Abstract
Lepus yarkandensis is a desert hare of the Tarim Basin in western China, and it has strong adaptability to drought environments. Cytochrome P450s (CYP450s) are important metabolic enzymes, and their metabolites (19-hydroxyeicosatetraenoic acid (19-HETE), 20-HETE, and epoxyeicosatrienoic acids (EETs)) are important for regulating kidney water absorption and sodium excretion. This study aimed to investigate the expression of CYP450 enzymes in the kidneys of L. yarkandensis. Our results revealed significant upregulation of CYP2E1 in L. yarkandensis kidneys compared with Oryctolagus cuniculus kidneys, particularly on the basolateral plasma membrane of proximal convoluted tubule (PCT) and proximal straight tubule (PST) cells, as well as in the outer medullary collecting duct and in the beginning and middle sites of the inner medullary collecting duct. In contrast, the expression of CYP2C, CYP2J, CYP4A11, and CYP4F3 was notably downregulated in the kidneys of L. yarkandensis, primarily in the basolateral plasma membrane of PCT and PST cells. Additionally, the levels of 19-HETE are greater, whereas those of 20-HETE and EETs are lower in the kidneys of L. yarkandensis than in those of O. cuniculus. The altered expression patterns of these CYP450 enzymes suggest that L. yarkandensis has a greater capacity for renal water reabsorption.
Similar content being viewed by others
Introduction
Lepus yarkandensis (Yarkand hare) inhabits the arid environments of the Tarim Basin, southern Xinjiang Uygur Autonomous Region of northwestern China, around the edge of the Takla Makan Desert. The Tarim Basin has a highly arid continental climate. The air is hot and dry in the summer and cold in the winter, and the temperature difference between day and night is substantial. Recently, an ecological niche model revealed the evolutionary history of the Quaternary period and indicated that habitat drought, desertification, and climate change affect the number and population distribution of L. yarkandensis1. Compared with that in Oryctolagus cuniculus, the high expression of aquaporins (AQPs) in the kidneys of L. yarkandensispromotes water reabsorption in renal tubules2. The high expression of lung AQPs in L. yarkandensisaccelerates water transport between capillaries and alveolar tubes to supplement water loss on the alveolar surface3. Moreover, the high expression of AQPs in the digestive tract of L. yarkandensisimproves the reabsorption efficiency of water in faeces4. Positive selection of the mitochondrial genes ND1 and ND6 may contribute to the adaptation of L. yarkandensisto arid environments5.
Cytochrome P450s (CYP450s) constitute a prominent heme protein family in the monooxygenase class that has significantly evolved to increase resistance to environmental stresses67;. Free arachidonic acid (AA) metabolites produced through the CYP450 enzyme pathway include primarily 19-HETE, 20-HETE, and EETs8. The main metabolite of CYP2E1 is 19-HETE, which is a potent vasodilator of glomerular blood vessels910;. The metabolite formed by the CYP4A and CYP4F subgroups is 20-HETE, which is the major metabolite of AA in the renal microvasculature and is a potent vasoconstrictor that increases sodium urinary excretion8. EETs are epoxy fatty acids that regulate the dynamic balance of water and electrolytes in the kidney11. EETs, the primary metabolites of CYP2C and CYP2J, which are essential in the cardiovascular system, can also regulate the water and electrolyte balance in animals. The inhibition of cytochrome P450 oxidase 2 J (CYP2J2) significantly reduces choroidal neovascularization in mouse models, highlighting the pivotal role of CYP2J2 in the process of angiogenesis12. Moreover, EETs can reduce kidney injury and have protective effects on the kidney13. The CYP2J2 gene in Camelus bactrianusmay play a vital role in the development of the cardiovascular system1415;. Human CYP2J2 or CYP2C11 may enhance the expansion effect of renal microvessels on acetylcholine. Inhibition of HNF-1α binding to the CYP2E1 promoter has been shown to reduce CYP2E1 expression in an LPS-induced AKI mouse model and regulate kidney injury16. CYP4A and CYP4F produce 20-HETE in mouse renal tubules and vascular segments, inhibiting the reabsorption of renal tubular sodium and chloride ions17. According to the above studies, the CYP450 enzymes and their metabolites affect the physiological function of the kidney in blood vessels and renal tubules, and they are essential for maintaining the dynamic balance of systemic fluids and electrolytes.
