Abstract
Doxorubicin (DOXO) is a powerful anthracycline chemotherapeutic drug, but its clinical usage has been limited by its deleterious effects on different organs, particularly hepatotoxicity. The aim of this study was to establish the combined effects of aerobic interval training (AIT) and curcumin supplementation on mitigating oxidative damage and endoplasmic reticulum (ER) stress-mediated apoptosis in a rat model of DOXO-induced hepatotoxicity. Fifty-six male Sprague–Dawley rats were randomly split into six groups: control (CON), vehicle, doxorubicin (Dox), doxorubicin + curcumin (Dox-C), doxorubicin + AIT (Dox-A), and doxorubicin + curcumin + AIT (Dox-AC). DOXO was intraperitoneally injected weekly (4 mg/kg/week) for five weeks. Curcumin supplementation (100 mg/kg/day) and AIT (4 min at 80–90% of VO2max intermitted by 3 min of active rest at 65–75% of VO2max) were conducted five times a week for six weeks. Finally, the hepatic tissue and blood samples were collected to assess histopathological changes, liver damage biomarkers, and the protein expression of oxidative stress, ER stress, and apoptosis markers. Tissue sections revealed that AIT and curcumin supplementation significantly improved hepatotoxicity induced by DOXO, as evidenced by the positive effects on histopathological alterations and serum markers of hepatic damage (P < 0.05). Both curcumin and AIT significantly reduced DOXO-triggered oxidative damage, ER stress, and apoptosis (P < 0.05), with the latter showing slightly higher effectiveness. Consequently, the combination of AIT with curcumin supplementation exhibits protective effects against chronic hepatotoxicity induced by DOXO, with AIT demonstrating relatively greater efficacy in increasing antioxidant capacity and reducing ER stress and apoptosis.
Similar content being viewed by others
Introduction
Doxorubicin (DOXO) is considered a powerful antineoplastic medicine frequently prescribed for many malignancies1. Although DOXO is a significantly efficacious chemotherapy drug, its clinical usage is hampered by its toxic impact on off-target cells2,3. DOXO could be the primary cause of hepatotoxicity in approximately 30% of breast cancer patients treated with this drug4. Following administration, DOXO is metabolized to doxorubicinol by the hepatic tissue, resulting in severe hepatic damage generally because of reactive oxygen species (ROS) formation5,6. Although several signaling pathways, such as mitochondrial dysfunction, redox imbalance, and cell death, have been proposed for DOXO-induced hepatotoxicity, the exact mechanisms involved in hepatotoxicity caused by DOXO are still lacking2.
The endoplasmic reticulum (ER) makes an essential contribution to calcium homeostasis, protein synthesis, and folding7. Several pathological conditions, including virus infection, the aggregation of damaged DNA, and the consumption of toxic drugs, may induce misfolded or unfolded proteins to aggregate in the ER lumen, initiating unfolded protein response (UPR)8. The ER membrane contains three sensor proteins that mediate ER stress: PERK, IRE1-ɑ, and ATF6. Under ER stress conditions, the abnormal accumulation of misfolded or unfolded proteins results in dissociating GRP78 from these proteins, leading to activating PERK/eIF2ɑ/ATF4/CHOP, IRE1-ɑ/JNK, and ATF6 signaling pathways and stimulating apoptosis mediated by these pathways9,10. Over the past years, DOXO has been documented to cause cytotoxicity by upregulating UPR markers and apoptosis induced by ER stress11,12,13. Moreover, one of the primary pathways behind DOXO-induced hepatotoxicity is oxidative damage, which triggers the production of misfolded proteins and initiates ER stress1,2,14. Accordingly, DOXO-induced oxidative stress may generate misfolded proteins, which activate caspase-dependent or non-dependent apoptosis caused by ER stress.
There is considerable evidence that endurance training has a promising potential for liver health, including its ameliorative effect on mitochondrial dysfunction15. Aerobic interval training (AIT), developed from athletic training programs, comprises high-intensity bouts, followed by active rest bouts16,17. AIT is not only time-efficient but also has the potential to elevate interest and be more enjoyable than traditional modalities18,19. Accordingly, AIT may be a more appropriate modality for patients undergoing chemotherapy. Aerobic training has been recently discovered to have a great capacity to alleviate DOXO-induced hepatotoxicity20,21. Moreover, high-intensity interval training (HIIT) and traditional methods, especially HIIT, have shown their promising effectiveness in reducing hepatic ER stress via downregulating the GRP78-mediated signaling pathways, including PERK, ATF4, and CHOP22. So far, however, there has been little discussion about the hepatoprotective impact of AIT on DOXO-induced ER stress. Besides AIT, curcumin, extracted from turmeric (curcuma longa), exerts anti-oxidative, anti-inflammatory, anti-cancer, anti-fibrotic, and anti-apoptosis actions23,24. Previously published studies have indicated that curcumin ameliorates hepatic injury induced by DOXO through decreasing oxidative stress and inflammation25. Furthermore, curcumin has been found to have a beneficial hepatoprotective impact on liver diseases by suppressing ER stress-mediated apoptosis26,27. However, the role of curcumin in attenuating DOXO-induced ER stress in the hepatic tissue remains largely unexamined.
To date, the connection between ER stress and DOXO-induced hepatic damage has not been well examined. Moreover, few empirical studies have discussed the synergistic effects of AIT and curcumin treatments in attenuating DOXO-induced hepatotoxicity by targeting the ER stress signaling pathway. Therefore, the current study seeks to provide evidence for the claim that AIT combined with or without curcumin supplementation is an effective therapeutic tool that can counteract oxidative damage and ER stress-mediated apoptosis in DOXO-intoxicated rats.
Materials and methods
Animals and ethical approval
Fifty-six male Sprague–Dawley rats (aged between 8 and 10 weeks, weighing 200 to 250 g), which were obtained from the Pasteur Institute of Iran, were used for the experiments. The ethics committee of the University of Tehran approved all procedures in this research (IR.UT.SPORT.REC.1402.073). Furthermore, all methods were conducted in accordance with the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments). Additionally, all methods were performed in accordance with the relevant guidelines and regulations as outlined in the editorial and publishing policies of the Scientific Reports journal. In brief, the animals were accommodated in pairs (3 rats per cage), cleaned rat cages, and remained in the same cage until the study concluded. In addition, the animals were provided with ad libitum access to standard chow and water, and were accommodated in an appropriately controlled environment, including 40–60% humidity, 22–25 °C temperature, 12:12 dark–light cycle, and with daily monitoring.
Study design
Following seven days of acclimatization, the animals were familiarized with a small animal treadmill for another week and randomly allocated to six groups (Fig. 1), as indicated below:
-
Control group (CON): The rats remained sedentary with no interventions (n = 8).
-
Vehicle group (Vehicle): The rats were injected intraperitoneally (i.p.) with normal saline solution (0.9%) once a week for five weeks as a placebo, were orally administered dimethyl sulfoxide (DMSO), a common solvent used in biomedical research as a vehicle and were placed on the stationary treadmill over six weeks (n = 8).
