Abstract
This study aims to identify biomarkers for reliably predicting diabetic kidney disease (DKD), systematically characterize serum metabolites and gut microbiota in DKD patients, and investigate the correlation between differential serum metabolites and gut microbiota. From September 2021 to January 2023, 90 subjects were recruited: 30 with DKD, 30 with type 2 diabetes mellitus (T2DM), and 30 normal controls (NCs). Serum metabolites, including 180 different metabolites, were analyzed using untargeted metabolomics UPLC-MS/MS, and gut microbiota were assessed via 16S rRNA sequencing. Differential metabolites were identified through univariate (t-test or Mann–Whitney U-test, P < 0.05) and multivariate analyses (OPLS-DA model, VIP > 1, P < 0.05), followed by selection using the least absolute shrinkage and selection operator (LASSO). The selected overlapping serum metabolites, along with DKD-associated differential gut microbiota, were used to develop a logistic regression prediction model for DKD based on six markers. In the DKD group compared to the DM and NC groups, 39 and 60 differential serum metabolites were identified, respectively (VIP > 1, P < 0.01). Among these, 36 serum metabolites, including alpha-Hydroxyisobutyric acid, were significantly elevated in DKD patients compared to those with DM. Of these, 28 metabolites showed a negative correlation with estimated glomerular filtration rate (eGFR), while 29 showed a positive correlation with urine albumin creatinine ratio (UACR). Patients with DKD were further categorized into subgroups (DKD middle and DKD early) based on eGFR (eGFR < 90 ml/min/1.73m2, eGFR ≥ 90 ml/min/1.73m2), revealing 23 differential metabolites. Dysbiosis of the gut microbiota was evident in DKD patients, with lower relative abundances of g_Prevotella and g_Faecalibacterium compared to the DM and NC groups. Subgroup analysis indicated decreased relative abundances of g_Prevotella and g_Faecalibacterium in the DKD middle group, along with a decrease in g_Klebsiella compared to the DKD early group, which correlated positively with DKD patients’ eGFR. There were 11 common metabolites among the three groups of differential metabolites. Among these, three serum metabolites—imidazolepropionic acid, adipoylcarnitine, and 1-methylhistidine—were identified as predictive serum metabolic markers. Disease prediction models (logistic regression models) were constructed based on these three metabolites combined with three genera of bacteria. These models demonstrated strong discriminatory power for diagnosing patients with DKD compared to patients with DM (area under the receiver operating characteristic curve (AUROC) = 0.939 and precision-recall curve (AUPR) = 0.940). The models also effectively discriminated between patients with DKD and NCs (0.976, 0.973). This study revealed distinctive serum metabolites and gut microbiota in patients with DKD. It demonstrated the potential utility of three specific serum metabolites and three genera of bacteria in diagnosing patients with DKD and assessing their renal dysfunction.
Similar content being viewed by others
Introduction
Diabetic kidney disease (DKD) is one of the most common microvascular complications of diabetes, affecting approximately 30%-40% of diabetic patients. It is typically characterized by albuminuria or a decrease in the estimated glomerular filtration rate (eGFR)1. Although renal biopsy is considered the gold standard for evaluating the presence and severity of DKD, its clinical application is limited by inherent issues such as invasiveness, sampling errors, and intra- and inter-observer variability2,3. Therefore, developing noninvasive, accurate, and reliable markers to assess the presence and progression of DKD has become crucial for continuous monitoring and treatment decisions in diabetic patients.
Recent advances in metabolomics and gut microbiota sequencing have expanded scientific exploration. Metabolomics has emerged as an effective tool for biomarker identification and exploring molecular mechanisms. Through meticulous qualitative and quantitative analyses, extensive knowledge of the overall metabolic signatures inherent in samples can be achieved4. 16S rRNA sequencing, the most widely used technique for gut microbiota detection, can identify all bacterial species, their abundance, and their phylogenetic positions in a sample5.
Changes in the serum metabolome and gut microbiota occur at different stages of DKD. A prospective cohort study of type 1 diabetes mellitus (T1DM) patients with stage 3 chronic kidney disease (CKD) revealed that certain metabolites, such as pseudouridine, significantly predict ESRD in those with the most rapid decline in eGFR. Additionally, the positive correlation between these metabolites and renal tubular injury indicators suggests their potential for DKD prognosis6. The microbial composition of DKD patients differs significantly from that of diabetic patients without DKD and healthy individuals7. The relative abundance of g_Escherichia-Shigella and g_Prevotella in fecal samples effectively distinguishes between diabetes and DKD patients. In conclusion, specific serum metabolites and gut microbiota could serve as biomarkers for DKD. However, their clinical application is limited by the variety and complexity of these markers.
Previous studies have identified distinct serum metabolomics and gut microbiota characteristics in DKD patients with ESRD (eGFR < 15 ml/min/1.73 m2) versus those without ESRD (eGFR ≥ 15 ml/min/1.73 m2). Specific serum metabolites linked to differential microbiota were primarily enriched in six metabolic pathways, with phenylalanine and tryptophan pathways most associated with DKD progression8. Additional research shows that tryptophan and polyamine metabolism, influenced by gut microbiota and their metabolites, contribute to renal fibrosis in CKD rats9,10. However, research on the relationship between altered serum metabolites and gut microbiota during the progression from DM to DKD is lacking. The interaction between these altered metabolites and gut microbiota, and their impact on DKD pathogenesis, remains underexplored.
Hu11 developed a predictive model based on six taxa and six metabolites to distinguish between adult-onset T1DM, T2DM, and healthy controls. However, no studies have combined gut microbiota and serum metabolites to predict the risk of DKD in DM patients while assessing disease severity at early or middle stages. In our study, we used non-targeted metabolomics UPLC-MS/MS technology and 16S rRNA sequencing to identify serum metabolites and gut microbiota profiles in our cohort. Detailed analyses identified distinct gut microbiota and serum metabolites related to DKD. The disease prediction models were rigorously evaluated. A multi-omics approach was employed to analyze differential serum metabolites and gut microbiota, determining their correlation and roles in DKD development. This study provides a scientific basis for early DKD identification and a theoretical foundation for understanding the "gut-kidney axis."
Results
Demographic and baseline characteristics of subjects
A total of 90 subjects were enrolled in this study based on the inclusion and exclusion criteria, categorized into three groups: 30 patients with DKD, 30 patients with DM, and 30 healthy individuals as NCs.
The NC group comprised 14 males and 16 females, with a median age of 54.00 years (48.00–59.25). The DM group included 15 males and 15 females, with a median age of 57.00 years (54.00–58.00). The DKD group consisted of 17 males and 13 females, with a median age of 60.50 years (53.50–71.00). Gender distribution did not significantly differ among the three groups (P > 0.05). The SCr level in the DKD group [79.95 (58.75–121.83)μmol/L] was significantly elevated compared to both the DM [57.00 (46.75–65.25)μmol/L] and NC groups [61.80 (54.13–76.60)μmol/L], (P < 0.001, P = 0.014, respectively). The estimated eGFR in the DKD group (76.44 ± 31.83 ml/min/1.73m2) was lower than that of the DM group (105.77 ± 6.54 ml/min/1.73m2) (P < 0.001). BUN levels were higher in the DKD group [6.98 (5.62–9.29)mmol/L] compared to both the DM [4.83 (4.49–5.75)mmol/L] and NC groups [4.87 (4.29–5.70)mmol/L] (P < 0.001 for both). Detailed general and clinical data are presented in Table 1.
Serum metabolomic analysis in patients with DKD
Qualification and quantification of serum metabolites
We conducted comprehensive analysis of 180 serum metabolites across three groups of patients with DKD. These metabolites included 41 fatty acids (FAs), 39 amino acids (AAs), 25 organic acids (OAs), 21 carnitines, 14 bile acids (BAs), 12 carbohydrates, 7 short-chain fatty acids (SCFAs), 5 phenylpropanoic acids (PAs), 4 indoles, 4 benzoic acids (BAs), 2 peptides, 2 phenols, 1 pyridine, 1 imidazole, 1 nucleotide, and 1 benzenoid. Subsequently, these metabolites were utilized for biomarker screening.
Comparison of serum metabolic profiles
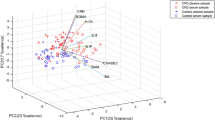
A principal component analysis (PCA) model was used to assess the serum metabolite profiles of the subjects. The results revealed distinct separation trends among the DKD, DM, and NC groups (P = 0.027), indicating significant differences in their overall serum metabolic profiles, as depicted in Fig. 1.
