelia devoid of outer surface proteins
50 ml of Borrelia growing in log phase were centrifuged (4000 rpm for 10 min), washed with Dulbecco’s phosphate-buffered saline (DPBS; KCl 200 mg/L, KH2PO4 200 mg/L, NaCl 8,000 mg/L, Na2HPO4 1150 mg/L) with 20% sucrose and then resuspended in DPBS containing 5 µg/ml of trypsin gold (Promega, Milan, Italy), keeping the solution at 37 °C, 10 min with gentle shaking. Borrelia were centrifuged at 6000 rpm for 10 min and the supernatant was discarded, the pellet was washed with DPBS to obtain Borrelia devoid of surface proteins.
Selection of phages displaying Cx7C peptides with affinity for the borrelial cell membrane
Twenty milliliters of Borrelia culture in early log phase were centrifuged (7000× g, 15 min, 22 °C), the pellet was resuspended in 1 ml of DMEM (Biowest, France) and 2 × 1011 phages from Cx7C combinatorial phage library were added (Ph.D.-Cx7C; New England Biolabs, NEB, USA). Cysteine on both sides of Cx7C peptides constrains the cyclic form, thanks to the disulfide bond. Phages were incubated for 2 h at 22 °C with constant shaking. Non-interacting phages were removed by centrifugation (12000× g, 8 min, 16 °C) followed by 5 times washing with 1 ml of PBS (12000× g, 8 min, 16 °C). Phages interacting with borrelial cell membrane phosphatidylcholine moiety were eluted by the addition of 60 units of phospholipase D (Sigma Aldrich, USA) diluted in Tris-buffered saline (TBS, pH 7.4) for 2 h at 37 °C followed by centrifugation at 12,000× g, 8 min, 22 °C. Note that, phospholipase D was used to elute phage particles bound on Borrelia, resulting in specific enrichment of Cx7C with a high affinity for phosphatidylcholine. Phospholipase D hydrolyzes phosphatidylcholine to produce phosphatidic acid and soluble choline. Phosphatidylcholine is one of only two major membrane phospholipids, along with phosphatidylglycerol of borrelial membrane (Fig. 1, panel A14), . Phages in supernatant were amplified in E.coli ER2738 (NEB), purified, and quantified by titration exactly as described in our publication64 and used in the second round of borrelial cell membrane specific phages selection (panning). During the second panning, phages were negatively panned against plasticware, culture medium proteins, and BMECs to rule out enrichment of non-specific phage clones. In brief, phages (2 × 1010 phages resuspended in 100 µl of PBS) were incubated in an empty tube for 1 h with constant shaking at 37 °C. The phage solution was then transferred to a tube coated with BSK-II medium (coating is achieved by incubating BSK-II medium at 4 °C overnight) and incubated for 1 h with constant shaking at 37 °C. The solution containing phages was then transferred to the BMECs fixed in an ELISA plate well and incubated for 1 h at 37 °C with constant shaking. After that, phage-containing solution (negatively panned phages) was used in the second round of panning against Borrelia. The second round of panning was carried out while maintaining all of the conditions described for the first round. The third round of panning was carried out maintaining the same settings as the second.
To confirm the binding affinity of phages (eluted after 3rd round of panning) to different forms of Borrelia, a dot blot was performed. Briefly, 2 µl of Borrelia in early log phase or cystic form (washed and resuspended in PBS, OD600 1.2) or shaved with trypsin were spotted on an activated PVDF membrane, air dried and 10 µl 4% paraformaldehyde was added on the spot. After 10 min membrane was washed with PBS, dried overnight and incubated in blocking solution (LI-COR Biosciences, USA) for 1 h. Membrane was then incubated with 1011 phages resuspended in blocking solution for 1 h with constant shaking. Membrane was washed 5 times with PBS containing 0.05% Tween 20 (PBS-T) each for 5 min. Bound phages were detected with anti-M13 pIII monoclonal antibody (1:1000, 1 h; NEB) and by donkey anti-mouse antibody conjugated with IRDye 770 (1:15000, 1 h; LI-COR Biosciences). Signal was captured at 800 nm (Odyssey CLx, LI-COR Biosciences). For negative control, phages were omitted from the reaction. For input control, phages were spotted on the membrane and detected by primary and secondary antibody as described above.
To separate phage clones, 0.5 µl of phage elute obtained from 3rd panning was mixed with 200 µl of E. coli ER2738 culture (OD600), incubated for 5 min, mixed with molten agar (50 °C) and overlaid on LB agar plates containing 1 mM IPTG (Fermentas, Lithuania) and 1 mM X-gal (Sigma, USA). After overnight incubation, 30 plaques were separately picked and individual phage clones were amplified in 1 ml of E. coli ER2738 culture following the manufacturer´s protocol (NEB, USA). Genomic DNA from each clone was isolated by heating at 95 °C for 10 min and subjected to amplification of fragment encompassing sequence of 7-mer peptide flanked by cysteine residues (designated as Cx7C) and C-terminal GGGS. Primer sequences and PCR conditions are presented in (Supplementary Table 2). Amplicons were gel purified and sequenced (supplementary information 2). As a positive control, DNA isolated from Cx7C library was used. The amino acid sequences of Cx7C peptides were deduced from nucleotide sequences and grouped based on sequence homology (Geneious v9.1, Biomatters, USA).
