Abstract
This study aims to investigate prognostic factors for visual recovery in patients with ethambutol-induced toxic optic neuropathy (EON) by analyzing visual field (VF) tests, retinal nerve fiber layer (RNFL) thickness on optical coherence tomography (OCT), and other variables. We retrospectively reviewed 46 eyes of 23 patients with EON who had been followed up for more than six months. Patients were categorized into recovery (n = 12) and non-recovery (n = 11) groups based on visual recovery at six months after discontinuation of ethambutol. In the recovery group, initial best-corrected visual acuity (BCVA) and VF tests were better (p < 0.001), body weight (p = 0.04) was higher and the number of patient with diabetes (p = 0.007) was lower than in the non-recovery group. Baseline BCVA, VF tests, inferior and nasal RNFL thickness were correlated with visual outcomes. Univariate analysis showed that younger age (p = 0.024), higher body weight (p = 0.033), better initial BCVA (p < 0.001), better VF tests (p = 0.007), no hypertension (p = 0.044) and no diabetes (p = 0.009) were significant associated with a good visual outcomes. Multivariate analysis confirmed baseline BCVA as the only significant prognostic factor. Better BCVA at baseline are associated with favorable visual outcomes in EON patients. VF parameters, age, hypertension, diabetes, and body weight could also be considered possible predictors. Although RNFL thickness has significant correlation with visual outcomes, it does not appear to be a reliable factor to predict visual recovery.
Similar content being viewed by others
Background
Ethambutol, an oral chemotherapeutic agent, was first introduced in 1961 and has since been used as the primary treatment for tuberculosis, along with isoniazid, rifampin, and pyrazinamide. It works by inhibiting the biosynthesis of the mycobacterial cell wall, leading to the death of the tuberculosis bacteria1. Ethambutol plays a vital role in treating tuberculosis, including in strains resistant to other antitubercular drugs. However, 1–5% of patients who receive the appropriate dose experience drug-induced toxicity, which affects the optic nerve within days to months of starting the medication, leading to symptoms such as vision impairment, visual field constriction, and color vision deficits2,3.
Ethambutol should be discontinued immediately if a patient develops visual impairment. Even after drug discontinuation, vision impairments, and field defects persist or worsen in two-thirds or more of cases. Therefore, early detection of ethambutol toxicity is critical for preventing permanent visual loss4,5,6. Many studies have investigated the structural changes and indicated that the thickness of the retinal nerve fiber layer (RNFL) on optical coherence tomography (OCT) is effective for the early detection of toxic optic neuropathy7,8.
However, comprehensive studies on the correlation between RNFL thickness, visual field (VF) test results, visual recovery, and prognostic factors that influence visual recovery are lacking. Therefore, this study aimed to evaluate the clinical efficacy of VF tests and RNFL thickness in predicting visual recovery in patients who developed optic neuropathy following ethambutol use.
Materials and methods
We retrospectively analyzed the medical records of 23 patients diagnosed with ethambutol-related toxic optic neuropathy at Yangsan Pusan National University Hospital between January 2020 and December 2022. The study adhered to the principles outlined in the Declaration of Helsinki and was approved by the Institutional Review Board of Pusan National University Yangsan Hospital (IRB number: 55–2024-050). Because this was a retrospective study, the requirement for informed consent was waived by the Institutional Review Board of Pusan National University Yangsan Hospital.
Patients were eligible for inclusion if they had been diagnosed with ethambutol-induced toxic optic neuropathy and had documented follow-up for more than 6 months. Only patients who had undergone assessments of visual acuity, dilated fundus examination, automated perimetry using a VF analyzer (Humphrey Field Analyzer; Carl Zeiss-Meditec, Dublin, CA, USA), and Cirrus HD OCT® (Carl Zeiss-Meditec, Oberkochen, Germany) of the peripapillary RNFL and macular ganglion cell-inner plexiform layer (GC-IPL) were included in the analysis. The visual field (VF) tests were conducted using the 30 − 2 testing protocol, which evaluates a broad central visual field including areas most likely affected in ethambutol-related toxic optic neuropathy. Only reliable data from the VF test results, which included false-positive error rates of less than 20%, false-negative error rates of less than 20%, and fixation loss rates of less than 20%, were considered for the analysis.