-
Doxorubicin group (Dox):The animals received weekly i.p. injections of DOXO (4 mg/kg/week) for five weeks28 and were orally administered DMSO as a vehicle (n = 10).
-
Doxorubicin + Curcumin group (Dox-C):The rats received i.p. injections of DOXO at a dosage of 4 mg/kg once weekly over five weeks and were given 100 mg/kg/day curcumin suspended in DMSO via oral gavage five days per week for six weeks25 (n = 10).
-
Doxorubicin + AIT group (Dox-A): The animals were given five i.p. injections of DOXO at 4 mg/kg/week, underwent AIT five days a week over six weeks, and were administered by DMSO as a vehicle (n = 10).
-
Doxorubicin + Curcumin + AIT group (Dox-AC): This group received weekly injections (i.p.) of DOXO at 4 mg/kg/week over five weeks, was subjected to AIT, and was administered 100 mg/kg/day of curcumin dissolved in DMSO five days weekly over six weeks (n = 10).
It is worth mentioning that normal saline solution (0.9%) was utilized as the vehicle for doxorubicin administration, while DMSO served as the vehicle for curcumin to ensure proper solubility and delivery.
Schematic illustration of the experimental design. After a five-week pilot study, the animals were acclimatized to a new environment and treadmill for two weeks. Afterward, they were randomly assigned to six groups. Forty-eight hours following the last intervention, they were sacrificed, followed by blood and tissue collection. Some components of the figure were created using images from Servier Medical Art (https://smart.servier.com), which is licensed under a Creative Commons Attribution 4.0 Unported License (https://creativecommons.org/licenses/by/4.0/). Abbreviations: AIT, aerobic interval training; DMSO, dimethyl sulfoxide; D, day; CON, control group; Dox, doxorubicin group; Dox-C, doxorubicin + curcumin group; Dox-A, doxorubicin + aerobic interval training group; Dox-AC, doxorubicin + curcumin + aerobic interval training group.
Hepatotoxicity induction
Hepatotoxicity has been observed in rats after receiving 3 mg/kg/week of DOXO injections over five weeks29. Additionally, male Sprague–Dawley rats demonstrated signs of hepatotoxicity after seven weeks of receiving a cumulative dosage of 14 mg/kg DOXO30. Furthermore, a pilot study was carried out to verify the induction of DOXO-induced hepatotoxicity using the selected procedure in our study. According to the findings of the pilot study and Trivedi et al. (2011) research, hepatotoxicity was induced by five i.p. injections of DOXO (Ebewe Pharma Co., Cat. No. A4866) over five weeks at 4 mg/kg/week28. Moreover, all weekly injections were conducted simultaneously on Fridays at a specific time of day. The vehicle group received 0.9% saline in the same volume and procedure as the Dox group.
Preparation of curcumin
To prepare the curcumin solution, 20 mg of curcumin powder (Sigma-Aldrich, Cat. No. C1386) was suspended in 1 ml of DMSO (Merck, Cat. No. 1.02952.1000). The curcumin solution was thoroughly mixed and orally administered to animals at 100 mg/kg five times weekly for six consecutive weeks25. It is essential to mention that the animals in the Vehicle, Dox, and Dox-A groups received DMSO solution (without curcumin) as a vehicle, with the same amount and protocol as the Dox-C group.
Aerobic interval training protocol
Following a week of acclimatization with the new environment, the animals were familiarized to the motorized treadmill as follows: During the initial two days, they were placed on the motionless treadmill to seek the treadmill lane for 5–10 min/day. In the following days, the treadmill’s velocity and duration were gradually enhanced from 5 to 10 m/min and 5 to 10 min/day, respectively. On the last day, the animals walked on the treadmill at 10 m/min for 10 min on a 5° slope31.
Following the familiarization period, the maximal oxygen consumption (VO2max) of the rats was calculated according to the relation between the running speed and VO2maxas described previously32. The AIT protocol consisted of a ten-minute warming up at 50–55% VO2max, seven high-intensity bouts (4 min each) at 80–90% VO2max, and a one-minute cool-down at 50–55% VO2max. Additionally, active recovery intervals (3 min each) at 65–75% VO2maxwere performed between each high-intensity bout33,34. Treadmill inclination was 25° and 5° during testing and training, respectively. The rats received a mild electric shock (0.05 mA) to maintain the treadmill’s pace. Moreover, the rats were subjected to AIT five times weekly (Saturday to Wednesday) for six weeks. Weekly training loads are presented in Supplementary Table 1 (Table S1).
Euthanasia and tissue collection
48 h following the last intervention and after 6h of fasting, an i.p. injection of 100 mg/kg ketamine (Rotexmedica, Germany) and 10 mg/kg xylazine (Alfasan, Netherlands) was used to anesthetize the rats, and blood samples were obtained from their hearts35. Afterward, they were euthanized by CO2 inhalation using a standard chamber (height, length, and width: 25.4, 48.26, and 22.86 cm, respectively) with a volume of 28 L. The chamber was filled at a fill rate of 5.6 L/min for 5 to 6 min. Afterward, death signs were examined, including ascertaining cardiac and respiratory arrest, lack of respiration, faded eye color, lack of heartbeat, lack of corneal reflex, no response to toe pinch, and presence of rigor mortis36. Following death verification, hepatic and soleus tissues were collected. A portion of the hepatic and soleus tissues were rapidly immersed in liquid nitrogen and thereafter stored at −80 °C, while another portion was immersed in 10% formalin.
The liver index measurement
The liver index was measured on the following equation:
Assessment of aerobic capacity
To assess the effectiveness of the AIT protocol, the maximal speed that the rats reached in the treadmill test (Vmax) and the levels of citrate synthase (CS) in the soleus muscle, as variables of aerobic capacity37,38, were measured. Forty-eight hours following the last training session, 100 mg of soleus muscle samples were homogenized in one ml of RIPA buffer (DNAbiotech, Cat. No. DB9719). The CS activity was examined by collecting the supernatant after centrifuging at 4000 rpm for 20 min at a low temperature. The CS activity was assessed using a high-quality ELISA kit (MyBioSource, Cat. No. MBS162118) and an ELISA reader (Biotek-Refelx800, USA) at 450 nm.
Histopathological evaluation
Hematoxylin and eosin staining (H&E)
The hepatic tissue samples were first embedded in paraffin blocks, followed by the cutting of five-μm-thick sections by a microtome. Following deparaffinization and rehydration, the liver sections were subjected to hematoxylin (Sigma-Aldrich, Cat. No. H9627) for seven seconds and then washed using distilled water for one minute. The tissue sections were subjected to lithium carbonate (Sigma-Aldrich, Cat. No. 1.05680) for two seconds, then stained with eosin (Sigma-Aldrich, Cat. No. HT110116) for three minutes. Finally, all photomicrographs of H&E staining were interpreted by an expert pathologist.