Building on the PCA model, we employed partial least squares discriminant analysis (PLS-DA) to delve deeper into the serum metabolic profiles of the three groups. This approach improved differentiation between the groups, highlighting substantial metabolic variations (P < 0.001), as illustrated in Fig. 2
Based on the PLS-DA model, an orthogonal partial least-squares discrimination analysis (OPLS-DA) model was further established for multi-dimensional analysis to preliminarily screen metabolites, contributing significantly to the differences in metabolic profiles among groups. Model reliability was validated via a 1000-time random permutation test, confirming robustness and significance. R2Y and Q2Y values were calculated for each permuted model, with results depicted in Fig. 3 illustrating clear separation trends between DKD and DM groups, DKD and NC groups, and among DKD subgroups. All models demonstrated Q2Y values > 0.2 and Y-axis intercepts < 0 in permutation test curves (Figure S1), indicating non-overfitting and statistically significant differences in serum metabolic profiles among groups. These findings underscore distinct metabolic differences between DKD and DM groups, DKD and NC groups, and among DKD subgroups.
Orthogonal partial least-squares discrimination analysis (OPLS-DA) for normol controls, patients with DM and DKD using the 180 serum metabolites. (A) DKD and NC groups; (B) DKD and DM groups; (C) DKD middle and DKD early groups.Abbreviations: NC, normal controls; DM, diabetic mellitus; DKD, diabetic kidney disease.
Correlation analysis between different serum metabolites and clinical indicators.
We utilized multidimensional analysis (OPLS-DA model) with a VIP threshold > 1 and P < 0.01 to identify 60 significantly different metabolites between the DKD and NC groups. Among these, 28 metabolites were decreased in the DKD group, while 32 were increased, predominantly belonging to the carnitine class (Table S1). Correlation analysis revealed that 15 of the elevated serum metabolites in DKD showed a positive correlation with SCr levels, whereas 7 of the decreased metabolites showed a negative correlation (Figure S2). When comparing the DKD and DM groups, we identified 39 significantly different metabolites (VIP > 1, P < 0.01), mainly amino acids. Among these, 3 metabolites were decreased in DKD, while 36, including serum α-hydroxyisobutyric acid, were increased (Table S2). Analysis showed that 28 of the elevated metabolites in DKD had a negative correlation with eGFR, and 29 showed a positive correlation with UACR, whereas 1 of the decreased metabolites correlated positively with eGFR and 2 negatively with UACR (Fig. 4). Further analysis within DKD subgroups revealed 23 significantly different metabolites between the DKD middle and DKD early groups (VIP > 1, P < 0.01), predominantly amino acids (Table S3). Among these, 5 metabolites were decreased in the DKD middle group, while 18 were increased. Analysis indicated that 13 of the elevated metabolites in the DKD middle group negatively correlated with eGFR, and 4 positively correlated with UACR, whereas 5 of the decreased metabolites showed a positive correlation with eGFR and 5 a negative correlation with UACR (Figure S3).
Interactions of disease-related metabolites and host clinical parameters in patients with DKD and DM. Correlations were calculated with Spearman’s correlation analysis. ***P < 0.001,** P < 0.01,* P < 0.05. Abbreviations: DM, diabetic mellitus; DKD, diabetic kidney disease; UACR, urine albumin creatinine ratio; Cr, serum creatinine; BUN, blood urea nitrogen; UA, uric acid; FPG, fasting plasma glucose; PPG, postprandial plasma glucose; HbA1c, hemoglobin A1c; TG, triglyceride; Hb, hemoglobin; Alb, albumin; eGFR, estimated glomerular filtration rate.
Analysis of gut microbiota
The rarefaction curves of all three study groups showed a plateauing trend, indicating that the sequencing depth of each sample closely matched the expected level. This suggests the adequacy and reliability of the sequencing data volume for subsequent analyses (Figure S4).
Analysis of alpha diversity
We analyzed the microbial diversity of the three groups. Significant differences were observed in the Simpson, Chao1, and ACE indices among the groups (P < 0.05) for alpha diversity. Specifically, the Simpson index was significantly lower in the DKD group compared to the NC group (P < 0.05) (Fig. 5A).
Faecal microbiome variations in NCs, patients with DM and DKD. (A)Alpha diversity; (B)Principal coordinates analysis (PCoA) based on Bray Curtis; (C)Non-metric multidimensional scaling (NMDS) analysis analysis based on Weighted Unifrac; (D)Composition and relative abundance of three groups at the bacterial phylum level; (E)Composition and relative abundance of three groups at the bacterial genus level. ***P < 0.001, ** P < 0.01,* P < 0.05.
Comparing the DKD middle group to the DKD early group, the ACE, Chao1, and Shannon indices were significantly lower (P < 0.05, P < 0.05, P < 0.05), while no significant difference was observed in the Simpson index (P = 0.091) (Figure S5A).
Analysis of beta diversity
To characterize the overall microbial features of the three groups, beta diversity comparison was performed using PERMANOVA. Principal coordinates analysis (PCoA) based on Bray–Curtis distance revealed significant differences in overall bacterial community structure among the groups (PERMANOVA test, DKD vs DM vs NC: P = 0.001) (Fig. 5B). Adonis analysis indicated significant differences between the DKD and NC groups (F = 3.241, P = 0.001), but not between the DKD and DM groups (F = 1.311, P = 0.076). Non-metric Multidimensional Scaling (NMDS) analysis based on Weighted Unifrac distance also confirmed significant differences among the groups (Stress = 0.076) (Fig. 5C). Adonis analysis further supported significant differences between the DKD and NC groups (F = 8.112, P = 0.003), while no significant differences were observed between the DKD and DM groups (F = 1.797, P = 0.119).
PCoA indicated no significant difference between the DKD middle and DKD early groups (P = 0.133). However, NMDS analysis revealed significant differences (Stress = 0.050) (Figure S5C). Despite this, Adonis analysis did not show significant differences (F = 0.512, P = 0.642).
Taxonomic changes in microbial composition
Next, we analysed the microbial composition at different taxonomic levels. The microbial composition at the phylum and genus levels were shown in (Fig. 5D-E) (Figure S5D-E).
LEfSe analysis identified differentially abundant microbial features among NCs, DKD patients, and DM patients. Specifically, 24 species were differentially abundant between DKD patients and DM patients (Fig. 6A), 32 species between DKD patients and NCs (Fig. 6B), and 39 species between DKD middle patients and DKD early patients (Fig. 6C) (LDA value > 2, P < 0.05) (Table S (4–6)).
Linear discriminant analysis (LDA) effect size (LEfSe) bar plot. (A) DKD and DM groups; (B) DKD and NC groups; (C) DKD middle and DKD early groups. The LEfSe was used to identify the species that significantly differed between groups. The bacteria with LDA value > 2 and P < 0.05 were considered differentially abundant. Abbreviations: NC, normal controls; DM, diabetic mellitus; DKD, diabetic kidney disease; LDA, linear discriminant analysis.
Additionally, we identified functional alterations in the gut microbiota of DKD patients. Pathways such as “Valine, leucine, and isoleucine degradation”, “Biofilm formation Vibrio cholerae”, “Glyoxylate and dicarboxylate metabolism”, and “Tryptophan metabolism” were significantly enriched in DKD compared to NCs and DM patients (LDA > 2, P < 0.05) (Figure S6(A-B)). Further analysis within DKD subgroups revealed significant enrichment in pathways including "β Lactam resistance", “Folate biosynthesis”, and “Lipopolysaccharide biosynthesis” in the DKD middle group compared to the DKD early group (LDA > 2, P < 0.05) (Figure S6C).
Correlation analysis between gut microbiota and clinical indicators
The correlation analysis of the 24 differential gut microbiota between the DKD and DM groups and the clinical indicators of the patients revealed significant associations. Specifically, g_Rikenella showed a positive correlation with UACR (r = 0.44, P < 0.001) and a negative correlation with eGFR (r = -0.41, P < 0.01). Conversely, g_Prevotella, g_Agathobacter, and g_Haemophilus exhibited a strong negative correlation with UACR (r = -0.33, P < 0.01; r = -0.36, P < 0.01; r = -0.33, P < 0.01) and a positive correlation with eGFR (r = 0.41, P < 0.01; r = 0.35, P < 0.01; r = 0.34, P < 0.01). Additionally, g_T34, f_Pasteurellaceae, o_Pasteurellales, o_Oscillospirales, and f_Ruminococcaceae were positively correlated with eGFR (r = 0.30, P = 0.02; r = 0.28, P = 0.03; r = 0.28, P = 0.03; r = 0.32, P = 0.01; r = 0.30, P = 0.02) and negatively correlated with UACR (r = -0.31, P = 0.02; r = -0.29, P = 0.02; r = -0.27, P = 0.02; r = -0.34, P = 0.03; r = -0.36, P < 0.01) (Fig. 7).
