Abstract
Long-lasting insecticidal nets and indoor residual sprays have played a major role in significantly reducing the burden of malaria. However, the management of mosquitoes resistant to current insecticides continues to be challenging. A promising new strategy is drug-based vector control where drugs are administered to vector hosts (human/animal), which renders the host blood toxic to mosquitoes—thereby reducing mosquito lifespan, fecundity and onward transmission of pathogens. Afoxolaner, fluralaner, and lotilaner are isoxazoline derivatives widely used as systemic insecticides for ectoparasite control in companion animals. Here, the mosquitocidal activity of these compounds against Anopheles gambiae s.s, Anopheles coluzzii, Aedes aegypti, and Anopheles funestus mosquitoes was evaluated. The effect of antibiotic treatment and different carbohydrates in artificial nectar meals on isoxazoline activity was also investigated. All isoxazolines tested showed rapid killing with little difference in susceptibility between different species and genera of mosquitoes. Fluralaner showed the most potent mosquitocidal effects with IC50 values ranging from 44 to 87 nM when mortality was assessed at 24 h post-feeding. Addition of the antibiotic cocktail or variations in sugar diet did not affect isoxazoline potency. In conclusion, data indicate potent and rapid mosquitocidal effects of isoxazolines that are likely unaffected by mosquito sugar feeding behaviour and microbiome dynamics.
Similar content being viewed by others
Introduction
Vector control is vital to control and potentially eliminate vector-borne diseases. In most African malaria-endemic countries, vector control for malaria relies on the use of long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS)1. These tools contributed to the estimated 586 million malaria cases that were averted in sub-Saharan Africa between 2000 and 20152. However, since 2015, this progress has stalled. Two of the major challenges that limit the impact of malaria vector control are widespread insecticide resistance across Anopheles populations, and a shift from indoor to outdoor vector resting and feeding behavior3.
Novel tools or approaches that are effective against pyrethroid-resistant or outdoor feeding/resting mosquitoes are urgently needed to supplement those currently available and are thought to be necessary to achieve elimination in most endemic areas4. Endectocides and ectocides are systemic insecticides/drugs administered to humans or animals that kill mosquitoes when ingested during blood feeding5. The mechanism of action of these drugs is distinct from that of commonly used topic insecticides such as organophosphates, organochlorides, carbamates, pyrroles (9), and they are effective against mosquitoes regardless of their susceptibility to insecticides or biting time or location. Recent data showed that ivermectin, an old drug used in onchocerciasis eradication campaigns for several years, is one of the most promising systemic insecticides. The mosquitocidal effect of ivermectin and its metabolites has been reported by several studies6. However, ivermectin has a short half-life of under 4 days in large mammals and 12–36 h in humans7,8 necessitating the use of multiple doses, a limitation that poses challenges in terms of logistics and drug compliance. A research approach to come out the short half-life the ivermectin was the insert long-lasting implants releasing mosquitocidal concentrations of ivermectin for 6 months have been developed and tested in rabbits and cattle9,10,11. Results are prominent up to 6 months after drug administration. However, the field application could be faced to numerous challenges including cost effective and real use in human.