Masson’s trichrome staining (MTC)
Following deparaffinization and rehydration, hepatic tissue sections were immersed in Bouin’s solution (Sigma-Aldrich, Cat. No. HT10132) at 56 °C in an autoclave for one hour. Tissue sections were washed with water and distilled water, followed by dipping in Weigert’s hematoxylin solution for 10–15 min. Following rewashing with distilled water, the hepatic sections were subjected to a Biebrich scarlet-acid fuchsine solution for 10–15 min. Afterward, the tissue sections were washed using distilled water and immersed in phosphomolybdic/phosphotungstic acids for 10–15 min, then treated with aniline blue (Sigma-Aldrich, Cat. No. B8563) solvent for 20–25 min. The sections were rewashed, subjected to 1% acetic acid (Merck, Cat. No. 100056) for 2–5 min, rewashed, dehydrated, and immersed in xylene. Photomicrographs of MTC staining were interpreted by a pathologist, and collagen deposition was quantified by Image J software (version 14.8).
TUNEL assay
Following deparaffinization and rehydration, hepatic tissue sections were taken into a Tris-buffered saline (TBS) solution obtained from Sigma-Aldrich (Cat. No. T5912) and heated in a microwave oven. Afterward, the hepatic tissue sections were immersed thrice in phosphate-buffered saline (PBS) obtained from Sigma-Aldrich (Cat. No. P4417) for 5 min, then immersed in 0.03% Triton (Sigma-Aldrich, Cat. No. T8787) for 30 min. Following rewashing with PBS, the hepatic tissue sections were treated with 10% goat serum (Sigma-Aldrich, Cat. No. G9023) for 45 min and incubated with a primary antibody (1:100 dilution) at 2–8 °C for 24 h. The slides were then washed four times, each for five minutes, with PBS and incubated with a secondary antibody (1:150 dilution) without light at 37 °C for 90 min. After rewashing, the hepatic sections were treated with DAPI (Sigma-Aldrich, Cat. No. D9542) for 20 min. Finally, immunofluorescent images were acquired by microscopy (Olympus Company, Japan). TUNEL-positive cells (apoptotic cells) were quantified by Image J software (version 14.8).
Biochemical assessment
High-quality standard kits (Parsazmun Company) were utilized to measure serum biomarkers of hepatic injury, including serum glutamic-oxaloacetic transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), total bilirubin (T-bilirubin), and albumin. The measurements were performed using a fully automatic biochemistry analyzer (Alpha Classic Company, Iran).
ELISA protocol
Serum lactate dehydrogenase (LDH) concentration
The supernatant was collected to assess LDH concentration after centrifuging serum at 2000–3000 rpm for 20 min. LDH concentration was assessed using a high-quality ELISA kit (MyBioSource, Cat. No. MBS2018912) and an ELISA assay reader (Biotek-Refelx800, USA) at 450 nm, and the findings are presented as U/l.
Measurement of oxidative stress
Following homogenizing the hepatic tissue and collecting the supernatant, the malondialdehyde (MDA) concentration was measured using a specific ELISA kit (ZellBio GmbH, Germany, Cat. No. RK09070), and the results are presented as μM/ml. The enzymatic activity of glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) in the hepatic tissue was evaluated using high-quality ELISA kits (GSH: ZellBio GmbH, Germany, Cat. No. RK04298; SOD: ZellBio GmbH, Germany, Cat. No. ZX-44108–96; CAT: Kiazist Company, Iran, Cat. No. KCAT96) and using an ELISA reader (Biotek-Refelx800, USA). The results for SOD, CAT, and GSH are reported as IU/ml, nM/min/ml, and mg/l, respectively.
Western blot analysis
After collecting and homogenizing hepatic tissue samples, we extracted the total protein from the supernatant, and subsequently, we measured the protein concentration based on the Lowry assay. Afterward, equal amounts of protein samples containing 20 μg were fractionated by electrophoresis on a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) at a constant voltage (90 V) for 2 h, followed by transferring to a polyvinylidene difluoride (PVDF) membrane obtained from Sigma-Aldrich (Car. No. MSPVDF04530301). Using a blocking buffer solution (Tween-20 (Sigma-Aldrich, Car. No. 8170721000), skim milk (Sigma-Aldrich), and Tris-buffered saline, glycerol, and distilled water), the PVDF membranes were blocked, followed by incubating with primary antibodies, including GRP78 (GeneTex, Cat. No. GTX113340), ATF6 (GeneTex, Cat. No. GTX15457), PERK (Cell Signaling Technology, Cat. No. #3192), phospho-PERK (Cell Signaling Technology, Cat. No. #12,185), ATF4 (GeneTex, Cat. No. GTX101943), eIF2ɑ (GeneTex, Cat. No. GTX101241), phospho-eIF2ɑ (GeneTex, Cat. No. GTX130006), CHOP (GeneTex, Cat. No. GTX112827), IRE1ɑ (GeneTex, Cat. No. GTX30005), phospho-IRE1ɑ (GeneTex, Cat. No. GTX132808), JNK (Biorbyt, Cat. No. orb129654), phospho-JNK (GeneTex, Cat. No. GTX52328), Bax (GeneTex, Cat. No. GTX109683), Bcl-2 (Biorbyt, Cat. No. orb10173), caspase-3 (Sigma-Aldrich, Cat. No. AB3623), caspase-12 (GeneTex, Cat. No. GTX37762), GAPDH (GeneTex, Cat. No. GTX100118) at a dilution of 1:1000 overnight at 4 °C. The PVDF membranes were incubated with secondary antibodies conjugated to HRP (Boster, Cat. No. BA1054; Santa Cruz Biotechnology, Cat. No. sc-516102) at room temperature for 2 h after being washed three times with TBST to eliminate any unbound primary antibodies. An enhanced chemiluminescent signals (ECL) substrate (Pars Tous, Cat. No. B111420) was employed to visualize protein bands, and then the protein bands were quantified using Image J software (version 14.8). An internal control (GAPDH) was utilized to normalize the data, and the findings were reported as relative protein expression.
Statistical analysis
Data are reported as mean ± standard deviation (SD). The Shapiro–Wilk and Brown-Forsythe tests were employed to verify the normality of the data and the homogeneity of variances, respectively. A one-way analysis of variance (ANOVA), followed by Tukey’s post hoc tests, was utilized to assess differences between groups, and a P-value < 0.05 was deemed statistically significant. In addition, repeated measures two-way ANOVA and Bonferroni’s post hoc test were adopted to analyze the differences between the initial and final body weight in different groups. The survival rates were also identified by the Kaplan–Meier method, and the differences among curves were calculated using the log-rank test. Moreover, GraphPad Prism software (version 10.2.3) was used to analyze the data.
Results
The CON and vehicle groups did not show any statistically significant differences with respect to aerobic capacity, histopathological changes, ELISA assays, biochemical tests, and western blot analysis. Accordingly, to simplify data interpretation, the Dox, Dox-C, Dox-A, and Dox-AC groups were compared to the CON group, and the vehicle group was eliminated from the analysis. In addition, the findings of the pilot study are reported in the supplementary data (Figure S1 and Table S2).