Interactions of disease-related bacteria and host clinical parameters in patients with DKD and DM. Correlations were calculated with Spearman’s correlation analysis. ***P < 0.001,** P < 0.01,* P < 0.05. Abbreviations: DM, diabetic mellitus; DKD, diabetic kidney disease; UACR, urine albumin creatinine ratio; Cr, serum creatinine; BUN, blood urea nitrogen; UA, uric acid; FPG, fasting plasma glucose; PPG, postprandial plasma glucose; HbA1c, hemoglobin A1c; CRP, C-reactive protein; TG, triglyceride; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; Hb, hemoglobin; Alb, albumin; eGFR, estimated glomerular filtration rate.
Construction of disease prediction model using serum metabolites and gut microbiota
Screening and identification of predictive serum metabolic markers
The specific screening process is illustrated in Fig. 8 Using both univariate analysis (t-test or Mann–Whitney U test, P < 0.05) and multivariate analysis (OPLS-DA, VIP > 1, P < 0.05), we identified 10 common metabolites out of 180 serum metabolites. These 10 metabolites were subsequently subjected to the LASSO algorithm. The top three serum metabolites were selected based on the ranking of non-zero LASSO coefficients: Imidazolepropionic acid, Adipoylcarnitine, and 1-Methylhistidine.
Workflow chart of feature selection. For a total of 180 metabolites, univariate analyses (Wilcoxon’s rank-sum test) and OPLS-DA were employed for three clinical aims (aim 1: DKD vs. NC, aim 2: DKD vs. DM, aim 3: DKD middle vs. DKD early). Ten metabolites with p < 0.05 and VIP > 1 in all three clinical aims were selected and fed into least absolute shrinkage and selection operator (LASSO) for three aims. The overlap of top 3 LASSO non-zero coefficients was selected to yield the final panel three metabolites. “AND” means the intersection of two or more sets.
We opted for a logistic regression model and applied tenfold cross-validation (CV) to assess the classification performance of the model on the subject cohort. The evaluation metrics used were CV-area under the receiver operating characteristic curve (CV-AUROC) and CV-area under the precision-recall curve (CV-AUPR).
The disease prediction model using these three metabolites exhibited robust discriminatory capability for diagnosing patients with DKD from those with DM (AUROC = 0.9, AUPR = 0.883), distinguishing between patients with DKD and NCs (AUROC = 0.841, AUPR = 0.725), and differentiating DKD early patients from DKD middle patients (AUROC = 0.894, AUPR = 0.933) (Fig. 9 (A-C)).
Disease classification based on the signatures of metabolome and (or) microbiome. Logistic regression model composed of metabolites, bacteria and their combination were constructed to discriminate patients with DKD from patients with DM (A) and from NCs (B). Logistic regression model composed of metabolites, bacteria and their combination in discriminating DKD middle patients from DKD early patients (C). Abbreviations: NC, normal controls; DM, diabetic mellitus; DKD, diabetic kidney disease; AUC, area under the curve.
Prediction of DKD and its stages based on differential gut microbiota
The logistic regression model evaluated the discriminatory power of differential bacterial genera between groups (DKD vs. DM, DKD vs. NC): g_Prevotella and g_Faecalibacterium (LDA ≥ 4, P < 0.05), and a significantly different bacterial genus between subgroups: g_Klebsiella (LDA ≥ 4, P < 0.05). The disease prediction model based on these three bacterial genera demonstrated strong discriminatory power to diagnose patients with DKD from patients with DM (AUROC = 0.69, AUPR = 0.772), to discriminate between patients with DKD and NCs (AUROC = 0.95, AUPR = 0.953), and to differentiate DKD early patients from DKD middle patients (AUROC = 0.759, AUPR = 0.837) (Fig. 9 (A-C)).
Prediction of DKD and its stages based on serum metabolites and bacterial genera
The disease prediction model, a logistic regression model, based on the selected three serum metabolites combined with three bacterial genera, exhibited excellent discriminatory ability to diagnose patients with DKD from patients with DM (AUROC = 0.939, AUPR = 0.940), discriminate between patients with DKD and NCs (AUROC = 0.976, AUPR = 0.973), and differentiate DKD early patients from DKD middle patients (AUROC = 1.000, AUPR = 1.000) (Fig. 9 (A-C)).
Correlation analysis between serum metabolites and gut microbiota
Pathway analysis of differential metabolites between DKD and DM groups
We conducted pathway analysis on differential metabolites between the DKD and DM groups (36 differential metabolites identified through both univariate and multivariate analyses: univariate analysis P < 0.05, and VIP > 1 and P < 0.05 in the OPLS-DA model for multivariate analysis). These metabolites were analyzed using MetaboAnalyst 5.0 (http://www.metaboanalyst.ca), referencing the KEGG database to generate pathway diagrams. Pathways with a P < 0.05 or an impact value ≥ 0.1 were considered significantly altered (Figure S7). Seven pathways met these criteria, including glycine, serine, and threonine metabolism; tryptophan metabolism; citrate cycle (TCA cycle); alanine, aspartate, and glutamate metabolism; phenylalanine metabolism; arginine and proline metabolism; and pentose phosphate pathway. These pathways play critical roles in the progression of DKD.
A related network (Fig. 10) illustrated alterations in seven metabolic pathways and associated serum metabolites in DKD patients. Compared to the DM group, DKD patients showed elevated levels of 5-aminolevulinic acid, pyruvic acid, and dimethylglycine in the glycine, serine, and threonine metabolism pathway, alongside lower tryptophan levels. The tryptophan metabolism pathway exhibited enrichment with indolelactic acid and kynurenine. The citrate cycle was enriched with pyruvic acid and isocitric acid. The phenylalanine metabolism pathway demonstrated higher levels of phenylacetylglycine, hippuric acid, and pyruvic acid. The alanine, aspartate, and glutamate metabolism pathway showed enrichment with N-acetylaspartic acid and pyruvic acid. The arginine and proline metabolism pathway was enriched with citrulline and 4-hydroxyproline. Additionally, the pentose phosphate pathway exhibited increased levels of gluconolactone and pyruvic acid.
Schematic diagram of phenylalanine metabolism, tryptophan metabolism, glycine, serine and threonine metabolism, citrate cycle (TCA cycle), alanine, aspartate and glutamate metabolism, arginine and proline metabolism, pentose phosphate pathway, and their relevant differential metabolite alterations during DKD occuration. The upregulated metabolites in the DKD group were labeled with red and downregulated metabolites in the DKD group with blue.
Integrating multi-omics analysis (correlation analysis between differential microbiota and metabolites in DKD and DM groups)
We further explored the association between gut microbiota and serum metabolites in relation to DKD using Spearman correlation coefficients. We selected 24 differentially abundant microbiota at various taxonomic levels between the DKD and DM groups (LDA > 2, P < 0.05) and identified 39 significantly different metabolites between the two groups using the OPLS-DA model (VIP > 1, P < 0.01). A Spearman correlation analysis was performed between these differential gut microbiota and serum metabolites, visualized as a correlation coefficient matrix heatmap (Figure S8).
Based on impact values and p values from KEGG analysis, glycine, serine, and threonine metabolism, tryptophan metabolism, citrate cycle, and phenylalanine metabolism emerged as pivotal pathways in the progression from DM to DKD. A metabolic network (Fig. 11) was created using enriched serum metabolites within these pathways and 11 genus-level microbiota selected from the 24 differentially abundant microbiota.
Integrating multiomics analysis. Metabolic pathway map of glycine, serine and threonine metabolism, tryptophan metabolism and phenylalanine metabolism. The metabolites expressed in red and blue were statistically different between DKD and DM groups. Red metabolites express upregulated in the DKD group; blue metabolites express downregulated in the DKD group. Other related metabolic pathways were expressed in solid wire frame.
Three metabolites (5-aminolevulinic acid, pyruvate, and dimethylglycine) were found at higher concentrations in the serum of the DKD group within the glycine, serine, and threonine metabolism pathway, while tryptophan levels were lower. Among the 11 differentially abundant microbiota at the genus level, g_Rikenella showed a positive correlation with 5-aminolevulinic acid (r = 0.29, P = 0.02), g_Eubacterium_hallii_group exhibited a positive correlation with pyruvate (r = 0.33, P = 0.01), and both g_Muribaculaceae and g_Rikenella demonstrated a positive correlation with dimethylglycine (r = 0.32, P = 0.01; r = 0.28, P = 0.03). Conversely, g_Agathobacter, g_Faecalibacterium, and g_Haemophilus were positively correlated with tryptophan (r = 0.30, P = 0.02; r = 0.33, P < 0.01; r = 0.26, P = 0.04).