In silico analysis of identified peptide sequences was performed to deduce AMP probability using Antimicrobial Peptide Scanner.2 (https://www.dveltri.com/ascan/v2/ascan.html), net charge identification, and water solubility (https://pepcalc.com). A possible match of identified sequences with the peptide uploaded to the public repository was checked with the peptide search tool of the UniProtKB database (https://www.uniprot.org/uniprotkb). Sequences were virtually scanned by ToxinPred (http://crdd.osdd.net/raghava/toxinpred/) to reveal the possible toxicity of peptides.
Individual phage clones were tested for binding affinity to Borrelia using dot blot as described above.
BBB homing peptide
Two truncated forms of OspA were produced (supplementary information 3) encompassing 10–11 and 12–16 beta sheets, exactly as described previously21. Both truncated forms and angiopep-2 (used as control as it is known BBB homing peptide, BOC Sciences, China) were labelled with Cyanine5.5 NHS ester (Lumiprobe, USA) following the manufacturer’s protocol. In short, required amount of Cyanine5.5 NHS ester was calculated based on formula: Amount of NH ester (mg) = 8 x amount of peptide (mg) x NH ester molar weight (Da) / molar weight of peptide (Da). The calculated amount of Cyanine5.5 NHS ester was incubated with 0.5 mg of peptides overnight at 4 °C. After incubation dialysis against PBS was performed overnight at 4 °C to remove unbound dye, and the concentration of conjugated peptide was determined by Bradford assay.
Construction of BBB model in-vitro
BMECs were cultured exactly as described in our earlier publication65. Briefly, 1 × 106 BMECs (cell line D3; Merck Millipore, USA) were seeded in a collagen-I (Corning, USA) coated T-75 cell culture flask (Sarstedt, Germany) in the complete endothelial medium at 37 °C, 95% humidity and 5% CO2 until confluence. The complete endothelial medium contains DMEM-F12 (Sigma-Aldrich, USA) supplemented with 1.4 µM hydrocortisone (Sigma-Aldrich), 10 ng/ml bFGF (Sigma-Aldrich), 5 mg/ml ascorbic acid (Sigma-Aldrich), 1x penicillin-streptomycin (Jena Bioscience, Germany), 10 mM HEPES (Biowest, France), 2 mM L-glutamin (Life Technologies, USA) and 10% fetal bovine serum (Biowest).
BBB model was cultured on cell-inserts. In brief, 5 × 105 BMECs were seeded in the luminal chamber of collagen-I coated 24-transwell Inserts (1 μm pores; cellQuart, Germany, transwell system depicted in supplementary information 4) containing 600 µl of complete endothelial medium in both luminal and abluminal chambers. The inserts were incubated at 37 °C in humidified CO2 incubator until a monolayer was formed. The formation of the monolayer was verified by measuring the transendothelial electrical resistance (TEER) between the luminal and abluminal chamber of inserts66. TEER was measured by pair of chopstick electrodes on a volt ohm meter EVOM (World Precision Instrument, USA). The resistance is inversely proportional to the growth area of the semipermeable membrane (cm2). The TEER value (Ω/cm2) was calculated using the formula:
Wherein:
Resistanceinsert =recorded resistance value between luminal and abluminal chambers of inserts in which the BMEC monolayer is fully grown.
Resistanceblank =recorded resistance value of semipermeable membrane in transwell without cells (only collagen coated).
When the observed TEER value was ~ 100 Ω/cm2, the barrier properties were confirmed by diffusion of CF 770 dextran67. Briefly, 0.1 µg/ml of CF 770 dextran was added to the luminal chamber of each insert, and its passage through the BBB model was recorded by scanning the contents of the abluminal chamber at 800 nm on Odyssey CLx Imaging System (LI-COR) at 1, 3, and 5 h. The concentration of CF 770 dextran in the abluminal chamber was determined by a standard curve prepared by a two-fold dilution of 0.2 µg/ml of CF 770 Dextran. Barrier properties were confirmed when the amount of CF 770 dextran that passed from the luminal to the abluminal chamber was lower than 6% of the amount added to the luminal chamber. Inserts with confirmed barrier properties (by both TEER and dextran) were used to test the passage of Cy5.5 labelled truncated fragments or angiopep-2-Cy5.5.
Assessment of crossing of labelled truncated fragments or angiopep-2 across BBB model
1 µg/ml of each of the truncated fragment labelled with Cy5.5 or angiopep-2-Cy5.5 or Dextran CF 770 (Biotium, USA) was added in the upper chamber (luminal) of the BBB model and incubated at 37 °C in cell culture incubator. Crossing of truncated fragments from the luminal to the abluminal chamber was measured by measuring the fluorescence at 1, 2, and 3 h of incubation. Fluorescence was measured on Odyssey CLx using wavelengths: 650/705 nm (for Cy5.5 labelled molecules) and 770/797 nm (for dextran CF 770). The concentration of each component in the abluminal chamber was calculated using standard curves. The percentage of crossing of BBB was calculated. The assay was performed in triplicate.
The N-terminal fragment of endothelial cell binding domain (O-BBB, NEKGETSEKTIVRANGTRLEYTDIKSDG), which demonstrated significant BBB crossing, was used to fuse with Cx7C. The tertiary structure of the O-BBB was predicted on ColabFold, which uses AlphaFold268.