The exclusion criteria for this analysis were as follows: presence of any ophthalmological abnormalities other than optic nerve disease, including glaucoma; any history of neurologic diseases leading to visual field defects; and specific conditions such as meningitis or central nervous system (CNS) tuberculosis. We analyzed the average peripapillary RNFL thickness and the thickness in each quadrant (superior, inferior, temporal, and nasal). The recovery group was defined as visual recovery 2 lines or more or final visual acuity 0.4 or more. The patients were divided into two groups based on their recovery states. Six months after the diagnosis, we evaluated the best-corrected visual acuity (BCVA), VF, RNFL thickness, and GC-IPL thickness of each patient.
Statistical analyses were performed using SPSS for Windows version 23.0 (SPSS Inc Corp., Armonk, NY). Descriptive statistics, including age, sex, visual acuity (logarithm of the minimum angle of resolution [logMAR]), duration from neurological symptom onset to surgery, pattern of VF defect, and presence of a central VF defect were calculated for all patients. Patient characteristics were compared using t-tests or chi-square tests. Pearson’s correlation coefficient was used to analyze the correlation between VF test results and RNFL thickness. Univariate and multivariate analyses were conducted for each variable to investigate factors associated with VF recovery. Statistical significance was defined as p < 0.05.
Results
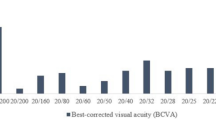
The study included 23 patients (46 eyes). Of these patients, 14 were male (28 eyes) and 9 were female (18 eyes). The mean age of the patients was 64.0 ± 18.0 years, and their body weight was 53.4 ± 7.9 kg. The BCVA at baseline was 0.78 ± 0.71 log MAR. The daily dose of ethambutol was 18.2 ± 4.0 mg, administered over 4.6 ± 3.0 months. The comorbidities were hypertension in nine patients, diabetes mellitus (DM) in six patients, dyslipidemia in two patients, and chronic kidney disease (CKD) in three patients (Table 1). All 23 patients in this study were treated for pulmonary tuberculosis. No cases of extrapulmonary tuberculosis, such as meningitis or CNS tuberculosis, were included.
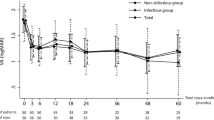
Of the 23 patients, 12 were assigned to the recovery group and 11 to the non-recovery group. In the recovery group, there was a statistically significant increase in body weight and a better initial BCVA (p = 0.038 and p < 0.001, respectively). The daily dose, usage duration, and total dose of ethambutol did not differ between the two groups. The mean deviation (MD), pattern standard deviation (PSD), and visual field index (VFI) in the VF revealed significantly greater impairments in the non-recovery group (all p < 0.001). However, RNFL thickness did not differ between the two groups. Significantly more patients had DM in the non-recovery group (p = 0.007) (Table 2).
Pearson correlation analysis was used to examine the relationship between BCVA, VF tests, and RNFL thickness at baseline and BCVA at 1, 3, and 6 months after discontinuation of ethambutol. The initial BCVA had a strong positive correlation with BCVA at 1, 3, and 6 months, with correlation coefficients of 0.879, 0.886, and 0.711, respectively (all p < 0.001). The PSD showed positive correlations of 0.422 at one month and 0.365 at three months, with each being statistically significant (p = 0.016 and p = 0.047, respectively). The VFI exhibited negative correlations of −0.539 at one month and − 0.556 at three months(p = 0.001 and p = 0.002, respectively). RNFL thickness showed a statistically significant negative correlation in the inferior quadrant, with a correlation coefficient of −0.536 (p = 0.006) one month after discontinuation of ethambutol. Additionally, in the nasal quadrant, the correlation coefficients were − 0.451 at one month and − 0.555 at three months, both showing statistically significant negative correlations (p = 0.024 and p = 0.011, respectively) (Table 3).