Two metabolites enriched in the tryptophan metabolism pathway, indole lactic acid and kynurenine, were found at higher levels in the DKD group, while tryptophan levels were lower. Among the 11 differentially abundant microbiota at the genus level, g_Eubacterium_hallii_group exhibited a positive correlation with pyruvate (r = 0.33, P = 0.01).
Three metabolites enriched in the phenylalanine metabolism (phenylacetyglutamine, hippuric acid, and pyruvate) were found at higher levels in the DKD group. Among the 11 differentially abundant microbiota at the genus level, g_ Parabacteroides, g_ Eubacterium_hallii_group, and g_Muribaculacee exhibited a positive correlation with hippuric acid (r = 0.46, P < 0.01; r = 0.35, P = 0.02; r = 0.33, P = 0.03), while g_Romboutsia showed a negative correlation (r = -0.35, P = 0.02). Additionally, g_Muribaculaceae, g_Rikenella, g_Parabacteroides, and g_Eubacterium_hallii_group were positively correlated with phenylacetylglutamine (r = 0.36, P < 0.01; r = 0.37, P < 0.01; r = 0.33, P = 0.01; r = 0.27, P = 0.03), whereas g_Faecalibacterium and g_Haemophilus demonstrated a negative correlation (r = -0.30, P = 0.02; r = -0.33, P = 0.01).
Discussion
The global incidence of DKD is on the rise. Analyzing changes in serum metabolites and gut microbiota in DKD patients, and identifying markers to accurately predict and assess disease severity, is crucial for treatment decisions and long-term monitoring. Our study integrates serum metabolomics with high-throughput sequencing of gut microbiota to develop a logistic regression prediction model for DKD. This diagnostic model significantly enhances discrimination compared to using either metabolites or microbiota alone. The "3 + 3" model effectively distinguishes DKD patients from NCs, DM patients, and differentiates between DKD patients at various stages simultaneously.
Through a multi-omics integration approach, we elucidated the connection between gut microbiota and serum metabolites, highlighting their role in the progression of DKD. Our findings underscore the significance of g_Rikenella, g_Muribaculaceae, g_Agathobacter, g_Parabacteroides, and g_Eubacterium_hallii_group in influencing phenylalanine, tryptophan, glycine, serine, and threonine metabolism pathways in DKD progression.
Explorations of diagnostic biomarkers in patients with DKD
DKD and serum metabolites
Our study reinforces the established correlations between serum metabolites and DKD, previously demonstrated in both DKD patients and animal models. Specifically, we identified significant changes in serum metabolites among DKD, NC, and DM groups, primarily involving carnitine-related and amino acid-related metabolites.
The correlation of fatty acids and their metabolic products, including carnitines, with DKD progression has been well-established in previous studies. Renal tissue studies in DKD patients have shown significant lipid deposition, exacerbating proteinuria and glomerulosclerosis. Changes in lipid metabolites such as carnitine and its derivatives have been identified as potential biomarkers for DKD12. Carnitine and its derivatives play a crucial role in beta-oxidation, and early mitochondrial dysfunction due to hyperglycemia is a key factor in DKD progression13. Therefore, alterations in carnitine levels may signify the onset of DKD. Our study uniquely identified adipoylcarnitine as positively correlated with DKD progression, serving as a novel indicator not previously reported. Sirolli14 analyzed plasma metabolites in DKD patients undergoing hemodialysis and found increased levels of short-chain and medium-chain acylcarnitines, alongside decreased propionylcarnitine, suggesting these as biomarkers for advanced DKD. Additionally, Hirayama15 observed elevated serum levels of γ-butyrobetaine (a precursor of L-carnitine) in DKD patients, correlating with renal hypoplasia and albuminuria occurrence.
Different types of amino acids and their derivatives have been established as potential biomarkers for DKD progression12. Niewczas16 reported higher serum levels of phenylacetylglutamine and p-cresol sulfate associated with increased ESRD risk in T2DM patients, as these uremic toxins derive from gut microbiota amino acid metabolism, common in CKD plasma17. High levels of these metabolites also correlate with DKD18. Zhang19 analyzed serum metabolites from 44 diabetic patients, noting associations between 4-hydroxy-L-proline and 6-aminocaproic acid (proline and lysine derivatives, respectively) with early DKD. Chou20 observed that plasma metabolites of tryptophan metabolism in T2DM patients positively correlated with albuminuria levels, with low plasma tryptophan correlating with rapid eGFR decline, consistent with our findings. Pena21 analyzed plasma from 90 T2DM patients, finding lower histidine levels in the albuminuria group versus the normal group. Histidine, known for its physiological benefits like scavenging radicals and anti-inflammatory effects, also supports CKD patients’ antioxidant capacity. In our study, 1-Methylhistidine showed a positive correlation with deteriorating renal function in DKD patients, contradicting previous findings22. This discrepancy may be attributed to compensatory increases, potentially serving an anti-inflammatory and antioxidant role.
Under healthy conditions, histidine undergoes metabolism primarily by gut microbiota to produce imidazole acetic acid methyl ester, imidazole acetate, glutamic acid, and cis-uric acid ester. However, in T2DM, dysbiosis of gut microbiota leads to the production of imidazole propionic acid (IMP), a common uremic toxin derived from histidine metabolism. Our study reveals a positive correlation between serum IMP levels and the progression of DKD, highlighting IMP as a potent indicator for predicting DKD progression. IMP, arising from histidine metabolism, has been implicated in impairing glucose tolerance by inhibiting insulin signaling pathways and activating inflammatory and oxidative stress pathways, thereby contributing to poor glycemic control and subsequent DKD progression. Furthermore, IMP exacerbates DKD progression through activation of the Toll-like receptor-4 (TLR4) pathway. Recent studies have confirmed that IMP activates TLR4 in renal interstitial cells of db/db mice23. Therefore, maintaining gut microbiota balance and reducing the release of harmful metabolites in the intestine are crucial for diabetic patients to mitigate DKD progression.
DKD and gut microbiota
Dysbiosis in gut microbiota increases the risk of DKD. Compared to the DM and NC groups, the relative abundances of g_Prevotella and g_Faecalibacterium were reduced in the DKD group. Additionally, the relative abundances of g_Prevotella, g_Klebsiella, and g_Faecalibacterium were lower in the DKD middle group compared to the DKD early group.
G_Prevotella, a Gram-negative bacterium, plays a crucial role in decomposing proteins and carbohydrates in the intestine. Previous studies have demonstrated an association between the decreased relative abundance of g_Prevotella and an increased risk of DKD7, consistent with our findings. G_Faecalibacterium, a Gram-negative bacterium from the Clostridiaceae family and Firmicutes phylum, with Faecalibacterium prausnitzii as its representative species, produces SCFAs in the intestine to maintain intestinal homeostasis and exert anti-inflammatory effects. A reduction in butyrate-producing bacteria like F. prausnitzii is associated with various diseases, including inflammatory bowel disease and Alzheimer’s disease, both of which are often accompanied by chronic inflammation, a key feature of DKD. Our findings suggest that changes in the relative abundance of g_Faecalibacterium correlate with the progression of DKD. Although direct evidence of a causal relationship between g_Faecalibacterium and DKD is lacking, the association merits further research given its vital role in intestinal health and its correlation with other chronic inflammatory diseases. G_Klebsiella, a genus of Gram-negative bacteria in the Enterobacteriaceae family, has been shown to cause various infections, including kidney infections. Certain strains of g_Klebsiella produce specific enzymes and toxins that damage kidney cells, exacerbating kidney diseases. Preventing and treating g_Klebsiella infections is crucial for patients with kidney diseases. However, no other studies have demonstrated a correlation between g_Klebsiella and DKD. In our study, the relative abundance of g_Klebsiella in the feces of DKD patients was reduced compared to DM patients and NCs. The abundance decreased further in middle-stage DKD patients compared to early-stage DKD patients. This reduction may be related to gut microbiota dysbiosis and intestinal barrier damage in DKD patients, leading to intestinal infections that trigger systemic inflammatory responses and cause kidney damage. Additionally, patients with kidney diseases such as DKD are more susceptible to intestinal infections due to factors like decreased immunity and metabolic disorders, resulting in gut microbiota dysbiosis.