Isoxazolines like afoxoloner, lotilaner, and fluralaner are ectoparasiticides currently used against fleas, ticks, and dust mites on dogs and cats. Repurposing these compounds for human ectoparasite control is currently being explored (clinicaltrials.gov NCT05387083)12. These drugs work as inhibitors of gamma-aminobutyric acid (GABA) and glutamate-gated chloride channels in the nervous system of invertebrates causing over excitation of the insect and arachnid nervous system10,11. This results in uncontrolled neural activity and finally death of insects and acarines13. Following the administration of a fluralaner chew to dogs, efficacy has been maintained against fleas in both field and laboratory studies for 12 weeks14. Isoxazolines show activity against antropophilic mosquitoes including Aedes mosquitoes that are not susceptible to ivermectin at doses that are safe to use in humans. Their activity is not affected by the resistance-to-dieldrin (rdl) mutations in the GABA receptor or by mutations in the kdr sodium channel or acetylcholine esterate genes that confer resistance to pyrethroids and carbamates, respectively12. Less is known about their metabolic fate in target mosquitoes. There is increasing evidence that the mosquito microbiome plays a role in detoxification and insecticide breakdown15,16. In addition, carbohydrates in nectar meals lead to a proliferation of the midgut microbiome17. The present study aims at assessing the mosquitocidal activity of S-fluralaner, S-afoxolaner, and S-lotilaner on Anopheles gambiae sensu stricto (s.s) Giles, 1900, Anopheles coluzzii. Coetzee, Della Torre, Sagnon, Schoute, and Costantini, 2013, Anopheles funestus Giles, 1900 and Aedes aegypti Linnaeus, 1762 mosquitoes reared under West-African conditions. Additionally, we assessed the impact of sugar feeding and antibiotic treatment on insecticidal effect through membrane feeding assays.
Results
Mosquitocidal effects of isoxazolines
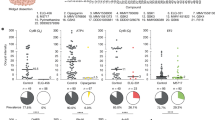
Fluralaner, afoxolaner and lotilaner demonstrated insecticidal activities against all tested strains of mosquitoes (Fig. 1; Table 1). When mosquito mortality was assessed at 24 h post-feeding, fluralaner exhibited the most potent activity with IC50 values ranging from 87 nM (95% CI: 68 − 10) against Anopheles funestus to 44.02 nM (33.09–54.95) against Anopheles gambiae s.s. The IC50 values were slightly lower at 72 h post-feeding and from 39.46 nM (20.98–57.94.56) to 22.66 nM (14.45–30.87) depending on the mosquito strain. At 24 h post-feeding, afoxolaner and lotilaner were less potent in comparison to fluralaner and showed IC50 values ranging 135.0 to 256.2 nM and 252.7 to 345.9 nM, respectively. For these compounds, IC50 values also dropped slightly when evaluated at later time points after feeding. This was most notable for afoxolaner where the IC50 values at 72 h after feeding were around 2.5 to 9 times less than at 24 h after feeding. This effect of incubation time on mosquitocidal potency was not as prominent for lotilaner. 100% mortality was reached at the highest dose 1 µM of fluralaner against all the 4 tested mosquito strains after 24 h of blood feeding. The mortality, after 24 h exposure with afoxolaner/lotilaner 1 µM, was more than 94% on all the 4 tested mosquito strains. The mortality was increased to 100% after 48 h of exposure (Figs. 1 and 2).
Insecticidal activity of fluralaner, afoxolaner and lotilaner against disease vectors. Survival (expressed as percentage of live engorged individuals) of Anopheles and Ae. aegypti mosquitoes at 24 h, 48 h and 72 h after feeding on compounds supplemented blood meals. Error bars indicate SDs based on at least five independent dose-response experiments in duplicate caps with approximately 20 mosquitoes each.
Effect of sugar diet on the mosquitocidal activity
Using afoxolaner and lotilaner as representative isoxazolines, we evaluated the impact of different sugar meals on their mosquitocidal activities. After taking a blood meal containing either afoxolaner or lotilaner, mosquitoes (An. coluzzi, An. gambiae s.s. and Ae. aegypti) were allowed to feed on either 5% glucose solution, 5% fructose solution, water, or not provided with anything. The mosquitocidal activity measured at 24 h, 48 h and 72 h post-feeding was not statistically significantly different when comparing between the sugar-feeding regimens for any mosquito colony (Fig. 3). These data indicate that the type of sugar ingested in the 3 days following a blood meal does not impact afoxolaner or lotilaner activity.