The univariate logistic regression analysis indicated associations between poor vision recovery and several factors: old age (Odds Ratio [OR], 1.05; p = 0.024), low body weight (OR, 0.913; p = 0.033), good visual acuity (OR, 135.3; p < 0.001), low MD (OR, 0.882; p = 0.007), high PSD (OR, 1.419; p = 0.008), low VFI (OR, 0.93; p = 0.006), as well as hypertension (OR, 3.6; p = 0.044) and diabetes (OR, 9.167; p = 0.009). However, multivariate analysis revealed that baseline BCVA was the only significant factor affecting visual recovery (Table 4).
Discussion
The exact mechanism of ethambutol-induced optic neuropathy (EON) is not clearly defined; however, ethambutol toxicity inhibits mitochondrial metabolism, affecting ganglion cells that require high mitochondrial respiratory activity9,10. Ethambutol toxicity is traditionally described as reversible upon discontinuation of the drug, and vision is believed to gradually recover over weeks to months. However, recovery is often incomplete, even in patients who report visual impairment after the cessation of ethambutol treatment3,11.
Some studies have investigated the prognostic factors for visual recovery in patients with EON. Older age has been associated with a higher risk of EON occurrence and poorer visual recovery2,12. In our study, older patients exhibited worse visual recovery rates in univariate analysis, suggesting that age-related vulnerability of the optic nerve might contribute to less favorable outcomes.
According to Kyncl et al.13, the dosage of ethambutol significantly affects the visual prognosis in patients with optic neuropathy. Their study highlighted that higher dose of ethambutol were associated with an increased risk of developing optic neuropathy and poorer visual recovery outcomes. On the other hand, Sadun and Wang demonstrated that even at recommended doses, ethambutol can cause optic neuropathy without a clear dose-response relationship14. In this study, daily dosage did not show a significant correlation with visual recovery, likely because most patients in our study were prescribed the same dosage. This implies that factors other than dosage, such as individual susceptibility and concurrent medical conditions, may play a substantial role in the development of EON and its prognosis.
Body weight is a potential prognostic factor. Some studies have indicated that ethambutol toxicity is more pronounced in individuals with lower body weight due to higher drug concentration relative to body mass2. In this study, patients with lower body weight showed poorer visual recovery.
Studies by Menon et al.5 and Jin et al.15. have highlighted that these comorbidities can adversely affect the visual prognosis in optic neuropathy. Hypertension and diabetes can lead to microvascular damage, exacerbating the effects of EON on the optic nerve and hindering recovery. In this study, hypertension and diabetes were more prevalent in the non-recovery group, supporting their role as risk factors for poor outcomes.
Our findings also emphasize the importance of initial BCVA and VF tests as significant predictors of visual recovery. Previous studies have consistently demonstrated that initial visual acuity is a critical factor in determining the prognosis of optic neuropathies. Menon et al.5 indicated that a better initial visual acuity is strongly associated with favorable visual outcomes in patients with optic neuropathy. Additionally, Chen et al.2 and Sivakumaran et al.3 emphasized the importance of VF assessments, highlighting that VF deterioration is associated with poorer visual recovery. Our study aligns with these findings, showing a strong correlation between initial BCVA and visual recovery, and between the initial VF and visual recovery, thus highlighting their importance as prognostic indicators. Furthermore, multivariate analysis confirmed that the initial BCVA was the most significant factor affecting visual recovery, underscoring its role as the most reliable predictor. Initial VF tests also provide crucial information that complements visual acuity in predicting long-term visual outcomes.