Diagnostic biomarkers of DKD
Metabolomics and sequencing have gradually emerged as non-invasive tools for the early diagnosis and prognosis of DKD, with various diagnostic markers identified to predict DKD occurrence12,24. Researchers have utilized liquid chromatography-mass spectrometry (LC–MS) to detect metabolites derived from gut microbiota in plasma, such as phenyl sulfate. This metabolite is associated with UACR in patients with T2DM and can predict an increase in UACR within two years in patients with microalbuminuria. Its predictive effectiveness surpasses that of soluble urokinase-type plasminogen activator receptor (suPAR)25. Additionally, the detection of g_Escherichia-Shigella and g_Prevotella in feces has been shown to distinguish DKD from diabetes. Previous studies have demonstrated that, compared to control groups, DKD patients exhibit an increased relative abundance of p_Verrucomicrobia, p_Proteobacteria, and p_Fusobacteria, along with elevated levels of endotoxin and inflammatory cytokines26. Animal experiments have revealed that in early-stage T2DM mice, the relative abundance of p_Firmicutes and p_Bacteroidetes increases, while the relative abundance of g_Bacteroides, g_Alistipes, and g_Parabacteroides decreases, compared to NCs27. Therefore, specific serum metabolites and gut microbiota profiles may serve as biomarkers for the early prediction and prognosis of DKD.
We identified a set of metabolites, including one carnitine metabolite, one amino acid metabolite, and one imidazole metabolite, all of which are negatively correlated with eGFR levels and positively correlated with UACR levels. Additionally, our study identified a set of gut microbiota markers with differential abundance between groups (DKD and DM, DKD and NCs): G_Prevotella, g_Faecalibacterium, and a significantly different genus between DKD subgroups (early and middle stages), g_Klebsiella. These three genera exhibit strong potential as biomarkers for disease discrimination.
Interaction between gut microbiota and serum metabolites in DKD progression
Metabolites act as intermediaries, reflecting the relationship between gut microbiota and disease. Given the intimate connection between gut microbiota and serum metabolites, a multi-omics integration analysis was employed to elucidate the relationship between gut microbiota and the progression of DKD. The results highlighted the potential roles of specific gut microbiota, including g_Muribaculaceae, g_Rikenella, g_Agathobacter, g_Eubacterium_hallii group, g_Faecalibacterium, g_Haemophilus, g_Parabacteroides, and g_Romboutsia, in DKD progression and their involvement in related amino acid metabolism.
In our study, we identified 11 distinct gut microbiota and 39 distinct serum metabolites between the DKD and DM groups. Three microbial-related metabolites from the phenylalanine metabolism pathway (hippuric acid [HA], phenylacetylglutamine, and pyruvic acid), two from the tryptophan metabolism pathway (indolelactic acid [ILA] and kynurenine), and three from the glycine, serine, and threonine metabolism pathway (5-aminolevulinic acid, dimethylglycine, and pyruvic acid) were positively correlated with DKD progression. In contrast, tryptophan was negatively correlated with DKD progression. Gut microbiota such as g_Parabacteroides, g_Eubacterium_hallii_group, and g_Muribaculaceae were positively correlated with high levels of HA and phenylacetylglutamine and were associated with DKD progression. Additionally, g_Muribaculaceae was positively correlated with ILA. G_Agathobacter, g_Faecalibacterium, and g_Haemophilus were positively correlated with tryptophan and negatively correlated with DKD progression. G_Rikenella was positively correlated with 5-Aminolevulinic acid and DKD progression. Both g_Muribaculaceae and g_Rikenella were positively correlated with dimethylglycine and DKD progression. These findings suggest that four metabolic pathways—glycine, serine, and threonine metabolism, tryptophan metabolism, citrate cycle, and phenylalanine metabolism—are strongly associated with the development of DKD. Specific gut microbiota may influence DKD progression through their impact on amino acid metabolism.
In this study, microbial-related serum metabolites [HA and phenylacetylglutamine] from the phenylalanine metabolism pathway were positively correlated with the deterioration of renal function in DKD patients. Abnormal serum levels of HA and phenylacetylglutamine have been previously reported in DKD patients8. Previous studies indicate that HA is involved in abnormal glucose and lipid metabolism and insulin resistance28,29,30. Elevated plasma HA levels and reduced urine HA levels are associated with an increased risk of diabetic CKD progression8. Serum phenylacetylglutamine levels serve as an early marker of renal function decline in diabetic patients31, aligning with our findings. Furthermore, our study showed that g_Parabacteroides, g_Eubacterium_hallii_group, and g_Muribaculaceae were positively correlated with serum HA and phenylacetylglutamine levels in both DM and DKD patients, suggesting a potential role for these microbiota in HA synthesis and phenylalanine metabolism. Previous research has linked f_Ruminococcaceae (a major SCFA-producing bacterium) with serum HA levels; however, our study did not find a positive correlation between f_Ruminococcaceae and serum HA levels. Although f_Ruminococcaceae was positively correlated with blood glucose, its relative abundance decreased in DKD patients compared to NCs and DM patients. Additionally, a positive correlation was observed between the relative abundance of f_Ruminococcaceae and renal function indicators in DKD and DM patients, contradicting previous reports32. In conclusion, the phenylalanine metabolism pathway is considered the most relevant pathway associated with DKD development in our study.
Consistent with previous studies33, ILA and kynurenine from the tryptophan metabolism pathway were positively correlated with the deterioration of renal function, while tryptophan showed the opposite trend. Serum metabolites from the tryptophan pathway are strongly associated with renal function and disease status. Research supports the idea that lower eGFR leads to higher serum levels of tryptophan pathway metabolites, including ILA and kynurenine34. Indoles and their derivatives, such as indoxyl sulfate and ILA, act as enteric-derived protein-bound uremic toxins with pro-oxidative and pro-inflammatory effects that can stimulate glomerular sclerosis and renal interstitial fibrosis35. However, the direct effects of ILA on glomeruli and renal interstitium have not been extensively studied. Tryptophan can be converted directly or indirectly into several indole-related compounds through microbial activities. In this study, we observed a positive correlation between the g_ Muribaculacee and serum ILA levels, indicating its potential significant role in ILA synthesis and tryptophan metabolism. A previous study has demonstrated that polysaccharides from Cortex Moutan can improve renal function in DKD rats by reconstituting the gut microbiota, specifically increasing relative abundance of Muribaculaceae and Lactobacillus, enhancing intestinal barrier function, reducing serum proinflammatory mediators, and increasing SCFAs levels, thereby alleviating DKD in rats36. However, the relationship between g_Muribaculacee and ILA synthesis, as well as their role in DKD progression, remains unexplored. We also noted a negative correlation between g_Agathobacter and serum levels of ILA, creatinine, and albuminuria, but a positive correlation with serum levels of tryptophan. This suggests that g_Agathobacter may influence DKD progression through the tryptophan metabolism pathway. Previous studies have indicated that the relative abundance of g_Agathobacter in DKD patients is significantly lower compared to diabetic patients and it positively correlates with eGFR while negatively correlating with microalbumin urinary, 24-h urine protein, and SCr37. Agathobacter, a Gram-positive anaerobe, produces SCFAs such as butyric acid and acetic acid as its main metabolites38. When the intestinal barrier is compromised, harmful bacteria and their metabolites may breach the barrier, triggering an immune system response39. The relationship between g_Agathobacter and ILA, tryptophan synthesis, and their role in DKD progression requires further investigation. In summary, our findings suggest that the tryptophan metabolism pathway may be intricately associated with the progression of DKD.