Synthesis of Cx7C fused with O-BBB
DNA encoding Cx7C was amplified from representative phage clone from each cluster that showed affinity to Borrelia. Primers used to amplify Cx7C fragment are listed in (Supplementary Table 2). DNA amplification, cloning into pQE-30 N-tag-II vector, electroporation in E. coli T5 SHuffle, clonal selection, protein overexpression and purification, assessment of purity by LDS-PAGE and MALDI-TOF-MS (Bruker Daltonics; Germany) were performed exactly as described in our methodological paper69. pQE-30 N-tag-II vector allows N-terminal fusion of O-BBB tag to Cx7C peptides. The fusion constructs consist of O-BBB at N-terminus of the Cx7C, flanked by GGGS at both ends (Fig. 2, panel D). The GGGS between O-BBB and Cx7C was placed to avoid any steric hindrance among two molecules, while GGGS at C-terminus was added to keep sequence similarity inherited from Ph.D.-Cx7C library (details are available in user manual from New England Biolabs, catalog number E8212S). Nine different Cx7C sequences were fused with O-BBB. These sequences were chosen based on the phage clones that exhibited binding affinity to Borrelia in the dot blot assay described above. Fused peptides were designated as Bor-1, Bor-8, Bor-9, Bor-11, Bor-16, Bor-18, Bor-23, Bor-26 and Bor-29.
The tertiary structures of the O-BBB fused Cx7C were predicted on ColabFold, which uses AlphaFold2 68.
Assessment of binding of Cx7C fusion peptides (bor peptides) to Borrelia
Fifty microliters of the Borrelia (washed and resuspended in PBS, OD600 0.3) were spotted in a 96-well plate, air dried overnight at room temperature, and fixed with 50 µl of 4% paraformaldehyde for 10 min. Nonspecific binding sites were blocked with 100 µl of 5% BSA in PBS-T and 2 µg of Bor peptides dissolved in 100 µl of PBS-T containing 1% BSA were added. After 1 h of incubation, wells were washed 5 times with 100 µl of PBS-T. Bor peptides bound on Borrelia were detected with HisProbe-HRP (1:1000 in PBS-T containing 0.5% BSA, Thermo Scientific, incubation 1 h at room temperature) and 1-Step Ultra TMB-ELISA substrate (100 µl/well, 25 min at room temperature, Thermo Scientific). 100 µl of 2M H2SO4 was added and readings were taken at 450 nm on ELISA plate reader (PerkinElmer, Victor X3, USA). The assay was performed in triplicates. For negative controls, either Borrelia or peptides were omitted from the assay. For input control 2 µg of Bor peptides were coated in well and detected by HisProbe-HRP and TMB. O-BBB was also included in the assay to see if it shows any non-specific binding to Borrelia.
Assessment of borreliacidal activity of peptides
The number of Borrelia in culture (in early log phase) was enumerated using by C6 flow cytometer exactly as described previously70. 2 × 103Borrelia were inoculated in 200 µl of fresh BSK-II medium containing Bor peptides or only O-BBB at varying concentrations (6.67, 3.33, 1.67 and 0 µM) and incubated at 33 °C for 5 days. After incubation, the number of Borrelia in the culture were enumerated by flow cytometry. Percent inhibition of Borrelia was calculated by taking into account the number of Borrelia in medium containing 0 µM of peptide. Doxycycline (50 µM to 5 nM) was used in the assay as a positive control. The Bor peptides with significant borreliacidal activity at 1.67 µM were tested further at lower concentrations (0.83, 0.41, 0.20, and 0.10 µM) while maintaining all other experimental parameters.
Assessment of inhibition of conversion of cystic form in to spirochetal form
Culture containing cysts was observed under the fluorescent microscope to check presence of any spirochetal form (Supplementary information 1). Borrelial cysts were counted using a C6 flow cytometery (Supplementary information 1). 2 × 103 cysts were incubated with Bor peptides at different concentrations (two-fold serial dilutions from 3.33 to 0.05 µM) in 200 µl of BSK-II medium devoid of rabbit serum for 16 h. Please note that absence of serum maintains the cystic form of Borrelia and are unable to convert readily in to spirochetal form. After incubation cultures were centrifuged at 6000 RPM for 15 min. Supernatant containing peptide was discarded and 200 µl of fresh complete BSK-II medium (containg rabbit serum) was added into the same tube and incubated for 15 days at 33 °C. In this assay, it was necessary to remove peptides after 16 h as presence of peptides in culture may inhibit newly formed spirochete in complete BSK-II medium, which may interfere the assessment of inhibitory effect of peptides exclusively on cyctic form. Doxycycline (50 nM) was used as a control, and the assay was carried out exactly as with Bor peptides. As a live control, the peptides were replaced by PBS. The enumeration of Borrelia in each treatment and controls was performed as described above. Percent inhibition of conversion of cystic form in to spirochetal form was calculated taking into account number of Borrelia present in PBS control.
Assessment concentration dependent binding of Bor-11, Bor-16, Bor-18 and Bor-26 to spirochetal form of Borrelia
ELISA was used to determine the concentration-dependent binding of Bor peptides to Borrelia. Borrelia growing in the early log phase were fixed to the microtiter wells with paraformaldehyde exactly as described above. All steps in ELISA were identical to those described above, with the exception of the peptide concentrations used in the assay ranging from 2.6 µM to 86 nM (two-fold serial dilution). Peptides were omitted from the assay as negative controls.
Assessment binding of Bor-11, Bor-16, Bor-18 and Bor-26 to different forms of Borrelia
Borrelia growing in the early log phase, its cystic form, and Borrelia devoid of surface proteins were fixed to the microtiter wells with paraformaldehyde. All steps in ELISA were identical to those described above, with the exception of the peptide concentration, which was 2 µg/well. Peptides were omitted from the assay as negative controls. As a control, an antibody raised against OspA (Abcam, UK) was used in the assay.