Survival rate and general health status
The animals’ survival rates were evaluated using Kaplan–Meier survival curves and a log-rank test (Fig. 2). Over the six weeks of the interventions, five of ten rats in the Dox group died, resulting in a survival rate of 50%. Interestingly, a higher survival rate was observed in the Dox-C (90%), Dox-A (80%), and Dox-AC (70%) groups. Furthermore, the survival animals in the Dox group exhibited symptoms and signs of bad general health status, including chromodacryorrhea, stiff hair, loss of appetite, diarrhea, progressive weight loss, and adynamia. As expected, these symptoms and signs were considerably reduced in the Dox-C, Dox-A, and Dox-AC groups, as indicated in Table 1. It is worth pointing out that the rats that survived from the Dox group developed ascites, as evidenced by a visibly swollen abdomen, which was subsequently verified during necropsy. These animals demonstrated a notable decrease in serum albumin levels, which could be linked to the ascites observed in the Dox group31,39. However, the ascites were markedly decreased in the Dox-C, Dox-A, and Dox-AC groups.
Body and liver weight changes and aerobic capacity
Initial body weights did not reveal any significant differences among the groups. The Dox (P < 0.0001), Dox-C (P < 0.001), Dox-A (P < 0.01), and Dox-AC (P < 0.001) groups demonstrated a significant weight loss at the end of the sixth week compared to the CON group. Furthermore, the CON group’s body weight increased significantly from week one to week six (+ 20.52%, P < 0.0001). In contrast, the Dox, Dox-C, Dox-A, and Dox-AC groups’ final body weight significantly reduced compared with these groups’ initial body weight (−21.85%, P < 0.0001; −15.56%, P < 0.001; −15.8%, P < 0.001; −15.39%, P < 0.001, respectively). Moreover, the Dox group exhibited a statistically significant reduction in the liver weight compared to the CON group (P < 0.05), but the other groups illustrated no significant differences. Also, no statistical differences in the liver index were found among all groups (Table 2).
The maximal speed that the animals reached in the treadmill test (Vmax) and the levels of CS in the soleus muscle were evaluated to assess aerobic capacity. As expected, the Dox group had significantly lower Vmax relative to the CON group (P < 0.01). In contrast, the Dox-A and Dox-AC groups exhibited a significant increase in Vmax compared to the CON and Dox groups (Dox-A vs. CON: P < 0.0001; Dox-AC vs. CON: P < 0.001; Dox-A and Dox-AC vs. Dox: P < 0.0001). Additionally, both the Dox-A and Dox-AC groups significantly increased Vmax in comparison with the Dox-C group (P < 0.0001 for both comparisons). Interestingly, the Dox-A group showed a statistically higher Vmax than the Dox-AC group (P < 0.05). Regarding CS activity, the Dox group exhibited significantly lower levels of CS in comparison with the CON group (P < 0.0001). The Dox-C (P < 0.05), Dox-A (P < 0.05), and Dox-AC (P < 0.001) groups significantly increased CS activity in comparison with the Dox group (Table 2). These results further emphasize the positive impact of the interventions on aerobic capacity.
Evaluation of hepatic damage
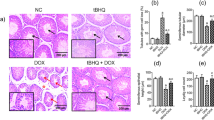
The results obtained from H&E staining revealed that the Dox group developed morphological changes, including degeneration in the hepatic tissue, necrosis, sinusoidal dilatation, hemorrhage, inflammation, apoptosis, kupffer cell proliferation, and lipid droplet accumulation, as shown in Fig. 3A to 3F. All interventions effectively improved morphological changes induced by DOXO injections. AIT combined with curcumin was more effective in ameliorating histopathological changes caused by DOXO than the Dox-C and Dox-A groups (Table 3). Concerning hepatic fibrosis, MTC staining demonstrated that the Dox group highly developed liver fibrosis in comparison with the CON group, as evidenced by significantly increased collagen deposition (P < 0.0001). The Dox-C, Dox-A, and Dox-AC groups displayed a significant reduction in collagen deposition in comparison with the Dox group (P < 0.0001 for all comparisons). In addition, the Dox-AC group exhibited significantly less collagen deposition than the Dox-C (P < 0.01) and Dox-A (P < 0.0001) groups. Interestingly, no statistically significant differences were detected between the Dox-AC and CON groups (Figs. 3a to 3f and 3G).
Liver histopathological changes in different groups. Representative hematoxylin and eosin (H&E) and Masson’s trichrome staining (MTC) photomicrographs of the hepatic tissue (400 × magnification). (A & a) CON; (B & b) Vehicle; (C & c) Dox; (D & d) Dox-C; (E & e) Dox-A; (F & f) Dox-AC; (G) the percentage of collagen deposition in different groups (n = 4/group). * P < 0.05 vs. the CON group; # P < 0.05 vs. the Dox group; $ P < 0.05 vs. the Dox-C group; & P < 0.05 vs. the Dox-A group. Abbreviations: CON, control group; Dox, doxorubicin group; Dox-C, doxorubicin + curcumin group; Dox-A, doxorubicin + aerobic interval training group; Dox-AC, doxorubicin + curcumin + aerobic interval training group.
The histopathological changes after DOXO injections were consistent with the serum markers of hepatic damage, with a significant increase in SGOT, SGPT, ALP, GGT, T-bilirubin, LDH, alongside a reduction in albumin in the Dox group in comparison with the CON group (P < 0.0001 for most markers, except P < 0.001 for albumin). The results, as shown in Table 4, indicate that the serum levels of SGOT (P < 0.0001), GGT (P < 0.001), SGPT (P < 0.01), T-bilirubin (P < 0.01), and ALP (P < 0.05) were statistically lower in the Dox-C group than in the Dox group. Furthermore, the Dox-A group revealed a significant reduction in SGOT (P < 0.0001), SGPT (P < 0.001), T-bilirubin (P < 0.05), and LDH (P < 0.0001) compared to the Dox group. The Dox-AC group was able to decrease the serum markers of hepatic injury and increase albumin levels compared to the Dox group (SGOT, SGPT, GGT, T-bilirubin, LDH: P < 0.0001; ALP: P < 0.01; albumin: P < 0.05). In addition, AIT combined with curcumin was able to decrease SGOT (P < 0.0001), SGPT (P < 0.001), GGT (P < 0.01), and LDH (P < 0.0001) compared to the Dox-C group. Likewise, in the Dox-AC group, in comparison with the Dox-A group, a significant decrease in SGOT (P < 0.0001), SGPT (P < 0.01), GGT (P < 0.0001), LDH (P < 0.01), and a significant increase in albumin (P < 0.05) were detected. Regarding the differences between the Dox-A and Dox-C groups, the results reveal that the serum levels of SGOT (P < 0.05) were significantly lower, and the serum levels of LDH (P < 0.0001) were statistically higher in the Dox-C than in the Dox-A group.