In our study, microbial-related serum metabolites [5-aminolevulinic acid and dimethylglycine] from the glycine, serine, and threonine metabolism pathways were positively correlated with the deterioration of renal function in DKD patients, a novel finding not previously reported. 5-aminolevulinic acid is implicated in endoplasmic reticulum stress in kidney cells40. Studies have demonstrated that 5-aminolevulinic acid may mitigate endoplasmic reticulum stress through redox pathways, potentially offering renal protective effects against lipotoxicity40. Elevated serum levels of 5-aminolevulinic acid observed in our study may indicate a compensatory mechanism, although the specific regulatory pathways warrant further investigation. Dimethylglycine has been identified as a potential biomarker for decreased renal function in CKD patients41, consistent with our findings. Furthermore, we observed a positive correlation between g_Rikenella and serum levels of 5-aminolevulinic acid and dimethylglycine in both DM and DKD patients, suggesting its significant role in glycine, serine, and threonine metabolism and the progression of DKD, a novel finding not previously reported. Current research suggests g_Rikenella as a potentially important intestinal probiotic, with implications for disease prevention and treatment. However, its impact on human health appears multifaceted, contingent upon specific disease contexts. Previous studies42,43 have reported decreased relative abundance of g_Rikenella in DKD animal models, negatively correlating with urine protein levels, inconsistent with our findings. Discrepancies could stem from dietary differences, dynamic gut microbiota changes, sample size limitations, or compensatory physiological adaptations. Additionally, our study revealed higher serum levels of α-hydroxysobutyric acid (a gut microbiota-derived metabolite via valine metabolism, linked to glucose metabolism and inflammatory damage) in DKD patients. It positively correlated with SCr and UACR in both DKD and DM patients, alongside the relative abundance of g_Rikenella. This underscores the complex interplay of gut microbiota and metabolic pathways in DKD progression. Further research with larger sample sizes is warranted to elucidate g_Rikenella's role in DKD. In summary, our findings suggest that glycine, serine, and threonine metabolism pathways are relevant to DKD development. These insights provide a basis for exploring microbiota-related metabolic dysfunctions in DKD progression and investigating new therapeutic strategies targeting protein-bound uremic toxins such as HA and ILA in DKD.
Limitations
Nevertheless, several limitations warrant consideration. Firstly, the study lacked comprehensive dietary intake records, precluding the interpretation of long-term dietary influences on gut microbiota and metabolic profiles. Secondly, renal function deterioration can potentially induce toxin accumulation, leading to partial dysbiosis of gut microbiota. Thus, further investigation is required to determine whether the identified markers are specific to early-stage DKD or result from microbiota and metabolic disruptions due to renal dysfunction, necessitating validation through in vivo experiments. Thirdly, the relatively small sample size and homogeneous characteristics of participants from Zhejiang Province may limit generalizability. Fourthly, the relationship between these biomarkers and DKD needs to be further confirmed due to the limited duration of the study. This study should serve as the foundation and bridge for our subsequent prognostic follow-up, population-based targeted metabolite cohort studies, and in vivo animal validation studies. Future studies should involve larger, multi-regional, and multi-center cohorts. Lastly, the analysis of gut microbiota relied on 16S rRNA sequencing; employing metagenomic sequencing could provide additional bacterial insights.
Methods
Subject recruitment
A total of 90 subjects were recruited from September 2021 to January 2023 at Ningbo No.2 Hospital (Ningbo, China). The subjects were divided into three groups: normal controls (NCs) (n = 30), DKD patients (n = 30), and T2DM patients (n = 30). DKD patients were further classified into early DKD (eGFR ≥ 90 ml/min/1.73m2) and middle DKD (eGFR < 90 ml/min/1.73m2). All patients met the 2017 American Diabetes Association (ADA) criteria for T2DM44. DKD was diagnosed in T2DM patients if they had either (1) macroalbuminuria or (2) microalbuminuria with diabetic retinopathy45. Subjects with any of the following conditions were excluded: primary or secondary kidney diseases unrelated to diabetes, systemic illnesses, severe gastrointestinal, hepatic, cerebrovascular, or cardiovascular conditions, pregnancy, history of malignant neoplasms, prior immunomodulator therapy, antibiotic or probiotic use within the past 3 months, or history of gastrointestinal surgery. All procedures adhered to the principles of the Declaration of Helsinki and were approved by the Human Ethics Committee of Ningbo No.2 Hospital (YJ-NBEY-KY-2023–013-01). Written informed consent was obtained from each participant at enrollment.
Clinical parameter measurements
Demographic and clinical data, including age, gender, body mass index (BMI) were collected using standardized procedures. In-depth interviews assessed smoking history, diabetes duration, and medication use (metformin or SGLT-2 inhibitors). Fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) levels were measured from morning and post-meal blood samples. Laboratory tests included complete blood count, haemoglobin A1c (HbA1c), metabolic panel (serum albumin, C-reactive protein (CRP) , lipid profile, uric acid (UA) , serum creatinine (SCr) , and blood urea nitrogen (BUN) ), urinary albumin creatinine ratio (UACR) , and eGFR calculated using the CKD-EPIScr formula. Subjects in the NC group underwent tests including complete blood count, metabolic panel, urinalysis, stool test, HBsAg (hepatitis B surface antigen) and anti-HCV (hepatitis C antibody). Procedures adhered to scientific and clinical guidelines to ensure reliable data.
Sample collection
Blood samples: All participants fasted overnight before blood collection. Approximately 5 ml of blood were drawn from the antecubital vein of each participant between 8 and 11 am. Samples were promptly transported to the laboratory, allowed to clot at room temperature for 30 min, and then centrifuged at 3500 rpm for 5 min at 4℃. Serum samples were meticulously separated, aliquoted, and stored in a -80℃ freezer until analysis.
Fecal samples: Fresh fecal matter was collected using sterile, airtight containers. Approximately 100 mg of feces were obtained from the center of the sample using a 1 mL filtered pipette tip, with this process repeated twice per participant. The samples, including the pipette tips, were then placed in 15 mL centrifuge tubes, ensuring the pipette tips were oriented with the round end downwards and the pointed end upwards. Immediately after collection, the fecal samples were chilled in an ice box and transported to the laboratory for aliquoting. Once aliquoted, the samples were stored at -80℃ until further analysis. The entire process, from collection to aliquoting, was completed within 30 min.
Metabolomic analysis of serum samples
Samples were analyzed at Metabo-Profile Biotechnology (Shanghai, China) and stored directly at -80°C until analysis. Untargeted metabolomic analysis was conducted using an ultra-performance liquid chromatography coupled with tandem mass spectrometry (UPLC-MS/MS) system (ACQUITY UPLC-Xevo TQ-S, Waters Corp., Milford, MA, USA). Quality control (QC) samples, created by combining equal amounts of each sample, were injected at regular intervals. Raw data files were acquired using UPLC-MS/MS. Peak integration, calibration, and quantitation for each metabolite were performed using MassLynx software (v4.1, Waters, Milford, MA, USA). Metabolites with a relative SD of > 30% across QC samples or present in < 80% of samples in any group were excluded from further analysis. Missing values were imputed with minimum values, and the abundance data were log2-transformed.
16S rRNA amplicon sequencing
DNA extraction and amplification, library sequencing and data processing were conducted by OEbiotech (Shanghai, China) Co., Ltd.
DNA extraction and amplification
Faecal samples were stored in a -80℃ freezer. Faecal DNA was extracted using the MagPure Soil DNA LQ Kit (Magan) following the manufacturer’s protocol, and its concentration and integrity were measured with the NanoDrop 2000 (Thermo Fisher Scientific, USA) and agarose gel electrophoresis. Extracted DNA was stored at -20℃. It was used as a template for PCR amplification of bacterial 16S rRNA genes with barcoded primers and Takara Ex Taq (Takara). For bacterial diversity analysis, the V3-V4 regions of the 16S rRNA genes were amplified with universal primers 343F (5’-TACGGRAGGCAGCAG-3’) and 798R (5’-AGGGTATCTAATCCT-3’)46.
Library construction and sequencing
The amplicon quality was visualized using agarose gel electrophoresis. The PCR products were purified with AMPure XP beads (Agencourt) and amplified in another round of PCR. After purification with AMPure XP beads again, the final amplicon was quantified using the Qubit dsDNA Assay Kit (Thermo Fisher Scientific, USA). The concentrations were adjusted for sequencing, which was performed on an Illumina NovaSeq 6000 with 250 bp paired-end reads (Illumina Inc., San Diego, CA; OE Biotech Company, Shanghai, China).
Bioinformatics analysis
Raw sequencing data were in FASTQ format. Paired-end reads were preprocessed using Cutadapt to detect and remove adapters. After trimming, the reads were filtered for low-quality sequences, denoised, merged, and chimeric reads were removed using DADA2 with the default parameters of QIIME2 (2020.11)47,48. The software then output representative reads and the amplicon sequence variant (ASV) abundance table. The representative read of each ASV was selected using the QIIME2 package. All representative reads were annotated and compared against the Silva database (Version 138) using q2-feature-classifier with default parameters.
QIIME2 software was used for alpha and beta diversity analysis. Microbial diversity in the samples was estimated using alpha diversity metrics, including the Chao1 and Shannon indices49,50. The ACE and Chao1 indices reflect microbial richness, while the Shannon and Simpson indices represent microbial diversity, influenced by both richness and evenness. The unweighted UniFrac distance matrix, performed using the R package, was used for unweighted UniFrac Principal coordinates analysis (PCoA) to estimate beta diversity. The R package was also used to analyze significant differences between groups using ANOVA, Kruskal–Wallis, T-test, and Wilcoxon statistical tests. The linear discriminant analysis effect size (LEfSe) method was used to compare taxonomy abundance spectra.