Evaluation of the effect of peptides on borrelial membrane
For detection of the borrelial membrane depolarization caused by the peptides, previously published protocol71 was adopted with minor changes. 10 ml of Borrelia (non-GFP) culture was centrifuged, the pellet was washed with PBS and resuspended in PBS to obtain OD600 1.0. 10 µl of this suspension was added to each well of the 96-well plate and mixed with 165 µl of PBS, 25 µl of 1 M glucose, and 5 µl of 5 µM DiBAC4(3) dye (Thermo Scientific). Immediately, Bor peptides (6.67 µg) were added to each well. The plate was immediately placed into a time-lapse imaging reader (Cytation 7, BioTek, Germany) with preset filters for DiBAC4(3) detection (490 nmEx and 516 nmEm). The measurement was carried out every 2 min during 4 h and data were processed using Gen5 V3.11.
To access effect of Bor peptides on membrane permeability a protocol published previously72 was modified. In brief, 10 ml of Borrelia (non-GFP) culture was centrifuged, the pellet was washed with DPBS and resuspended in DPBS to obtain OD600 1.0. 10 µl of this suspension was added to each well of a 96-well plate and propidium iodide (30 µM; ThermoFisher Scientific, United States) was added to each well. Bor peptides (6.67 µg) were added to each well immediately. One well was retained without the addition of any peptide to form a negative control. Whereas, Triton X-100 (0.1%, Sigma-Aldrich, Germany) was added in positive control well. Fluorescence of PI was measured on Cytation7 (BioTek, USA) every 2 min until 2 h at 535 nmEx and 617 nmEm.
Assessment of cell toxicity (XTT assay)
HEK293T cells were grown in a 96-well culture plate (Sigma Aldrich) in DMEM high glucose medium (Thermo Scientific) enriched with 2mM L-glutamine (Sigma Aldrich) and 5% fetal bovine serum (BioWest) at 37 °C with 5% CO2 until 70% confluence. Cells were either used for XTT or in cell proliferation assay.
Cell metabolism was determined through XTT (AppliChem, Germany) following the manufacturer’s instructions as described in our earlier publication73. Briefly, HEK293T cells were cultivated in 96-well plate. At 70% confluence, the cells were added with peptides (Bor-11, 16, 18 and 26) at various concentrations (3.33 and 0 µM). Cells were incubated for 24 h at 37 °C and 5% CO2. After incubation, 50 µl XTT reagent was directly added, incubated for 3 h, and measured at 450 nm. For controls, peptides were either replaced by PBS or Triton X (0.1%). The assay was conducted in six replicates. Metabolic activity was calculated using the formula: \(\left[ {\left( {{\text{absorbanc}}{{\text{e}}_{{\text{sample}}}} - {\text{ absorbanc}}{{\text{e}}_{{\text{blank}}}}} \right){\text{ }} \times {\text{ 1}}00} \right]/\left( {{\text{absorbanc}}{{\text{e}}_{{\text{negative control}}}} - {\text{ absorbanc}}{{\text{e}}_{{\text{blank}}}}} \right)\). Here PBS served as negative control.
Cell proliferation assay
For proliferation assay, peptides (Bor-11, 16, 18, and 26) at varying concentrations (3.33, 1.67, and 0 µM of peptides diluted in complete DMEM medium) were added on the freshly seeded HEK293T in 96-well plate. Cells without peptides served as growth control. Cells were incubated at 37 °C in a humidified CO2 incubator for 96 h and cell counting was performed at every 24 h with high contrast bright field microscopy on Cytation7 imaging system (BioTek, USA). Cell enumeration and cell morphology were documented with Gen Image 5 ver 3.11 software. Technical parameters are presented in https://www.agilent.com/cs/library/applications/automated-kinetic-imaging-assay-cell-proliferation-5994-2593EN-agilent.pdf). Correlation between the number of cells in treatments and negative control was determined by Pearson correlation coefficient (r)74.
Hemolysis assay
Bor-11, 16, 18, and 26 peptides were evaluated for their hemocompatibility using the method described earlier75. In brief, 30 ml of healthy sheep blood was collected in the presence of 2500 IU heparin (Zentiva, Slovakia) by licensed veterinarian at the animal rearing house of the University of Veterinary Medicine and Pharmacy, Košice. The blood was centrifuged at 657 × g for 5 min and the separated erythrocytes were washed three times with 0.9% saline and dispersed in 150 ml of 0.9% saline. Erythrocyte suspension (1 ml) was incubated with peptides at varying concentrations (300, 100, and 50 ng) for 1 h, 3 h, and 5 h at 37 °C. 1 ml of erythrocyte suspension either mixed with 2% Triton X-100 (Sigma) or 0.9% saline serving as positive and negative controls, respectively in the assay. All the samples were gently mixed every 30 min. At the end of incubation, the samples were centrifuged (657 × g for 5 min) and the supernatant was collected in new tubes and allowed to oxidize hemoglobin at room temperature. The absorbance of oxyhemoglobin was measured at 414 nm. Hemolysis was calculated using the formula: % hemolysis = (absorbancesample - absorbancenegative control) / (absorbancepositive control - absorbancenegative control) x 100. The entire assay was performed in triplicates.
Binding of Bor peptides to endothelial cells
The ability of Bor peptides to bind to endothelial surface was tested by cell-ELISA. In short, the monolayer of the BMECs cultured in 96 wells was washed once with PBS and fixed with 4% paraformaldehyde for 10 min. Blocking of nonspecific binding sites, incubation of 2 µg of Bor peptides with cells, and detection of bound peptides with HisProbe-HRP and TMB-ELISA substrate were performed as ELISA assay described above. The assay was performed in triplicates. For negative controls, either cells or peptides were omitted from the assay. O-BBB was used as a control.