Markers of oxidative stress in the hepatic tissue
The findings obtained from the preliminary analysis of oxidative stress markers are illustrated in Table 5. According to the table, the Dox group significantly increased the MDA concentration and reduced the CAT, SOD, and GSH activity in comparison with the CON group (P < 0.0001 for all comparisons). The Dox-C, Dox-A, and Dox-AC groups significantly lowered the concentration of MDA (P < 0.001, P < 0.0001, and P < 0.0001, respectively) and increased the activity of CAT (P < 0.01, P < 0.0001, and P < 0.0001, respectively), SOD (P < 0.05, P < 0.0001, and P < 0.0001, respectively), and GSH (P < 0.05, P < 0.001, and P < 0.0001, respectively) in comparison with the Dox group. Moreover, the activity of SOD (P < 0.01) and CAT (P < 0.0001) enzymes were significantly higher in the Dox-A group than in the Dox-C group. Regarding the combined effect of AIT and curcumin supplementation, the results indicated that the Dox-AC group was able to decrease the MDA concentration and increase the activity of CAT and GSH compared to the Dox-C and Dox-A groups (MDA: P < 0.0001, and P < 0.01, respectively; CAT: P < 0.0001, and P < 0.05, respectively; GSH: P < 0.001, and P < 0.05, respectively). Furthermore, the Dox-AC group had a significantly higher activity of SOD than the Dox-C group (P < 0.001).
Biomarkers of ER stress in the hepatic tissue
To focus on the key findings, we report the p-PERK/PERK, p-JNK/JNK, p-eIF2ɑ/eIF2ɑ, and p-IRE1ɑ/IRE1ɑ ratios in the main manuscript (Fig. 4). Additional data for p-PERK, PERK, p-JNK, JNK, p-eIF2ɑ, eIF2ɑ, p-IRE1ɑ, and IRE1ɑ, all normalized to GAPDH, are available in the supplementary data (Figure S2). As expected, western blot analysis revealed that the Dox group significantly increased the expression of all ER stress markers in comparison with the CON group (P < 0.0001 for all comparisons). The Dox-C, Dox-A, and Dox-AC groups demonstrated significant reductions in the expression of ER stress markers compared to the Dox group (GRP78: P < 0.01, P < 0.0001, and P < 0.0001, respectively; ATF6: P < 0.0001 for all comparisons; p-IRE1ɑ/IRE1ɑ ratio: P < 0.01, P < 0.0001, and P < 0.0001, respectively; p-JNK/JNK ratio: P < 0.05, P < 0.0001, and P < 0.0001, respectively; p-PERK/PERK ratio: P < 0.01, P < 0.0001, and P < 0.0001, respectively; p-eIF2ɑ/eIF2ɑ: P < 0.01, P < 0.0001, and P < 0.0001, respectively; ATF4: P < 0.05, P < 0.0001, and P < 0.0001, respectively; CHOP: P < 0.001, P < 0.0001, and P < 0.0001, respectively). Furthermore, no significant difference between the Dox-C and Dox-A groups was evident, except for p-JNK/JNK ratio (P < 0.05). The Dox-AC group revealed significantly lower protein expression of ER stress markers compared to the Dox-C group, except for the protein expression of CHOP (GRP78, ATF6, p-IRE1α/IRE1α, p-JNK/JNK, p-PERK/PERK, ATF4: P < 0.01; p-eIF2α/eIF2α: P < 0.001), whereas the Dox-AC group did not yield an additional effect on the protein expression of ER stress markers compared to the Dox-A group.
Protein expression of endoplasmic reticulum stress markers in the hepatic tissue (A) representative western blot analysis of ER stress markers in the hepatic tissue; (B) p-IRE1ɑ/IRE1ɑ ratio; (C) ATF6/GAPDH; (D) p-JNK/JNK ratio; (E) p-PERK/PERK ratio; (F) GRP78/GAPDH; (G) ATF4/GAPDH; (H) p-eIF2ɑ/eIF2ɑ ratio; (I) CHOP/GAPDH. Data are presented as mean ± SD (n = 4/group). * P < 0.05 vs. the CON group; # P < 0.05 vs. the Dox group; $ P < 0.05 vs. the Dox-C group. Abbreviations: ATF4, activating transcription factor 4; ATF6, activating transcription factor 6; CHOP; C/EBP homologous protein; eIF2ɑ, eukaryotic initiation factor 2ɑ; GRP78, the 78-kDa glucose-regulated protein; IRE1ɑ, inositol-requiring transmembrane kinase/endoribonuclease 1α; JNK, c-Jun N-terminal Kinase; PERK, protein Kinase RNA-Like ER Kinase; CON, control group; Dox, doxorubicin group; Dox-C, doxorubicin + curcumin group; Dox-A, doxorubicin + aerobic interval training group; Dox-AC, doxorubicin + curcumin + aerobic interval training group.
Evaluation of apoptosis
TUNEL staining revealed more TUNEL-positive cells in the animals’ hepatic tissue in the Dox group than in the CON group (P < 0.0001, Figs. 5A and B). The Dox-C, Dox-A, and Dox-AC groups displayed significantly lower TUNEL-positive cells than the Dox group (P < 0.0001 for all comparisons). Furthermore, The Dox-AC group exhibited a significant reduction in TUNEL-positive cells compared to the Dox-C group (P < 0.01).
Evaluation of apoptosis in the hepatic tissue. (A and B) representative TUNEL staining photomicrographs of the hepatic tissue and the quantitative results for apoptosis in the liver (200 × magnification); (C and D) representative western blot analysis of apoptosis biomarkers and quantitative data in the hepatic tissue. Data are reported as mean ± SD (n = 4/group). * P < 0.05 vs. the CON group; # P < 0.05 vs. the Dox group; $ P < 0.05 vs. the Dox-C group; & P < 0.05 vs. the Dox-A group. Abbreviations: Bax, Bcl2-associated X protein; Bcl-2, B-cell lymphoma 2; CON, control group; Dox, doxorubicin group; Dox-C, doxorubicin + curcumin group; Dox-A, doxorubicin + aerobic interval training group; Dox-AC, doxorubicin + curcumin + aerobic interval training group.
Concerning apoptosis-related proteins in the hepatic tissue, western blot analysis illustrated that, relative to the CON group, the Dox group revealed increased expression of Bax, caspase-3, and caspase-12 and reduced expression of Bcl-2 (P < 0.0001 for all comparisons). The Dox-C, Dox-A, and Dox-AC groups significantly reduced the expression of Bax (P < 0.05, P < 0.01, and P < 0.001, respectively), caspase-3 (P < 0.05, P < 0.001, and P < 0.0001, respectively), and caspase-12 (P < 0.05, P < 0.01, and P < 0.0001, respectively) and increased the expression of Bcl-2 (P < 0.0001 for all comparisons) compared to the Dox group. Moreover, the Dox-AC group exhibited a decreased expression of caspase-3 compared to the Dox-C (P < 0.01) and Dox-A (P < 0.05) groups, as presented in Figs. 5C and D.