Ethambutol-induced toxic optic neuropathy results from mitochondrial dysfunction leading to defects in the papillomacular bundle. Therefore, temporal RNFL thickness is significantly diminished compared to other areas of the optic disc nerve fiber layer16. Ambika et al.17 reported that a reduction in temporal RNFL thickness strongly correlates with poor visual recovery. Lee et al.18. reported that a reduction in the temporal GC-IPL thickness was negatively correlated with the degree of vision recovery. However, we found no significant correlation between temporal RNFL thickness and visual recovery. Nevertheless, the inferior and nasal RNFL thicknesses showed a significant correlation with recovery. In a previous longitudinal analysis, initial RNFL swelling occurred within three months after symptom onset and then decreased overtime18,19,20. These observations suggest changes in RNFL thickness over the disease course, wherein swelling and thinning occur as part of the natural progression of optic neuropathy. We believe that these results occurred because patients in the recovery group were tested at a stage where the temporal RNFL was already thin, whereas those in the non-recovery group were examined during a phase when their temporal RNFL was still thick. These studies suggest that temporal RNFL thinning is not always the most reliable indicator at all stages of optic neuropathy as its profile varies across different clinical stages of the disease.
Our regression analysis showed that the RNFL thickness was not significantly associated with visual recovery. This finding can be attributed to the variability in RNFL thickness at different stages of the disease. Longitudinal changes in the initial swelling following thinning could lead to inconsistent results when using RNFL thickness as a predictive measure.
This study has certain limitations. First, its retrospective design inherently limits control over data collection and introduces potential bias. Second, the small sample size may constrain the interpretation of results. Third, this study did not include macular ganglion cell layer thickness analysis due to limitations in available data. Fourth, while the 30 − 2 visual field test was used to assess visual field changes in this study, it has limitations in detecting a central or cecocentral scotoma. Although the 30 − 2 test provided clinically meaningful data, future studies should consider incorporating 10 − 2 testing for a more comprehensive evaluation of central visual field defects. Additionally, data about the accessibility of healthcare, general health and nutritional status which may have influence the visual outcomes were not included in this study. These contextual differences make direct comparisons with other populations or countries challenging. Furthermore, there is a possibility that cases of toxic optic neuropathy in patients who were not referred from internal medicine to ophthalmology were missed, which may have affected the overall findings. Future studies with prospective designs, larger cohorts, and ganglion cell layer thickness analysis are needed to strengthen and expand upon these findings.
In conclusion, the BCVA at baseline is the only significant prognostic factor for visual recovery in patients with EON. Additionally, VF results, age, hypertension, diabetes, and body weight could be considered possible factors in predicting visual outcomes. Although RNFL thickness has significant correlation with visual outcomes, it might not appear to be a reliable factor to predict visual recovery. Therefore, caution should be exercised when using OCT findings in isolation to predict visual prognosis.
Data availability
The datasets generated and analyzed in the current study are available from the corresponding author on reasonable request.
References
Carr, W. et al. Interim Guidance: 4-Month Rifapentine-Moxifloxacin Regimen for the treatment of drug-susceptible pulmonary tuberculosis - United States 2022. MMWR Morb Mortal. Wkly. Rep. 71, 285–289 (2022).
Chen, S. C., Lin, M. C. & Sheu, S. J. Incidence and prognostic factor of ethambutol-related optic neuropathy: 10-year experience in southern Taiwan. Kaohsiung J. Med. Sci. 31, 358–362 (2015).
Sivakumaran, P., Harrison, A. C., Marschner, J. & Martin, P. Ocular toxicity from ethambutol: a review of four cases and recommended precautions. N Z. Med. J. 111, 428–430 (1998).
Lee, E. J. et al. Incidence and clinical features of ethambutol-induced optic neuropathy in Korea. J. Neuroophthalmol. 28, 269–277 (2008).
Menon, V., Jain, D., Saxena, R. & Sood, R. Prospective evaluation of visual function for early detection of ethambutol toxicity. Br. J. Ophthalmol. 93, 1251–1255 (2009).