Statistics
All statistical analyses were performed using SPSS Statistics 26.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 7.0. A P value < 0.05 was considered statistically significant. Results were expressed as means with standard deviation (SD) for normally distributed continuous variables, median values (interquartile ranges) for non-normally distributed continuous variables, and frequencies and percentages for categorical variables. ANOVA or Student’s t-test was used for comparisons of normally distributed continuous variables. The Mann–Whitney U-test or Kruskal–Wallis test was used for non-normally distributed continuous variables. For categorical variables, the chi-square test was used.
Serum metabolite marker selection was statistically analyzed using univariate analysis, orthogonal partial least squares discriminant analysis (OPLS-DA), and the least absolute shrinkage and selection operator (LASSO). Logistic regression assessed their predictive efficacy in DKD progression via the receiver operating characteristic curve (ROC) analysis. Enrichment analysis of differential metabolites was conducted using MetaboAnalyst 5.0(http://www.metaboanalyst.ca), presenting results in a bubble chart of enriched KEGG pathways(Kyoto Encyclopedia of Genes and Genomes, https://www.kegg.jp). For the pathway diagrams, we referred to the comprehensive KEGG database51,52,53. Statistical significance was defined based on a P value < 0.05 or a pathway impact value ≥ 0.1, indicating significant pathway alterations.
To characterize the gut microbiota in patients with DKD, rarefaction curves and rank-abundance curves ensured adequate sample size and sequencing depth. Alpha diversity assessed community richness and diversity, while beta diversity measured differences in ASV composition between samples. Linear discriminant analysis effect size (LEfSe) identified taxa with significantly different relative abundances, with P values corrected using the Benjamini and Hochberg false discovery rate (FDR). Taxa with linear discriminant analysis (LDA) values > 2.0 and P < 0.05 were considered differentially abundant. Additionally, functional gene analysis of distinct species was conducted using the KEGG database to analyze metabolic pathway differences.
Additionally, correlation and regression analyses were conducted on selected variables including serum metabolites, gut microbiota composition, and laboratory indicators to comprehensively understand their interrelationships. Differences in correlations between variables were analyzed using the psych package (version 1.9.12) and visualized with the corrplot software package using heatmaps.
Conclusions
Our study reveals significant alterations in serum metabolites and gut microbiota among patients with DKD. Untargeted metabolomics analysis identified three serum metabolic markers (imidazolepropionic acid, adipoylcarnitine, and 1-Methylhistidine). A predictive model incorporating these markers and three bacterial genera (Prevotella, Faecalibacterium, and Klebsiella) accurately distinguishes DKD patients from DM patients and NCs, and differentiates early and intermediate stages of DKD. The study delves into the intricate network of interactions among gut microbiota, microbiota-related serum metabolites, and clinical indicators in DKD patients, shedding new light on their roles enriched in four metabolic pathways implicated in DKD progression.
Data availability
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bio-informatics 2021) in National Genomics Data Center (Nucleic Acids Res 2022), China National Center for Bioinformation / Beijing Institute of Genomics, Chinese Academy of Sciences (GSA-Human: HRA008360) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa-human.
References
Gupta, S., Dominguez, M. & Golestaneh, L. Diabetic Kidney Disease: An Update. Med. Clin. North Am. 107, 689–705. https://doi.org/10.1016/j.mcna.2023.03.004 (2023).
Alicic, R. Z., Rooney, M. T. & Tuttle, K. R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 12, 2032–2045. https://doi.org/10.2215/CJN.11491116 (2017).
Gesualdo, L, Fiorentino, M, Conserva, F & Pontrelli, P. Should we enlarge the indication for kidney biopsy in patients with diabetes? The pro part. Clin Kidney J 17, sfad266, https://doi.org/10.1093/ckj/sfad266 (2024).
Klepacki, J, Klawitter, J, Klawitter, J, Karimpour-Fard, A, Thurman, J, Ingle, G et al. Amino acids in a targeted versus a non-targeted metabolomics LC-MS/MS assay. Are the results consistent? Clin Biochem 49, 955–961, https://doi.org/10.1016/j.clinbiochem.2016.06.002 (2016).
Li, Y. et al. Difference in Intestinal Flora and Characteristics of Plasma Metabonomics in Pneumoconiosis Patients. Metabolites https://doi.org/10.3390/metabo12100917 (2022).
Niewczas, M. A. et al. Circulating Modified Metabolites and a Risk of ESRD in Patients With Type 1 Diabetes and Chronic Kidney Disease. Diabetes Care 40, 383–390. https://doi.org/10.2337/dc16-0173 (2017).
Tao, S. et al. Understanding the gut-kidney axis among biopsy-proven diabetic nephropathy, type 2 diabetes mellitus and healthy controls: an analysis of the gut microbiota composition. Acta Diabetol. 56, 581–592. https://doi.org/10.1007/s00592-019-01316-7 (2019).
Zhang, Q. et al. The Role of Gut Microbiota and Microbiota-Related Serum Metabolites in the Progression of Diabetic Kidney Disease. Front Pharmacol. https://doi.org/10.3389/fphar.2021.757508 (2021).
Liu, J. R. et al. Gut microbiota-derived tryptophan metabolism mediates renal fibrosis by aryl hydrocarbon receptor signaling activation. Cell Mol. Life Sci. 78, 909–922. https://doi.org/10.1007/s00018-020-03645-1 (2021).
Feng, Y. L. et al. Microbiome-metabolomics reveals gut microbiota associated with glycine-conjugated metabolites and polyamine metabolism in chronic kidney disease. Cell Mol. Life Sci. 76, 4961–4978. https://doi.org/10.1007/s00018-019-03155-9 (2019).
Hu, J. et al. Distinct signatures of gut microbiota and metabolites in different types of diabetes: a population-based cross-sectional study. EClinicalMedicine https://doi.org/10.1016/j.eclinm.2023.102132 (2023).
Pereira, P. R. et al. Metabolomics as a tool for the early diagnosis and prognosis of diabetic kidney disease. Med. Res. Rev. 42, 1518–1544. https://doi.org/10.1002/med.21883 (2022).
Cleveland, K. H. & Schnellmann, R. G. Pharmacological Targeting of Mitochondria in Diabetic Kidney Disease. Pharmacol. Rev. 75, 250–262. https://doi.org/10.1124/pharmrev.122.000560 (2023).
Sirolli, V. et al. Toward personalized hemodialysis by low molecular weight amino-containing compounds: future perspective of patient metabolic fingerprint. Blood Transfus. 10(Suppl 2), s78-88. https://doi.org/10.2450/2012.012s (2012).
Hirayama, A. et al. Metabolic profiling reveals new serum biomarkers for differentiating diabetic nephropathy. Anal. Bioanal. Chem. 404, 3101–3109. https://doi.org/10.1007/s00216-012-6412-x (2012).
Niewczas, M. A. et al. Uremic solutes and risk of end-stage renal disease in type 2 diabetes: metabolomic study. Kidney Int. 85, 1214–1224. https://doi.org/10.1038/ki.2013.497 (2014).
Hung, S. C., Kuo, K. L., Wu, C. C. & Tarng, D. C. Indoxyl Sulfate: A Novel Cardiovascular Risk Factor in Chronic Kidney Disease. J. Am. Heart Assoc. https://doi.org/10.1161/jaha.116.005022 (2017).
Koppe, L., Fouque, D. & Soulage, C. O. Metabolic Abnormalities in Diabetes and Kidney Disease: Role of Uremic Toxins. Curr. Diab. Rep. 18, 97. https://doi.org/10.1007/s11892-018-1064-7 (2018).
Zhang, H. et al. Identification of Potential Serum Metabolic Biomarkers of Diabetic Kidney Disease: A Widely Targeted Metabolomics Study. J. Diabetes Res. 2020, 3049098. https://doi.org/10.1155/2020/3049098 (2020).
Chou, C. A., Lin, C. N., Chiu, D. T., Chen, I. W. & Chen, S. T. Tryptophan as a surrogate prognostic marker for diabetic nephropathy. J. Diabetes Investig. 9, 366–374. https://doi.org/10.1111/jdi.12707 (2018).
Pena, M. J. et al. Urine and plasma metabolites predict the development of diabetic nephropathy in individuals with Type 2 diabetes mellitus. Diabet. Med. 31, 1138–1147. https://doi.org/10.1111/dme.12447 (2014).