Insecticidal activity of afoxolaner and lotilaner under different sugar feeding regimes. The figures show IC50 values against mosquitoes indicated on the x-axis mosquitoes at 24 h, 48 h and 72 h after feeding on compound- supplemented blood meals. Error bars data indicate 95% confidence intervals from experiments with duplicate or triplicate cups containing approximately 20 mosquitoes each.
Effect of antibiotic treatment on mosquitocidal activity
To assess the effect of microbiome on the activity of afoxolaner, An. gambiae mosquitoes were treated with a combination of antibiotics (15 µg/ml gentamicin, 100 units/ml penicillin and 100 units/ml streptomycin) before, during, and after a blood meal that was supplemented with different concentrations of afoxolaner. No statistically significant difference was observed in mortality between the antibiotic-treated and untreated groups after 24 h, 48 h and 72 h post blood meal (Fig. 4). The IC50 values [95% CI] of afoxolaner in the antibiotic treated vs. untreated mosquitoes were not different at 24 h (treated = 0.109 µM [0.037–0.275], untreated = 0.095 µM [0.043–0.195]), 48 h (treated = 0.045 µM [0.020–0.098], untreated = 0.047 µM [0.015–0.136]), or 72 h (treated = 0.030 µM [0.012–0.071], untreated = 0.026 µM [0.006–0.091]).
Insecticidal activity of afoxolaner on Anopheles gambiae s.s with and without antibiotics treatment. Survival (expressed as percentage of live engorged individuals) of Anopheles mosquitoes at 24 h, 48 h and 72 h after feeding on compound-supplemented blood meals. Error bars data indicate SDs from at least three independent dose-response experiments conducted in duplicate or triplicate cups containing approximately 20 mosquitoes each.
Discussion
Isoxazolines fluralaner, afoxolaner and lotilaner were tested against An. coluzzii Goden and An. gambiae s.s. Kisumu, An. funestus and Ae. aegypti strains to assess their insecticidal proprieties by allowing mosquitoes to feed on blood supplemented with drugs at different concentrations through a synthetic membrane. An. gambiae, An. coluzzii and An. funestus are the major vectors involved in the transmission of malaria parasites to humans and reared in the laboratory and Ae. aegypti is the major vector for dengue transmission in Burkina Faso18,19,20,21. Malaria and dengue fever remain the major vector-borne-diseases in Burkina Faso and scientific community is working to find out novel strategies to enhance the fight against.
All isoxazolines showed rapid killing of the mosquito colonies tested. Furthermore, the speed of killing over the first 72 h was not affected by the drug concentration, as IC50 values did not vary considerably within this time window. Given the rapid speed of killing, isoxazolines may affect both mosquito fecundity as well as onward pathogen transmission. The most potent insecticidal activity was observed with fluralaner with IC50 values ranging from 23 to 87 nM depending on the mosquito species and timepoint after feeding. The IC50 values of fluralaner were around 15–50% of IC50 of afoxolaner and lotilaner. For the latter two compounds, we tested the S-enantiomers whereas fluralaner was tested as a racemic mixture. The potency of fluralaner may further improve with a stereopure compound. The data reported here are in line with previous work from our labs that compared mosquitocidal properties of afoxolaner and fluralaner against various laboratory strains of Anopheles, Ae. aegypti, and Culex pipiens22 reared in European insectaries. This indicates that isoxazoline potency was likely unaffected by mosquito rearing location and associated differences in microbiome.
Microbiome composition could vary substantially between individuals, species, life stages, and locations of mosquito collection and rearing23,24. Diversity in microbiomes contribute to variation in pupation rate and other life-history traits as well as antimicrobial responses25. Mosquito survival may be affected by the influence of the microbiome on adult nutrition, fitness, homeostasis, metabolism, interaction with other microbes or pathogens and insecticide resistance26. Recent studies revealed a key role of the Anopheles mosquitoes gut microbiome on insecticide resistance. Dada et al.., revealed associations between the microbiota and phenotypic resistance to the insecticide fenitrothion27. Also, Bernard et al., demonstrated that the gut bacterial populations of Anopheles arabiensis s.s. are a crucial determinant of their life histories28. Gut-associated microbiota may indirectly affect mosquito lifespan by facilitating sugar digestion. Bacteria in the gut use fructose, the major sugar component of nectar, as an energy source to produce other nutrients for the mosquito host29. The produced nutrients could increase or decrease mosquito susceptibility to insecticides. Moreover, floral nectar and extra-floral plant fluids contain sucrose and other sugars, including glucose, mannose, galactose, and fructose, which are used by female mosquitoes. These carbohydrates impact mosquito fecundity and survival30,31.