Serum stability of the Bor peptides
To test the stability of peptides in serum, 1.67 µM of each Bor peptide was incubated with an equal volume of human serum (complement inactivated at 56 °C for 30 min) for 1, 3, and 6 h. After each time point, the mixture was transferred to BSK-II medium containing 2 × 103Borrelia (in early log phase). For control reactions, peptides were replaced either with doxycycline (50 nM), or PBS, or heat inactivated normal human serum (equal volume). The cultures were incubated for 5 days at a total volume of 200 µl. Following incubation, the number of Borrelia in each treatment was measured using flow cytometry, as described before. Assay was performed in triplicates.
Crossing of Bor peptides through in-vitro BBB model and borreliacidal effect after crossing the barrier
A BBB model was cultured on 24-well cell inserts, as described above. Borrelia in early log phase were counted using flow cytometry, and 2 × 104 bacteria were inoculated into the abluminal chamber of cell inserts. After 60 min (37 °C, 5%, CO2), 4.1 µM of each peptide (approximately 6.25 µg) diluted in 200 µl of complete endothelial medium was added in each luminal chamber. 500 ng of CF770 Dextran was also added into the luminal chamber. As a negative control, peptides were replaced by PBS and as positive control doxycycline (0.11 µg/ml) was added to the luminal chamber. Please note that throughout the experiment, the luminal chamber contained 400 µl of the medium, while abluminal chamber had 600 µl. Medium was devoid of penicillin and streptomycin standardly used in cell culture. Inserts were incubated for 5 h (37 °C, 5%, CO2), and TEER was measured to determine whether the BBB integrity was compromised during the incubation. Abluminal content was scanned at 800 nm to measure % crossing of dextran. The abluminal content (500 µl) of each insert was transferred to a 0.5 ml sterile tube and incubated for 6 days at 37 °C and 5% CO2. The borrelicidal effect was determined by measuring the number of Borrelia grown in the tube using flow cytometry (CytoFLEX, Beckman Coulter, US), as previously described.
Data availability
Data generated in this study are available from the corresponding author upon reasonable request.
References
Shapiro, E. D. & Wormser, G. P. Lyme disease in 2018: what is new (and what is not). JAMA 320, 635–636. https://doi.org/10.1001/jama.2018.10974 (2018).
Radolf, J. D., Strle, K., Lemieux, J. E. & Strle, F. Lyme disease in humans. Curr. Issues Mol. Biol. 42, 333–384. https://doi.org/10.21775/cimb.042.333 (2021).
Thompson, D., Sorenson, J., Greenmyer, J., Brissette, C. A. & Watt, J. A. The lyme disease bacterium, Borrelia burgdorferi, stimulates an inflammatory response in human choroid plexus epithelial cells. PLoS One 15, e0234993. https://doi.org/10.1371/journal.pone.0234993 (2020).
Duray, P. H. & Steere, A. C. Clinical pathologic correlations of lyme disease by stage. Ann. N Y Acad. Sci. 539, 65–79. https://doi.org/10.1111/j.1749-6632.1988.tb31839.x (1988).
Koedel, U., Fingerle, V. & Pfister, H. W. Lyme neuroborreliosis-epidemiology, diagnosis and management. Nat. Rev. Neurol. 11, 446–456. https://doi.org/10.1038/nrneurol.2015.121 (2015).
Rauer, S. et al. Lyme neuroborreliosis. Dtsch. Arztebl Int. 115, 751–756. https://doi.org/10.3238/arztebl.2018.0751 (2018).
Miklossy, J. et al. Persisting atypical and cystic forms of Borrelia burgdorferi and local inflammation in Lyme neuroborreliosis. J. Neuroinflamm. 5, 40. https://doi.org/10.1186/1742-2094-5-40 (2008).
Mihajlovic, M. & Lazaridis, T. Antimicrobial peptides in toroidal and cylindrical pores. Biochim. Biophys. Acta 1798, 1485–1493. https://doi.org/10.1016/j.bbamem.2010.04.004 (2010).
Cudic, M. & Otvos, L. Jr. Intracellular targets of antibacterial peptides. Curr. Drug Targ. 3, 101–106. https://doi.org/10.2174/1389450024605445 (2002).
Sambri, V. et al. Comparative in vitro activity of five cathelicidin-derived synthetic peptides against Leptospira, Borrelia and Treponema pallidum. J. Antimicrob. Chemother. 50, 895–902. https://doi.org/10.1093/jac/dkf220 (2002).
Socarras, K. M., Theophilus, P. A. S., Torres, J. P., Gupta, K. & Sapi, E. Antimicrobial activity of bee venom and melittin against Borrelia burgdorferi. Antibiot. (Basel). 6. https://doi.org/10.3390/antibiotics6040031 (2017).
Isogai, E., Isogai, H., Takahashi, K., Kobayashi-Sakamoto, M. & Okumura, K. Antimicrobial activity of three tick defensins and four mammalian cathelicidin-derived synthetic peptides against Lyme disease spirochetes and bacteria isolated from the midgut. Exp. Appl. Acarol. 49, 221–228. https://doi.org/10.1007/s10493-009-9251-5 (2009).
Rajamani, L. M., Goh, V., Beuerman, T. L. E. & Verma, R. W. N.K. https://patentimages.storage.googleapis.com/57/ad/65/9e8082bfba1d65/US11396531.pdf (2022).