Discussion
Hepatotoxicity is a prevalent side-effect induced by DOXO, a potent anthracycline chemotherapeutic agent, which presents significant challenges in cancer treatment2,4. The fundamental mechanisms underlying hepatotoxicity induced by DOXO include free radical damage, DNA damage, mitochondrial dysfunction, and apoptosis1. Furthermore, earlier investigations have proven that ER stress-mediated apoptosis has a pivotal role in DOXO-induced cytotoxicity11,13. Given the growing interest in complementary strategies to alleviate hepatotoxicity caused by chemotherapy, this study, therefore, set out to evaluate the promising protective impacts of AIT and curcumin supplementation on DOXO-induced hepatotoxicity by focusing on oxidative stress and apoptosis caused by ER stress. The current study illustrated that AIT and curcumin administration profoundly mitigated DOXO-induced hepatic injury in male rats by alleviating oxidative damage, ER stress, and apoptosis.
Several studies have illustrated that hepatotoxicity caused by DOXO is initiated by triggering genes, accounting for oxidative stress response, DNA damage, and cell death2. Oxidative stress is the primary molecular mechanism associated with hepatic damage caused by DOXO, mainly owing to the formation of ROS in the hepatic tissue40,41. Our study confirms that the animals subjected to DOXO exhibited noteworthy increases in lipid peroxidation, as determined by MDA measurement, and a considerable reduction in anti-oxidative markers, including GSH, SOD, and CAT. However, both AIT and curcumin treatments reversed the reduction in GSH, SOD, and CAT and the increase in MDA in hepatocytes following DOXO injections. Additionally, the combination of AIT and curcumin administration yielded additional impacts on mitigating oxidative stress. Our experiments corroborate previous results, such as those conducted by Santos-Alves et al. (2019), demonstrating that endurance training effectively ameliorated hepatic oxidative stress induced by DOXO, as evidenced by reducing MDA activity30. Also, Ibrahim Fouad and colleagues (2022) have indicated that curcumin has an anti-oxidative property to improve DOXO-induced oxidative damage through dampening in MDA and increasing SOD activity25. Although the results align with recent studies, Boeno’s (2023) findings do not support our findings42. A possible explanation for these results may be a low training period, which was ten days in Boeno and colleagues’ study. Several studies have mentioned that a training period of at least 3 to 4 weeks is required to produce physiological adaptations43,44,45. Another explanation is that they examined the preconditioning effect of endurance training, but we assessed the effects of AIT during DOXO injections. One interesting finding in our study is that AIT seems to have more anti-oxidant properties than curcumin, as demonstrated by the differences in the enzymatic activity of CAT and SOD. This finding may stem from the limited oral curcumin bioavailability, which is influenced by its inadequate absorption in the small intestine and its rapid hepatic metabolism, followed by its removal by the gallbladder46. These data revealed that both AIT and curcumin administration, particularly AIT, suppress DOXO-induced oxidative stress and reverse the decreased anti-oxidant capacity caused by this drug, resulting in improved liver function and reduced hepatic damage.
Recently, it has been assumed that oxidative stress makes a key contribution to triggering and initiating ER stress47. Excessive ROS production causes disturbing ER protein folding, initiating ER stress, followed by UPR stimulation and apoptosis induction. Hence, there is a crosstalk between oxidative damage and ER stress, which makes an important contribution to inducing apoptosis48. In the current study, DOXO administration was found to result in ER stress-induced apoptosis by increasing GRP78, ATF6, p-PERK, p-IRE1ɑ, p-eIF2ɑ, ATF4, p-JNK, CHOP, caspase-12, caspase-3, pro-apoptotic Bax expression, and reducing anti-apoptotic Bcl-2 in the hepatic tissue. These results are in accord with previous studies indicating that DOXO-induced ER stress causes the stimulation of apoptosis markers12,13. The upregulation of CHOP increases mitochondrial permeabilization, leading to the translocation of Bax from the cytosol to mitochondria and, subsequently, cytochrome c release, followed by the stimulation of caspase-3, which eventually results in apoptosis. Under ER stress, CHOP can also downregulate the levels of anti-apoptotic proteins, such as Bcl-249. Moreover, prolonged ER stress induces the upregulation of caspase-12, followed by caspase-9 stimulation through increasing cytosolic calcium levels. Caspase-9 eventually triggers caspase-3 and induces apoptosis50,51. Notably, the current study revealed that ER stress in the liver accompanied by hepatic apoptosis were mitigated in the animals exposed to AIT and curcumin administration. The combination of AIT with curcumin supplementation yielded further improvement compared to curcumin treatment. However, despite the promising efficacy of curcumin, AIT was determined to be more beneficial in mitigating ER stress caused by DOXO. These findings, similar to the results of oxidative stress, may partly be explained by the low oral bioavailability of curcumin, as mentioned before46. Moreover, these results suggest the promising potential of AIT for attenuating oxidative stress, followed by ER stress induced by DOXO. Our findings align with the research conducted by Sepúlveda-Fragoso et al. (2022), who suggested that AIT has a protective impact on ER stress induced by fatty liver disease via reducing the expression of GRP78, p-eIF2ɑ, and ATF452. Our findings were also reported by Laorodphun and colleagues (2022), indicating that curcumin reverses the adverse effect of gentamicin on the renal tissue by decreasing the expression of GRP78, CHOP, and caspase-1253. Concerning the combined effect of AIT and curcumin supplementation, a few studies, such as those performed by Cho et al. (2020), showed that resistance training combined with curcumin administration effectively ameliorated ER stress induced by diabetes in the rats’ brains54. However, our results are partly contradictory to findings reported in Passoss et al. (2022) study demonstrating a partially protective impact of endurance training on some markers of ER stress caused by nonalcoholic steatohepatitis. These authors did not show any protective effect of aerobic training on the protein expression of Bip, p-PERK, ATF6, ATF4, and p-IRE1ɑ. The significance of exercise intensity in physiological adaptation can partly explain these results, as mentioned by these authors55. According to our knowledge, this is the first study to illustrate that AIT and curcumin supplementation synergistically mitigate chronic hepatotoxicity caused by DOXO. A considerable amount of research has explored the role of exercise preconditioning in counteracting hepatic damage induced by DOXO, and most of them have used a single injection to induce hepatotoxicity. According to these data, we can infer that both AIT and curcumin could be considered a potential nonpharmacological tool to prevent the deleterious side effects caused by DOXO. In light of the crosstalk between free radical damage and ER stress in apoptosis, it is likely that AIT and curcumin supplementation may attenuate DOXO-induced oxidative stress, which can lead to reducing the dissociation of GRP78 from PERK, IRE1ɑ, and ATF6, rendering these three major ER transmembrane proteins inactive. This process decreases the activation of ATF6 and the phosphorylation of PERK and IRE1ɑ, thereby suppressing the PERK/eIF2ɑ/ATF4/CHOP, IRE1ɑ/JNK, and downstream of the ATF6 signaling pathways, followed by inhibiting apoptosis. These impacts of AIT and curcumin interventions finally ameliorate hepatotoxicity, as evidenced by histopathological analysis and evaluation of serum markers of hepatic damage. Given the promising results of AIT and curcumin supplementation in mitigating DOXO-induced hepatotoxicity, these interventions could potentially be considered for future therapeutic strategies in patients undergoing chemotherapy, particularly for liver protection.