In our studies, a cocktail of the broad-spectrum bactericidal antibiotic gentamycin, the gram-negative narrow-spectrum antibiotic streptomycin and the broad-spectrum antifungal penicillin has been used to clear a large spectra endogenous bacteria present in mosquitoes18,32. We did not observe an effect on antibiotic treatment on isoxazoline potency, suggesting that changes in microbiome composition have no effect on isoxazoline metabolism, pharmacokinetics or pharmacodynamics. Though a limitation of our approach is that we did not quantify changes in microbiome composition in the treated mosquitoes, so we cannot confirm the affect of the antibiotics on the microbiome. However, we used an antibiotic cocktail and dosages that have been shown to effectively change microbiome dynamics in mosquitoes previously33. In addition, we did not observe an effect of different sugar diets on isoxazoline potency and speed of killing, though there may have been an effect if the mosquitoes were supplied with the different sugar diets for a longer period before the experiments.
Lotilaner is currently in clinical development for prevention of Lyme disease (clinicaltrials.gov #NCT05387083). These studies will provide important insight into the safety, tolerability, pharmacokinetics and pharmacodynamics of lotilaner. These learnings may stimulate further development of lotilaner for drug-based vector control. The data presented here indicate that isoxazolines show the potent and rapid killing of vectors relevant for malaria and dengue transmission, and that this action is independent of mosquito habitat and feeding behavior.
Methods
Mosquito colonies
The insecticide susceptible reference sensu stricto (s.s.) strain Kisumu, the local Anopheles coluzzii strain Goden, and Anopheles funestus s.s. established since 2004 at Centre de recherche et de formation sur le paludisme (CNRFP) insectary in Burkina Faso, were used. Aedes aegypti strain Bora Bora maintained in the insectary since 2022 at CNRFP, was also used. All strains were maintained in insectary rearing conditions of 27 ± 2 °C temperature, 70–80% humidity with 12:12 h light and dark cycle34. Mosquitoes were maintained with a 5% sugar solution.
Preparation of drugs for membrane feeding assays
Powders of S-afoxolaner, S-lotilaner and racemic fluralaner were initially dissolved in DMSO to prepare stock concentrations of 10 mM, and aliquots frozen at − 20 °C. On the day of mosquito feeding, a frozen aliquot was thawed and serially diluted in DMSO and subsequently in DMEM to the required concentrations. Anticoagulated blood collected from a human volunteer in a Lithium heparin vacutainer was mixed with each drug to achieve a range of concentrations (1000 nM, 100 nM, 10 nM and 1 nM) and a final DMSO concentration of 0.1%. Vehicle control DMSO was used in all experiments at the concentration of 0.1%.
Assessing mosquitocidal activity in membrane feeding assays
Three to five day old female mosquitoes were starved overnight prior to blood feeding. Approximately 20 mosquitoes were placed in gauze-sealed feeding cups. Feeding cups were prepared in duplicate per drug concentration and control. Drug-spiked blood (600 µl) was delivered in a glass feeders attached to a circulating water bath (37 °C), and mosquitoes were allowed to feed on the blood through para-film membranes for 15 min or until engorged as described previously for standard membrane feeding assays35. Afterwards, unfed mosquitoes were removed and blood-fed mosquitoes were maintained under standard insectary rearing conditions with 5% sugar solution, while daily mortality was recorded for up to 3 days post treatment. At least three experiments in duplicate feeding cups were performed with the test compounds against each investigated mosquito strain.