Kerstholt, M., Netea, M. G. & Joosten, L. A. B. Borrelia burgdorferi hijacks cellular metabolism of immune cells: consequences for host defense. Ticks Tick. Borne Dis. 11, 101386. https://doi.org/10.1016/j.ttbdis.2020.101386 (2020).
Gwynne, P. J., Clendenen, L. H., Turk, S. P., Marques, A. R. & Hu, L. T. Antiphospholipid autoantibodies in Lyme disease arise after scavenging of host phospholipids by Borrelia burgdorferi. J. Clin. Invest. 132 https://doi.org/10.1172/JCI152506 (2022).
Dong, X. Current strategies for brain drug delivery. Theranostics 8, 1481–1493. https://doi.org/10.7150/thno.21254 (2018).
Zhang, W. et al. Differential expression of receptors mediating receptor-mediated transcytosis (RMT) in brain microvessels, brain parenchyma and peripheral tissues of the mouse and the human. Fluids Barriers CNS 17 https://doi.org/10.1186/s12987-020-00209-0 (2020).
Zhou, X., Smith, Q. R. & Liu, X. Brain penetrating peptides and peptide-drug conjugates to overcome the blood-brain barrier and target CNS diseases. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 13, e1695. https://doi.org/10.1002/wnan.1695 (2021).
Bencurova, E., Mlynarcik, P. & Bhide, M. An insight into the ligand-receptor interactions involved in the translocation of pathogens across blood-brain barrier. FEMS Immunol. Med. Microbiol. 63, 297–318. https://doi.org/10.1111/j.1574-695X.2011.00867.x (2011).
Pulzova, L. et al. OspA-CD40 dyad: ligand-receptor interaction in the translocation of neuroinvasive Borrelia across the blood-brain barrier. Sci. Rep. 1, 86. https://doi.org/10.1038/srep00086 (2011).
Mlynarcik, P. et al. Deciphering the interface between a CD40 receptor and borrelial ligand OspA. Microbiol. Res. 170, 51–60. https://doi.org/10.1016/j.micres.2014.09.003 (2015).
Mochnacova, E. et al. Simple and rapid pipeline for the production of cyclic and linear small-sized peptides in E. Coli. Protein Expr Purif. 191, 106026. https://doi.org/10.1016/j.pep.2021.106026 (2022).
Merilainen, L., Herranen, A., Schwarzbach, A. & Gilbert, L. Morphological and biochemical features of Borrelia burgdorferi pleomorphic forms. Microbiol. (Reading) 161, 516–527. https://doi.org/10.1099/mic.0.000027 (2015).
Brorson, O. et al. Destruction of spirochete Borrelia burgdorferiround-body propagules (RBs) by theantibiotic Tigecycline. PNAS 106, 18656–18661. https://doi.org/10.1073/pnas.0908236106 (2009).
Sapi, E. et al. Evaluation of in-vitro antibiotic susceptibility of different morphological forms of Borrelia burgdorferi. Infect. Drug Resist. 4, 97–113. https://doi.org/10.2147/IDR.S19201 (2011).
Kortela, E. et al. Oral doxycycline compared to intravenous ceftriaxone in the treatment of lyme neuroborreliosis: a multicenter, equivalence, randomized, open-label trial. Clin. Infect. Dis. 72, 1323–1331. https://doi.org/10.1093/cid/ciaa217 (2021).
Chung, M. K. et al. Systematic comparisons between Lyme disease and post-treatment Lyme disease syndrome in the U.S. with administrative claims data. EBioMedicine 90, 104524. https://doi.org/10.1016/j.ebiom.2023.104524 (2023).
Dersch, R., Sommer, H., Rauer, S. & Meerpohl, J. J. Prevalence and spectrum of residual symptoms in Lyme neuroborreliosis after pharmacological treatment: a systematic review. J. Neurol. 263, 17–24. https://doi.org/10.1007/s00415-015-7923-0 (2016).
Hodzic, E., Imai, D., Feng, S. & Barthold, S. W. Resurgence of persisting non-cultivable Borrelia burgdorferi following antibiotic treatment in mice. PLoS One 9, e86907. https://doi.org/10.1371/journal.pone.0086907 (2014).
Di Domenico, E. G. et al. The emerging role of microbial biofilm in Lyme neuroborreliosis. Front. Neurol. 9, 1048. https://doi.org/10.3389/fneur.2018.01048 (2018).
Sapi, E. et al. Characterization of biofilm formation by Borrelia burgdorferi in vitro. PLoS One 7, e48277. https://doi.org/10.1371/journal.pone.0048277 (2012).
Brorson, O. & Brorson, S. H. In vitro conversion of Borrelia burgdorferi to cystic forms in spinal fluid, and transformation to mobile spirochetes by incubation in BSK-H medium. Infection 26, 144–150. https://doi.org/10.1007/BF02771839 (1998).
Epand, R. M. & Vogel, H. J. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta. 1462, 11–28. https://doi.org/10.1016/s0005-2736(99)00198-4 (1999).
Gee, M. L. et al. Imaging the action of antimicrobial peptides on living bacterial cells. Sci. Rep. 3, 1557. https://doi.org/10.1038/srep01557 (2013).
Malanovic, N. & Lohner, K. Gram-positive bacterial cell envelopes: the impact on the activity of antimicrobial peptides. Biochim. Biophys. Acta 1858, 936–946 (2016).