Yang, J., Liu, D. & Liu, Z. Integration of Metabolomics and Proteomics in Exploring the Endothelial Dysfunction Mechanism Induced by Serum Exosomes From Diabetic Retinopathy and Diabetic Nephropathy Patients. Front Endocrinol. (Lausanne) https://doi.org/10.3389/fendo.2022.830466 (2022).
Liu, L. et al. Metabolic Homeostasis of Amino Acids and Diabetic Kidney Disease. Nutrients https://doi.org/10.3390/nu15010184 (2022).
Jung, C. Y. & Yoo, T. H. Pathophysiologic Mechanisms and Potential Biomarkers in Diabetic Kidney Disease. Diabetes Metab. J. 46, 181–197. https://doi.org/10.4093/dmj.2021.0329 (2022).
Kikuchi, K. et al. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat. Commun. 10, 1835. https://doi.org/10.1038/s41467-019-09735-4 (2019).
Lin, J. R. et al. Gut microbiota and diabetic kidney diseases: Pathogenesis and therapeutic perspectives. World J. Diabetes 13, 308–318. https://doi.org/10.4239/wjd.v13.i4.308 (2022).
Cai, T. T. et al. Resveratrol Modulates the Gut Microbiota and Inflammation to Protect Against Diabetic Nephropathy in Mice. Front Pharmacol. 11, 1249. https://doi.org/10.3389/fphar.2020.01249 (2020).
Liu, D. et al. Fuzhuan Brick Tea Attenuates High-Fat Diet-Induced Obesity and Associated Metabolic Disorders by Shaping Gut Microbiota. J. Agric. Food Chem. 67, 13589–13604. https://doi.org/10.1021/acs.jafc.9b05833 (2019).
Yuan, X. et al. Gut Microbiota of Chinese Obese Children and Adolescents With and Without Insulin Resistance. Front Endocrinol. (Lausanne) https://doi.org/10.3389/fendo.2021.636272 (2021).
Liu, Y. K. et al. A salivary microbiome-based auxiliary diagnostic model for type 2 diabetes mellitus. Arch. Oral. Biol. https://doi.org/10.1016/j.archoralbio.2021.105118 (2021).
Barrios, C. et al. Gut-Microbiota-Metabolite Axis in Early Renal Function Decline. PLoS One https://doi.org/10.1371/journal.pone.0134311 (2015).
Lecamwasam, A. et al. Gut Microbiome Composition Remains Stable in Individuals with Diabetes-Related Early to Late Stage Chronic Kidney Disease. Biomedicines https://doi.org/10.3390/biomedicines9010019 (2020).
Hasegawa, S. & Inagi, R. Harnessing Metabolomics to Describe the Pathophysiology Underlying Progression in Diabetic Kidney Disease. Curr. Diab. Rep. 21, 21. https://doi.org/10.1007/s11892-021-01390-8 (2021).
Cheng, Y. et al. The relationship between blood metabolites of the tryptophan pathway and kidney function: a bidirectional Mendelian randomization analysis. Sci. Rep. 10, 12675. https://doi.org/10.1038/s41598-020-69559-x (2020).
Rysz, J. et al. The Impact of CKD on Uremic Toxins and Gut Microbiota. Toxins (Basel) https://doi.org/10.3390/toxins13040252 (2021).
Zhang, M. et al. Moutan Cortex polysaccharide ameliorates diabetic kidney disease via modulating gut microbiota dynamically in rats. Int. J. Biol. Macromol. 206, 849–860. https://doi.org/10.1016/j.ijbiomac.2022.03.077 (2022).
Zhang, L. et al. The Intestinal Microbiota Composition in Early and Late Stages of Diabetic Kidney Disease. Microbiol. Spectr. https://doi.org/10.1128/spectrum.00382-23 (2023).
Rosero, JA, Killer, J, Sechovcová, H, Mrázek, J, Benada, O, Fliegerová, K et al. Reclassification of Eubacterium rectale (Hauduroy et al. 1937) Prévot 1938 in a new genus Agathobacter gen. nov. as Agathobacter rectalis comb. nov., and description of Agathobacter ruminis sp. nov., isolated from the rumen contents of sheep and cows. Int. J. Syst. Evol. Microbiol. 66, 768–773, https://doi.org/10.1099/ijsem.0.000788 (2016).
Tamanai-Shacoori, Z. et al. Roseburia spp.: a marker of health?. Future Microbiol. 12, 157–170. https://doi.org/10.2217/fmb-2016-0130 (2017).
Hamada, S. et al. Five-Aminolevulinic Acid (5-ALA) Induces Heme Oxygenase-1 and Ameliorates Palmitic Acid-Induced Endoplasmic Reticulum Stress in Renal Tubules. Int. J. Mol. Sci. https://doi.org/10.3390/ijms241210151 (2023).
Benito, S. et al. LC-QTOF-MS-based targeted metabolomics of arginine-creatine metabolic pathway-related compounds in plasma: application to identify potential biomarkers in pediatric chronic kidney disease. Anal. Bioanal. Chem. 408, 747–760. https://doi.org/10.1007/s00216-015-9153-9 (2016).
Cai, H. D., Su, S. L., Guo, J. M. & Duan, J. A. Effect of Salviae Miltiorrhizae Radix et Rhizoma on diversity of intestinal flora in diabetic nephropathy rats. Zhongguo Zhong Yao Za Zhi 46, 426–435. https://doi.org/10.19540/j.cnki.cjcmm.20200723.402 (2021).
Hong, J. et al. Jiangtang Decoction Ameliorates Diabetic Kidney Disease Through the Modulation of the Gut Microbiota. Diabetes Metab. Syndr. Obes. 16, 3707–3725. https://doi.org/10.2147/dmso.S441457 (2023).
Standards of Medical Care in Diabetes-2017 Abridged for Primary Care Providers. Clin Diabetes 35, 5–26, https://doi.org/10.2337/cd16-0067 (2017).
KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am. J. Kidney Dis. https://doi.org/10.1053/j.ajkd.2006.12.005 (2007).
Nossa, C. W. et al. Design of 16S rRNA gene primers for 454 pyrosequencing of the human foregut microbiome. World J. Gastroenterol. 16, 4135–4144. https://doi.org/10.3748/wjg.v16.i33.4135 (2010).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857. https://doi.org/10.1038/s41587-019-0209-9 (2019).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. https://doi.org/10.1038/nmeth.3869 (2016).
Chao, A. & Bunge, J. Estimating the number of species in a stochastic abundance model. Biometrics 58, 531–539. https://doi.org/10.1111/j.0006-341x.2002.00531.x (2002).
Hill, T. C., Walsh, K. A., Harris, J. A. & Moffett, B. F. Using ecological diversity measures with bacterial communities. FEMS Microbiol. Ecol. 43, 1–11. https://doi.org/10.1111/j.1574-6941.2003.tb01040.x (2003).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951. https://doi.org/10.1002/pro.3715 (2019).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucl. Acids Res. 51, D587-d592. https://doi.org/10.1093/nar/gkac963 (2023).
Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucl. Acids Res. 28, 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Acknowledgements
We thank the staff and participants of the study for their important contributions and all the residents in Ningbo, Zhejiang who participated in this cohort study. At the same time, we are also grateful for the support of the Zhejiang Provincial Natural Science Foundation of China (LY20H05005), Medical Scientific Research Foundation of Zhejiang Province, China (2023KY283, 2024KY039), Project of Ningbo Leading Medical & Health Discipline(2022-S03), National Natural Science Foundation of China (82200782), and Xiangshan County Science and Technology Plan Project—Research Fund Project(2023C6014).
Author information
Authors and Affiliations
Contributions
Conceptualization, Y.H. and K.C. (Kedan Cai); methodology, Y.H. and X.N.; formal analysis, Y.H. and Q.C..; investigation, Y.Q. and K.C. (Kanan Chen); resources, G.Z. and M.Z.; data curation, Y.H. and N.X.; writing—original draft preparation, Y.H.; writ-ing—review and editing, Y.H. and K.C. (Kedan Cai); visualization, X.B. and J.W.; supervision, K.C. (Kedan Cai) and Y.M.; project administration, K.C. (Kedan Cai) and Q.L.; funding acquisition, K.C. (Kedan Cai) and Q.L. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Ningbo No. 2 Hospital (protocol code #PJ-NBEY-KY-2017–055-01 (6 December 2017)).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, Y., Ni, X., Chen, Q. et al. Predicting diabetic kidney disease with serum metabolomics and gut microbiota. Sci Rep 15, 12179 (2025). https://doi.org/10.1038/s41598-025-91281-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-91281-9