Effect of sugar diet on mosquitocidal activity
The impact of sugar on drug activity was assessed in An. coluzzi, An. gambiae s.s and Ae. aegypti. Each mosquito species received afoxolaner and lotilaner at a range of concentrations (1000 nM, 100 nM, 10 nM and 1 nM). Drugs were fed to cups of ~ 20, 3–5 day old female mosquitoes in duplicate, in a 600uL blood meal as described above. After feeding each condition was then provided with a different sugar feeding diet (5% glucose solution, 5% fructose solution, water, or nothing).All groups of mosquitoes were maintained under the standard rearing conditions and the number and percentage of dead mosquitoes was recorded every day until the day 3 post feeding.
Effect of antibiotic treatment on mosquitocidal activity
To assess the impact of the mosquito microbiome on drug activity, 3–4 day old female An. gambiae mosquitoes were divided into two groups; either treated with antibiotics or untreated. The treated group was provided with 5% glucose solution supplemented with 15 µg/ml gentamicin and 100 units/ml penicillin and 100 units/ml streptomycin combination 3 days before the blood meal to clear the endogenous bacteria present in mosquitoes36. Mosquitoes were then fed with blood supplemented with Afoxolaner at different concentrations (1000 nM, 100 nM, 10 nM and 1 nM) that included the same antibiotic cocktail and after blood feeding were provided with 5% glucose solution supplemented with the antibiotic cocktail, as above. The untreated group received the same drug-spiked blood meals, but they did not receive the antibiotic cocktail at any time. Each condition was assessed in duplicate with 20 mosquitoes per cup. Unfed mosquitoes were removed from the cups and mortality was monitored for 3 days.
Data Analysis for Viability Assays The Inhibitory Concentration 50% (IC50) was used to determine which molecule is the most potent and to look at delayed effects at 72 h. IC50 values were calculated by applying a four-parameter logistic regression model using a least squares method to find the best fit using the GraphPad Prism 5.0 and CDD Vault software packages. P-value < 0.05 was considered as statistically significant.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Benelli, G. & Beier, J. C. Current vector control challenges in the fight against malaria. Acta Trop. 174, 91–96 (2017).
Bhatt, S. et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211 (2015).
Sanou, A. et al. Insecticide resistance and behavioural adaptation as a response to long-lasting insecticidal net deployment in malaria vectors in the cascades region of Burkina Faso. Sci. Rep. 11, 17569 (2021).
Griffin, J. T. et al. Reducing Plasmodium falciparum malaria transmission in Africa: A model-based evaluation of intervention strategies. PLoS Med. 7(8), e1000324. https://doi.org/10.1371/journal.pmed.1000324 (2010).
Selvaraj, P., Suresh, J., Wenger, E. A., Bever, C. A. & Gerardin, J. Reducing malaria burden and accelerating elimination with long-lasting systemic insecticides: a modelling study of three potential use cases. Malar. J. 18, 307 (2019).
Kobylinski, K. C. et al. Ivermectin metabolites reduce Anopheles survival. Sci. Rep. 13, 1–8 (2023).
Chaccour, C., Lines, J. & Whitty, C. J. M. Effect of ivermectin on Anopheles gambiae mosquitoes fed on humans: the potential of oral insecticides in malaria control. J. Infect. Dis. 202(1), 113–116. https://doi.org/10.1086/653208 (2010).
Crump, A. & Omura, S. Ivermectin ‘Wonder drug’ from Japan: The human use perspective. Proc. Jpn. Acad. Ser. B: Phys. Bio. Sci. Series B 87(2), 13–28. https://doi.org/10.2183/pjab.87.13 (2011).