Wang, G., Li, X. & Wang, Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 44, D1087–1093. https://doi.org/10.1093/nar/gkv1278 (2016).
Greco, I. et al. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 10, 13206. https://doi.org/10.1038/s41598-020-69995-9 (2020).
Lim, L. M. et al. Resurgence of colistin: a review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacotherapy 30, 1279–1291. https://doi.org/10.1592/phco.30.12.1279 (2010).
Chen, C. H., Bepler, T., Pepper, K., Fu, D. & Lu, T. K. Synthetic molecular evolution of antimicrobial peptides. Curr. Opin. Biotechnol. 75, 102718. https://doi.org/10.1016/j.copbio.2022.102718 (2022).
Greber, K. E., Dawgul, M., Kamysz, W. & Sawicki, W. Cationic net charge and counter ion type as antimicrobial activity determinant factors of short lipopeptides. Front. Microbiol. 8, 123. https://doi.org/10.3389/fmicb.2017.00123 (2017).
Evans, B. J., King, A. T., Katsifis, A., Matesic, L. & Jamie, J. F. Methods to enhance the metabolic stability of peptide-based PET radiopharmaceuticals. Molecules 25 https://doi.org/10.3390/molecules25102314 (2020).
Gang, D., Kim, D. W. & Park, H. S. Cyclic peptides: promising scaffolds for biopharmaceuticals. Genes (Basel) 9 https://doi.org/10.3390/genes9110557 (2018).
Shinbara, K., Liu, W., van Neer, R. H. P., Katoh, T. & Suga, H. Methodologies for backbone macrocyclic peptide synthesis compatible with screening technologies. Front. Chem. 8, 447. https://doi.org/10.3389/fchem.2020.00447 (2020).
Sainath Rao, S., Mohan, K. V. & Atreya, C. D. A peptide derived from phage display library exhibits antibacterial activity against E. Coli and Pseudomonas aeruginosa. PLoS One 8, e56081. https://doi.org/10.1371/journal.pone.0056081 (2013).
Pini, A. et al. Antimicrobial activity of novel dendrimeric peptides obtained by phage display selection and rational modification. Antimicrob. Agents Chemother. 49, 2665–2672. https://doi.org/10.1128/AAC.49.7.2665-2672.2005 (2005).
Flachbartova, Z. et al. Inhibition of multidrug resistant Listeria monocytogenes by peptides isolated from combinatorial phage display libraries. Microbiol. Res. 188–189, 34–41. https://doi.org/10.1016/j.micres.2016.04.010 (2016).
Lin, K. C. et al. A dodecapeptide (YQVTQSKVMSHR) exhibits antibacterial effect and induces cell aggregation in Escherichia coli. Appl. Microbiol. Biotechnol. 94, 755–762. https://doi.org/10.1007/s00253-011-3857-3 (2012).
Bishop-Hurley, S. L., Schmidt, F. J., Erwin, A. L. & Smith, A. L. Peptides selected for binding to a virulent strain of Haemophilus influenzae by phage display are bactericidal. Antimicrob. Agents Chemother. 49, 2972–2978. https://doi.org/10.1128/AAC.49.7.2972-2978.2005 (2005).
Bishop-Hurley, S. L., Rea, P. J. & McSweeney, C. S. Phage-displayed peptides selected for binding to campylobacter jejuni are antimicrobial. Protein Eng. Des. Sel. 23, 751–757. https://doi.org/10.1093/protein/gzq050 (2010).
Jiang, Y., Zhang, J., Meng, F. & Zhong, Z. Apolipoprotein E peptide-directed chimeric polymersomes mediate an ultrahigh-efficiency targeted protein therapy for glioblastoma. ACS Nano 12, 11070–11079. https://doi.org/10.1021/acsnano.8b05265 (2018).
Oller-Salvia, B. et al. MiniAp-4: a venom-inspired peptidomimetic for brain delivery. Angew Chem. Int. Ed. Engl. 55, 572–575. https://doi.org/10.1002/anie.201508445 (2016).
Al-Azzawi, S., Masheta, D., Guildford, A., Phillips, G. & Santin, M. Designing and characterization of a novel delivery system for improved cellular uptake by brain using dendronised apo-E-derived peptide. Front. Bioeng. Biotechnol. 7, 49. https://doi.org/10.3389/fbioe.2019.00049 (2019).
Sakamoto, K., Shinohara, T., Adachi, Y., Asami, T. & Ohtaki, T. A novel LRP1-binding peptide L57 that crosses the blood brain barrier. Biochem. Biophys. Rep. 12, 135–139. https://doi.org/10.1016/j.bbrep.2017.07.003 (2017).
Jacquot, G. et al. Optimization and in vivo validation of peptide vectors targeting the LDL receptor. Mol. Pharm. 13, 4094–4105. https://doi.org/10.1021/acs.molpharmaceut.6b00687 (2016).
Andre, S. et al. Development of an LDL receptor-targeted peptide susceptible to facilitate the brain access of diagnostic or therapeutic agents. Biology (Basel) 9 https://doi.org/10.3390/biology9070161 (2020).
Arranz-Gibert, P. et al. A MALDI-TOF-based method for studying the transport of BBB shuttles-enhancing sensitivity and versatility of cell-based in vitro transport models. Sci. Rep. 9, 4875. https://doi.org/10.1038/s41598-019-40973-0 (2019).
Majerova, P. et al. Novel blood-brain barrier shuttle peptides discovered through the phage display method. Molecules 25 https://doi.org/10.3390/molecules25040874 (2020).