Although our findings have indicated a profound effect of AIT and curcumin administration against DOXO-induced hepatotoxicity, several limitations of this study need to be addressed. First, the lack of cancer induction prior to DOXO administration can be assumed as a limitation. Nevertheless, no available study has been performed regarding the potential protective impact of AIT and curcumin treatment against DOXO-induced hepatic damage that has used this approach. Future research on this topic is strongly encouraged. Additionally, mechanistic studies are necessary to better understand the mechanisms responsible for the relationship between oxidative stress and ER stress in activating apoptosis. Besides, because of lower DOXO clearance in women than in men, women are more susceptible to drug-induced hepatic damage56,57. Hence, further research is recommended to determine the gender differences concerning this topic. Furthermore, considering the challenges associated with curcumin’s bioavailability, studies investigating different delivery methods, such as liposomal formulations or nanoparticle-based systems, should be prioritized to improve curcumin’s therapeutic efficacy. This would ensure that future clinical applications of curcumin are optimized for maximum bioavailability and sustained therapeutic effects. Finally, further studies should be undertaken to ascertain the feasibility, therapeutic effectiveness, and safety of these interventions during chemotherapy in humans suffering from neoplasm.
Conclusion
Our study specifically aimed to examine the protective role of AIT and curcumin supplementation in counteracting DOXO-induced hepatotoxicity. Our findings clearly indicated that both AIT and curcumin supplementation could mitigate hepatic injury, oxidative stress and ER stress-mediated apoptosis in DOXO-intoxicated rats. However, despite the promising efficacy of curcumin, AIT seemed to be more effective in ameliorating these outcomes, as well as biomarkers of oxidative stress, ER stress, and apoptosis.
Data availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
Change history
08 April 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-95422-y
References
Renu, K. et al. Toxic effects and molecular mechanism of doxorubicin on different organs – an update. Toxin Rev. 41(2), 650–674 (2022).
Prasanna, P. L., Renu, K. & Valsala Gopalakrishnan, A. New molecular and biochemical insights of doxorubicin-induced hepatotoxicity. Life Sci. 250, 117599 (2020).
Rawat, P. S. et al. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 139, 111708 (2021).
Damodar, G. et al. An evaluation of hepatotoxicity in breast cancer patients receiving injection Doxorubicin. Ann. Med. Health Sci. Res. 4(1), 74–79 (2014).
Shivakumar, P. et al. A study on the toxic effects of doxorubicin on the histology of certain organs. Toxicol. Int. 19(3), 241–244 (2012).
Alherz, F. A. et al. The potential beneficial role of Ginkgetin in doxorubicin-induced hepatotoxicity: Elucidating the underlying claim. Biomed. Pharmacother. 165, 115010 (2023).
Harding, H. P., Zhang, Y. & Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397(6716), 271–274 (1999).
Lemmer, I. L. et al. A guide to understanding endoplasmic reticulum stress in metabolic disorders. Mol. Metab. 47, 101169 (2021).
Liu, X. & Green, R. M. Endoplasmic reticulum stress and liver diseases. Liver Res. 3(1), 55–64 (2019).
Lukas, J. et al. Role of endoplasmic reticulum stress and protein misfolding in disorders of the liver and pancreas. Adv. Med. Sci. 64(2), 315–323 (2019).
Yarmohammadi, F. et al. Endoplasmic reticulum stress in doxorubicin-induced cardiotoxicity may be therapeutically targeted by natural and chemical compounds: A review. Pharmacol. Res. 164, 105383 (2021).
Kim, B. S. et al. Sacubitril/valsartan reduces endoplasmic reticulum stress in a rat model of doxorubicin-induced cardiotoxicity. Arch. Toxicol. 96(4), 1065–1074 (2022).
Kaymak, E. et al. Thymoquinone alleviates doxorubicin induced acute kidney injury by decreasing endoplasmic reticulum stress, inflammation and apoptosis. Biotech. Histochem. 97(8), 622–634 (2022).
Ong, G. & Logue, S. E. Unfolding the interactions between endoplasmic reticulum stress and oxidative stress. Antioxidants 12(5), 981 (2023).
Fletcher, J. A. et al. Impact of various exercise modalities on hepatic mitochondrial function. Med. Sci. Sports Exerc. 46(6), 1089–1097 (2014).
Wisløff, U. et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115(24), 3086–3094 (2007).
Haram, P. M. et al. Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovasc. Res. 81, 723–732 (2009).
Bartlett, J. D. et al. High-intensity interval running is perceived to be more enjoyable than moderate-intensity continuous exercise: Implications for exercise adherence. J. Sports Sci. 29(6), 547–553 (2011).
Calverley, T. A. et al. HIITing the brain with exercise: Mechanisms, consequences and practical recommendations. J. Physiol. 598(13), 2513–2530 (2020).
Ahmadian, M., Dabidi Roshan, V. & Leicht, A. S. Age-related effect of aerobic exercise training on antioxidant and oxidative markers in the liver challenged by doxorubicin in rats. Free Radic. Res. 52, 775–782 (2018).
Hinkley, J. M. et al. Exercise Training Prevents Doxorubicin-induced Mitochondrial Dysfunction of the Liver. Med. Sci. Sports Exerc. 51(6), 1106–1115 (2019).
Yuan, Z. et al. HIIT and MICT attenuate high-fat diet-induced hepatic lipid accumulation and ER stress via the PERK-ATF4-CHOP signaling pathway. J. Physiol. Biochem. 78(3), 641–652 (2022).
Rezaee, R. et al. Curcumin: A potentially powerful tool to reverse cisplatin-induced toxicity. Pharmacol. Res. 117, 218–227 (2017).
Benzer, F. et al. Curcumin ameliorates doxorubicin-induced cardiotoxicity by abrogation of inflammation, apoptosis, oxidative DNA damage, and protein oxidation in rats. J. Biochem. Mol. Toxicol. https://doi.org/10.1002/jbt.22030 (2018).
Ibrahim Fouad, G. & Ahmed, K. A. Curcumin ameliorates doxorubicin-induced cardiotoxicity and hepatotoxicity via suppressing oxidative stress and modulating iNOS, NF-κB, and TNF-α in rats. Cardiovasc. Toxicol. 22(2), 152–166 (2022).
Shakeri, A. et al. Curcumin and its analogues protect from endoplasmic reticulum stress: Mechanisms and pathways. Pharmacol. Res. 146, 104335 (2019).