Pooda, S. H. et al. Proof-of-concept study for a long-acting formulation of ivermectin injected in cattle as a complementary malaria vector control tool. Parasit. Vect. 16, 1–12. https://doi.org/10.1186/s13071-022-05621-z (2023).
Chaccour, C. et al. Screening for an ivermectin slow-release formulation suitable for malaria vector control. Malar. J. 14, 1–9. https://doi.org/10.1186/s12936-015-0618-2 (2015).
Chaccour, C. J. et al. Targeting cattle for malaria elimination: Marked reduction of Anopheles arabiensis survival for over six months using a slow- release ivermectin implant formulation. Parasit. vect. 11, 1-9 (2018).
Miglianico, M. et al. Repurposing isoxazoline veterinary drugs for control of vector-borne human diseases. Proc. Natl. Acad. Sci. U S A. 115, E6920–E6926 (2018).
Jiang, S., Tsikolia, M., Bernier, U. R. & Bloomquist, J. R. Mosquitocidal activity and mode of action of the isoxazoline fluralaner. Int. J. Environ. Res. Public. Health https://doi.org/10.3390/ijerph14020154 (2017).
Rohdich, N., Roepke, R. K. & Zschiesche, E. A randomized, blinded, controlled and multi-centered field study comparing the efficacy and safety of Bravecto™ (fluralaner) against Frontline™ (fipronil) in flea- and tick-infested dogs. Parasit. Vect. 7, 1–5. https://doi.org/10.1186/1756-3305-7-83 (2014).
Pelloquin, B. et al. Overabundance of Asaia and Serratia bacteria is Associated with Deltamethrin Insecticide susceptibility in Anopheles coluzzii from Agboville, Côte d’Ivoire. Microbiol. Spectr. 9, 1–16 (2021).
Soltani, A., Vatandoost, H., Oshaghi, M. A., Enayati, A. A. & Chavshin, A. R. The role of midgut symbiotic bacteria in resistance of Anopheles Stephensi (Diptera: Culicidae) to organophosphate insecticides. Pathog Glob Health. 111, 289–296 (2017).
Wang, M. et al. HHS Public. Access. 35, (2021).
Kaboré, D. P. A. et al. Mosquito (Diptera: Culicidae) populations in contrasting areas of the western regions of Burkina Faso: Species diversity, abundance and their implications for pathogen transmission. Parasit. Vect. 16, 1–11. https://doi.org/10.1186/s13071-023-06050-2 (2023).
Niang, A. et al. Entomological baseline data collection and power analyses in preparation of a mosquito swarm−killing intervention in south–western Burkina Faso. Malar. J. 20, 1–12. https://doi.org/10.1186/s12936-021-03877-x (2021).
Dabiré, R. K. et al. Population dynamics of Anopheles gambiae s. l. in Bobo-Dioulasso city: Bionomics, infection rate and susceptibility to insecticides. Parasit. Vect. 5, 1–9. https://doi.org/10.1186/1756-3305-5-127 (2012).
Soma, D. D. et al. Insecticide resistance status of malaria vectors Anopheles gambiae (s. l.) of southwest Burkina Faso and residual efficacy of indoor residual spraying with microencapsulated pirimiphos-methyl insecticide. Parasit. Vect. 14, 1–9. https://doi.org/10.1186/s13071-020-04563-8 (2021).
Miglianico, M. et al. Repurposing isoxazoline veterinary drugs for control of vector-borne human diseases. Proc. Natl. Acad. Sci. U S A. 115(29), E6920-E6926 (2018).
Novakova E, Woodhams DC, Rodríguez-Ruano SM, Brucker RM, Leff JW, Maharaj A, Amir A, Knight R and Scott J. Mosquito Microbiome Dynamics, a Background for Prevalence and Seasonality of West Nile Virus. Front. Microbiol. 8, 526. https://doi.org/10.3389/fmicb.2017.00526 (2017)
Muturi, E. J. et al. Mosquito microbiota cluster by host sampling location. Parasit. vect. 11, 1-12 (2018).