Kim, K. L. & Park, S. P. Visual function test for early detection of ethambutol induced ocular toxicity at the subclinical level. CutanOculToxicol 35, 228–232 (2016).
Chai, S. J. & Foroozan, R. Decreased retinal nerve fiber layer thickness detected by optical coherence tomography in patients with ethambutol-induced optic neuropathy. Br. J. Ophthalmol. 91, 895–897 (2007).
Pavan Taffner, B. M., Mattos, F. B., Cunha, M. C. & Saraiva, F. P. The use of optical coherence tomography for the detection of ocular toxicity by ethambutol. PLoS ONE. 13, e0204655 (2018).
Chamberlain, P. D., Sadaka, A., Berry, S. & Lee, A. G. Ethambutol Optic Neuropathy CurrOpinOphthalmol 28, 545–551 (2017).
Pan, B. X. et al. Mathematically modeling the involvement of axons in Leber’s hereditary optic neuropathy. Invest. Ophthalmol. Vis. Sci. 53, 7608–7617 (2012).
Tsai, R. K. & Lee, Y. H. Reversibility of ethambutol optic neuropathy. J. OculPharmacolTher. 13, 473–477 (1997).
Chan, R. & Kwok, A. Ocular toxicity of ethambutol. Hong Kong Med. J. 12, 56–60 (2006).
Kyncl, M., Fus, M. & Lestak, J. Ethambutol-induced optic neuropathy. Biomed. Pap Med. Fac. Univ. Palacky Olomouc Czech Repub. 167, 288–293 (2023).
Sadun, A. A. & Wang, M. Y. Ethambutol optic neuropathy: how we can prevent 100,000 new cases of blindness each year. J. Neuroophthalmol. 28, 265–268 (2008).
Jin, K. W., Lee, J. Y., Rhiu, S. & Choi, D. G. Longitudinal evaluation of visual function and structure for detection of subclinical ethambutol-induced optic neuropathy. PLoS ONE. 14, e0215297 (2019).
Wang, M. Y. & Sadun, A. A. Drug-related mitochondrial optic neuropathies. J. Neuroophthalmol. 33, 172–178 (2013).
Ambika, S., Lakshmi, K. P., Gopal, M. & Noronha, O. V. Visual outcomes of toxic optic neuropathy secondary to Ethambutol: a retrospective observational study from India, an endemic country. Indian J. Ophthalmol. 70, 3388–3392 (2022).
Lee, J. Y., Choi, J. H., Park, K. A. & Oh, S. Y. Ganglion Cell Layer and Inner Plexiform Layer as predictors of Vision Recovery in Ethambutol-Induced Optic Neuropathy: a longitudinal OCT analysis. Invest. Ophthalmol. Vis. Sci. 59, 2104–2109 (2018).
Borrelli, E. et al. Changes in Choroidal Thickness follow the RNFL Changes in Leber’s Hereditary Optic Neuropathy. Sci. Rep. 6, 37332 (2016).
Lee, W. J., Hong, E. H., Park, H. M. & Lim, H. W. Traumatic optic neuropathy-associated progressive thinning of the retinal nerve fiber layer and ganglion cell complex: two case reports. BMC Ophthalmol. 19, 216 (2019).
Acknowledgements
This manuscript has not been published or presented elsewhere in part or its entirety and is not under consideration by another journal.
Funding
This study was supported by Research institute for Convergence of biomedical science and technology, Pusan National University Yangsan Hospital (30-2024-004).
Author information
Authors and Affiliations
Contributions
K.S.J and C.H.Y. were responsible for the study design and acquisition of clinical information. H.K.E. and K.S.J. prepared the manuscript. H.K.E. and Y.H.J. were responsible for data analysis. L.J.E. and C.H.Y. reviewed the manuscript. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, K.E., Yoon, H., Choi, H. et al. Factors affecting visual outcome in patients with toxic optic neuropathy caused by ethambutol. Sci Rep 15, 6257 (2025). https://doi.org/10.1038/s41598-025-90663-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-90663-3