Afrin, R. et al. Curcumin ameliorates streptozotocin-induced liver damage through modulation of endoplasmic reticulum stress-mediated apoptosis in diabetic rats. Free Radic. Res. 49(3), 279–289 (2015).
Trivedi, P. P. et al. Cardioprotective effects of hesperetin against doxorubicin-induced oxidative stress and DNA damage in rat. Cardiovasc. Toxicol. 11(3), 215–225 (2011).
Renu, K. et al. Elevated lipolysis in adipose tissue by doxorubicin via PPARα activation associated with hepatic steatosis and insulin resistance. Eur. J. Pharmacol. 843, 162–176 (2019).
Santos-Alves, E. et al. Physical exercise positively modulates DOX-induced hepatic oxidative stress, mitochondrial dysfunction and quality control signaling. Mitochondrion 47, 103–113 (2019).
Sequeira, C. M. et al. Aerobic exercise training attenuates doxorubicin-induced ultrastructural changes in rat ventricular myocytes. Life Sci. 264, 118698 (2021).
Høydal, M. A. et al. Running speed and maximal oxygen uptake in rats and mice: Practical implications for exercise training. Eur. J. Cardiovasc. Prev. Rehabil. 14(6), 753–760 (2007).
Jiang, H.-K. et al. Aerobic interval training attenuates mitochondrial dysfunction in rats post-myocardial infarction: roles of mitochondrial network dynamics. Int. J. Mol. Sci. 15(4), 5304–5322 (2014).
Jiang, H. K. et al. Aerobic interval training protects against myocardial infarction-induced oxidative injury by enhancing antioxidase system and mitochondrial biosynthesis. Clin. Exp. Pharmacol. Physiol. 41(3), 192–201 (2014).
Kohler, I. et al. Is carbon dioxide (CO2) a useful short acting anaesthetic for small laboratory animals?. Lab. Anim. 33(2), 155–161 (1999).
Underwood, W. and R. Anthony, AVMA guidelines for the euthanasia of animals: 2020 edition. Retrieved on March, 2020. 2013(30): p. 2020–1.
Agarwal, D. et al. Chronic exercise modulates RAS components and improves balance between pro- and anti-inflammatory cytokines in the brain of SHR. Basic Res. Cardiol. 106(6), 1069–1085 (2011).
de Lima, E. A. et al. Aerobic exercise, but not metformin, prevents reduction of muscular performance by AMPk activation in mice on doxorubicin chemotherapy. J. Cell. Physiol. 233(12), 9652–9662 (2018).
Zaccherini, G., Tufoni, M. & Bernardi, M. Albumin administration is efficacious in the management of patients with cirrhosis: A systematic review of the literature. Hepatic Med.: Evid. Res. 12, 153–172 (2020).
Wali, A. F. et al. Naringenin regulates doxorubicin-induced liver dysfunction: Impact on oxidative stress and inflammation. Plants 9(4), 550 (2020).
Costa Godinho, L. R. L. et al. Creatine supplementation potentiates exercise protective effects against doxorubicin-induced hepatotoxicity in mice. Antioxidants 12(4), 823 (2023).
Boeno, F. P. et al. Effects of exercise preconditioning on doxorubicin-induced liver and kidney toxicity in male and female rats. Int. J. Mol. Sci. 24(12), 10222 (2023).
Vega, R. B. et al. Molecular mechanisms underlying cardiac adaptation to exercise. Cell Metab. 25(5), 1012–1026 (2017).
Herrod, P. J. J. et al. The time course of physiological adaptations to high-intensity interval training in older adults. Aging Med. (Milton) 3(4), 245–251 (2020).
Wen, D. et al. Effects of different protocols of high intensity interval training for VO(2)max improvements in adults: A meta-analysis of randomised controlled trials. J. Sci. Med. Sport 22(8), 941–947 (2019).
Dei Cas, M. R. & Ghidoni,. Dietary curcumin: correlation between bioavailability and health potential. Nutrients https://doi.org/10.3390/nu11092147 (2019).
Yuzefovych, L. V. et al. Mitochondrial DNA damage via augmented oxidative stress regulates endoplasmic reticulum stress and autophagy: Crosstalk, links and signaling. PLoS One 8(12), e83349 (2013).
Cao, S. S. & Kaufman, R. J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 21(3), 396–413 (2014).
Hu, H. et al. The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front. Immunol. 9, 3083 (2018).
Morishima, N. et al. An endoplasmic reticulum stress-specific caspase cascade in apoptosis: CYTOCHROME c-INDEPENDENT ACTIVATION OF CASPASE-9 BY CASPASE-12*. J. Biol. Chem. 277(37), 34287–34294 (2002).
Nakagawa, T. et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403(6765), 98–103 (2000).
Sepúlveda-Fragoso, V. et al. Comparison between aerobic exercise training and enalapril treatment as tools to improve diet-induced metabolic-associated fatty liver disease: Effects on endoplasmic reticulum stress markers. Life Sci. 311, 121136 (2022).
Laorodphun, P. et al. Curcumin protects rats against gentamicin-induced nephrotoxicity by amelioration of oxidative stress, endoplasmic reticulum stress and apoptosis. Pharmaceutical Biol. 60(1), 491–500 (2022).
Cho, J. A. et al. Exercise and curcumin in combination improves cognitive function and attenuates er stress in diabetic rats. Nutrients https://doi.org/10.3390/nu12051309 (2020).
Passos, E. et al. Physical exercise positively modulates nonalcoholic steatohepatitis-related hepatic endoplasmic reticulum stress. J. Cell. Biochem. 123(10), 1647–1662 (2022).
Amacher, D. E. Female gender as a susceptibility factor for drug-induced liver injury. Hum. Exp. Toxicol. 33(9), 928–939 (2014).
Dobbs, N. A. et al. Gender affects doxorubicin pharmacokinetics in patients with normal liver biochemistry. Cancer Chemother. Pharmacol. 36(6), 473–476 (1995).
Acknowledgements
This research, which was part of student theses, received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
A. M. Z. and S. N. M. N. and M. S. carried out the experiment; A. M. Z. and S. N. M. N. wrote the main manuscript; A. M. Z. and S. N. M. N. processed the experimental data, performed the analysis, drafted the main manuscript, and designed the figures; A. M. Z. and S. N. M. N. helped supervise the project. A. M. Z., S. N. M. N., and K. R. conceived the original idea. S. C., M. R. K., and K. R. supervised the project; All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in Table 1, where the row “Chromodacryorrhea” was incorrectly given as “++ for Dox group”.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zobeydi, A.M., Mousavi Namavar, S., Sadeghi Shahdani, M. et al. Mitigating doxorubicin-induced hepatotoxicity in male rats: The role of aerobic interval training and curcumin supplementation in reducing oxidative stress, endoplasmic reticulum stress and apoptosis. Sci Rep 15, 6604 (2025). https://doi.org/10.1038/s41598-025-91133-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-91133-6