Dickson, L. B. et al. Carryover effects of larval exposure to different environmental bacteria drive adult trait variation in a mosquito vector. Sci. Adv. 3(8), e1700585 (2017).
Cansado-utrilla, C., Zhao, S. Y., Mccall, P. J., Coon, K. L. & Hughes, G. L. The microbiome and mosquito vectorial capacity: Rich potential for discovery and translation. Microbiome 9(1), 1–11 (2021).
Dada, N., Sheth, M., Liebman, K., Pinto, J. & Lenhart, A. Whole metagenome sequencing reveals links between mosquito microbiota and insecticide resistance in malaria vectors. Sci. Rep. 8, 1–13 (2018).
Barnard, K., Jeanrenaud, A. C. S. N., Brooke, B. D. & Oliver, S. V. The contribution of gut bacteria to insecticide resistance and the life histories of the major malaria vector Anopheles arabiensis (Diptera: Culicidae). Sci. Rep. 9, 1–11 (2019).
Guégan, M. et al. Who is eating fructose within the Aedes albopictus gut microbiota? Environ. Microbiol. 22(4), 1193–1206 (2020).
Manda, H. et al. Effect of discriminative plant-sugar feeding on the survival and fecundity of Anopheles gambiae. Malar. J. 6, 1–11 (2007).
Manda, H. et al. Discriminative feeding behaviour of Anopheles gambiae s.s. on endemic plants in western Kenya. Med. Vet. Entomol. 21, 103–111 (2007).
Mackey, A. J., Wang, Z. X., Toure, A. M. & Beier, J. C. Bactericidal effects of sugar-fed antibiotics on resident midgut bacteria of newly emerged Anopheline mosquitoes (Diptera: Culicidae). J. Med. Entomol. 37(2) 246–249 (2000).
Fofana, A. et al. Analyzing gut microbiota composition in individual Anopheles mosquitoes after experimental treatment. iScience 24, 103416 (2021).
Elisa, S., Elisa, P. S., Wirtz, R., Avery, M. & Benedict, M. Methods in Anopheles Research Second Edition. 1–8 (2010).
Ponnudurai, T. et al. Infectivity of cultured Plasmodium falciparum gametocytes to mosquitoes. Parasitology 2, 165–173. https://doi.org/10.1017/S0031182000062065 (1989).
Kalappa, D. M. et al. Influence of midgut microbiota in Anopheles stephensi on Plasmodium berghei infections. Malar. J. 17, 1–8 (2018).
Acknowledgements
The authors wish to thank Arnab Chatterjee (Scripps Institute) for providing compounds and Didier Leroy, Esperanza Herreros and Jeremy Burrows (Medicines for Malaria Venture) for stimulating discussions. Marnix Vlot is acknowledged for help with data visualisations. This work was cofunded by the Product Development Partnership fund of the Dutch Ministry of Foreign Affairs. TB and KAC were supported by a European Research Council (ERC) Consolidator Grant to TB (ERC-CoG 864180; QUANTUM).
Author information
Authors and Affiliations
Contributions
TB, KAC, GWM, JMB performed the study design. SH ON ZS, GWM performed data collection and laboratory analysis. SH, KAC and GWM supported data analysis and manuscript preparation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All experiments were performed in accordance with relevant guidelines and regulations. The protocol was approved by the CNRFP Institutional Ethics Committee (N°2019-06-000004) and the national Ethics Committee (N°2020-01-006). Before blood sampling, written informed consent was obtained from each volunteer and/or their legal guardian.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Soré, H., Guelbeogo, W.M., Zongo, S. et al. The mosquitocidal activity of isoxazoline derivatives afoxolaner, lotilaner, and fluralaner are not affected by mosquito sugar or antibiotic treatment. Sci Rep 15, 11830 (2025). https://doi.org/10.1038/s41598-025-90990-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-90990-5