Kim, J. Y., Choi, W. I., Kim, Y. H. & Tae, G. Brain-targeted delivery of protein using chitosan- and RVG peptide-conjugated, pluronic-based nano-carrier. Biomaterials 34, 1170–1178. https://doi.org/10.1016/j.biomaterials.2012.09.047 (2013).
Zhang, H., Gerson, T., Varney, M. L., Singh, R. K. & Vinogradov, S. V. Multifunctional peptide-PEG intercalating conjugates: programmatic of gene delivery to the blood-brain barrier. Pharm. Res. 27, 2528–2543. https://doi.org/10.1007/s11095-010-0256-x (2010).
Neves, V. et al. Novel peptides derived from dengue virus capsid protein translocate reversibly the blood-brain barrier through a receptor-free mechanism. ACS Chem. Biol. 12, 1257–1268. https://doi.org/10.1021/acschembio.7b00087 (2017).
Jiang, N. et al. Blood-brain barrier penetration of an Abeta-targeted, arginine-rich, d-enantiomeric peptide. Biochim. Biophys. Acta 1858, 2717–2724. https://doi.org/10.1016/j.bbamem.2016.07.002 (2016).
Moriarty, T. J. et al. Real-time high resolution 3D imaging of the lyme disease spirochete adhering to and escaping from the vasculature of a living host. PLoS Pathog. 4, e1000090. https://doi.org/10.1371/journal.ppat.1000090 (2008).
Samuels, D. S. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol. Biol. 47, 253–259. https://doi.org/10.1385/0-89603-310-4 (1995).
Mertinkova, P. et al. Development of peptides targeting receptor binding site of the envelope glycoprotein to contain the West Nile virus infection. Sci. Rep. 11, 20131. https://doi.org/10.1038/s41598-021-99696-w (2021).
Hruskovicova, J. et al. Engineering the single domain antibodies targeting receptor binding motifs within the domain III of west Nile virus envelope glycoprotein. Front. Microbiol. 13, 801466. https://doi.org/10.3389/fmicb.2022.801466 (2022).
Srinivasan, B. et al. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 20, 107–126. https://doi.org/10.1177/2211068214561025 (2015).
Cho, H. et al. Three-dimensional blood-brain barrier model for in vitro studies of neurovascular pathology. Sci. Rep. 5, 15222. https://doi.org/10.1038/srep15222 (2015).
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682. https://doi.org/10.1038/s41592-022-01488-1 (2022).
Mochnacova, E. et al. Simple and rapid pipeline for the production of cyclic and linear small-sized peptides in E. Coli. Protein Expr. Purif. 191, 106026. https://doi.org/10.1016/j.pep.2021.106026 (2021).
Tkacova, Z. et al. Comprehensive mapping of the cell response to Borrelia bavariensis in the brain microvascular endothelial cells in vitro using RNA-Seq. Front. Microbiol. 12, 760627. https://doi.org/10.3389/fmicb.2021.760627 (2021).
Clementi, E. A., Marks, L. R., Roche-Hakansson, H. & Hakansson, A. P. Monitoring changes in membrane polarity, membrane integrity, and intracellular ion concentrations in Streptococcus pneumoniae using fluorescent dyes. J. Vis. Exp. e51008 https://doi.org/10.3791/51008 (2014).
Clementi, E. A., Marks, L. R., Roche-Håkansson, H. & Håkansson, A. P. Monitoring changes in membrane polarity, membrane integrity, and intracellular ion concentrations in Streptococcus pneumoniae using fluorescent dyes. J. Visual. Exp. https://doi.org/10.3791/51008 (2014).
Mertinková;, P. et al. Development of peptides targeting receptor binding site of the envelope glycoprotein to contain the West Nile virus infection. Sci. Rep. https://doi.org/10.1038/s41598-021-99696-w (2021).
Social Science Statistics. https://www.socscistatistics.com/tests/pearson/default2.aspx
Rahman, M. A. & Ochiai, B. Fabrication and hemocompatibility of carboxy-chitosan stabilized magnetite nanoparticles. Microsyst. Technol. 24 (2018).
Acknowledgements
The authors thank Ing. Martina Cepkova and Ing. Viera Rusnakova for their technical assistance. Research was funded by grants APVV-22-0084, APVV-18-0259, VEGA1/0348/22 and EURONANOMED2021-105.
Author information
Authors and Affiliations
Contributions
MB and EM designed experiments. EM and MB performed phage display and panning. EM and JJ overexpressed Bor peptide and purified. SDS-PAGE, MALDI and other quality control of Bor was performed EM. EM, KK, KB and JJ performed ELISA, XTT, proliferation and hemolysis assays. EM and KK performed Borrelia culturing. KB and MB constructed BBB model in-vitro and performed translocation assays for dextan, angiopep-2, and various OspA truncated forms. MB performed protein modeling. EM performed membrane depolarization assay, time to kill assay and EC50 assays. KB, TM, JJ, KK and MB performed assays for the crossing of Bor across BBB model followed by borreliacidal test. Statistical and bioinformatic analyses were performed by EM and MB. MB and EM prepared the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mochnáčová, E., Bhide, K., Kucková, K. et al. Antimicrobial cyclic peptides effectively inhibit multiple forms of Borrelia and cross the blood-brain barrier model. Sci Rep 15, 6147 (2025). https://doi.org/10.1038/s41598-025-90605-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-90605-z
Keywords
This article is cited by
-
Complex Peptide Injectables: Development and Challenges
International Journal of Peptide Research and Therapeutics (2025)