CYP450s are crucial metabolic enzymes that play a vital role in the detoxification of exogenous substances, cellular metabolism, and the maintenance of homeostasis. The AA metabolites generated via CYP450s are essential for regulating renal water absorption and sodium excretion. Although the CYP450 enzymes involved in the AA pathway have been studied in Homo sapiens, Rattus norvegicus, and Camelus bactrianus, the expression levels of the CYP450 enzymes in L. yarkandensis have not been reported, and the role of these enzymes in the adaptation to extremely arid environments needs to be further explored. This study compared the expression of CYP450 enzymes in the kidneys of Lepus yarkandensis with that in the kidneys of Oryctolagus cuniculus and analysed the role of CYP450s in the adaptation of L. yarkandensis to arid and water-deficient desert habitats, providing a theoretical basis for the survival mechanism of desert animals in special environments.
Results
CYP2E1 expression in the kidneys of O. cuniculus and L. yarkandensis
CYP2E1 distribution in the kidneys of O. cuniculus and L. yarkandensis
We performed an IHC analysis of the distribution of CYP2E1 in the kidneys of O. cuniculus and L. yarkandensis. In the proximal convoluted tubules (PCTs), CYP2E1 was distributed on the basolateral plasma membrane of PCT cells (Fig. 1A and B). The mean optical density values revealed that the expression of CYP2E1 in L. yarkandensis PCTs increased (Fig. 1C) (P < 0.01). In the proximal straight tubules (PSTs), CYP2E1 was distributed on the basolateral plasma membrane of PST cells (Fig. 1D and E), and the expression of CYP2E1 in L. yarkandensis PSTs significantly increased, similar to that in PCTs (Fig. 1F) (P < 0.01). In the outer medullary collecting duct (OMCD), CYP2E1 was distributed on the basolateral plasma membrane of OMCD cells (Fig. 1G and H), and the mean optical density values were similar to those of the first two tissues (Fig. 1I) (P < 0.05). At the beginning of the inner medullary collecting duct (IMCD1), CYP2E1 was distributed on the basolateral plasma membrane of IMCD1 cells (Fig. 1J and K), and the expression of CYP2E1 was significantly greater in L. yarkandensis than in O. cuniculus (Fig. 1L) (P < 0.01). In the middle inner medullary collecting duct (IMCD2), CYP2E1 was distributed on the basolateral plasma membrane of IMCD2 cells (Fig. 1M and N), and the expression of CYP2E1 was significantly greater in L. yarkandensis than in O. cuniculus (Fig. 1O) (P < 0.01). The results revealed that CYP2E1 was more distributed on the basolateral plasma membrane of PCT, PST, OMCD, IMCD1, and IMCD2 in L. yarkandensis than in O. cuniculus.
CYP2E1 distribution in O. cuniculus and L. yarkandensis kidneys. Paraffin section (6 μm) of the kidneys of O. cuniculus and L. yarkandensis. The sections were incubated with an anti-CYP2E1 antibody. Scale bar: 50 μm. CYP2E1 is located at PCT (A and B), PST (D and E), OMCD (G and H), IMCD1 (J and K), and IMCD2 (M and N) in O. cuniculus and L. yarkandensis. In L. yarkandensis, the protein abundance of CYP2E1 was significantly increased in PCT, PST, OMCD, IMCD2, and IMCD2 (C, F, I, L, and O). Each group consisted of six animals, with three technical replicates per animal. *P < 0.05; **P < 0.01.
CYP2E1 mRNA and protein expression in the kidneys of O. cuniculus and L. yarkandensis
The expression levels of CYP2E1 mRNA and protein in the kidneys of O. cuniculus and L. yarkandensis were determined via qRT‒PCR and western blotting. As shown in Fig. 2, the qRT‒PCR data analysis with GAPDH as an internal reference revealed that the expression level of CYP2E1 mRNA in kidneys was significantly greater (P < 0.01) in L. yarkandensis than in O. cuniculus (Fig. 2A). The expression of CYP2E1 in the kidney was detected using GAPDH as the internal reference, and a band at 57 kDa was detected (top of Fig. 2B). The average optical density value indicated that the expression of CYP2E1 was significantly upregulated in the kidney of L. yarkandensis compared with that in the kidney of O. cuniculus (bottom of Fig. 2B) (P < 0.01). The results revealed that the CYP2E1 mRNA and protein expression levels in kidneys were significantly upregulated in L. yarkandensis than in O. cuniculus.
CYP2E1 mRNA and protein expression levels in the kidneys of O. cuniculus and L. yarkandensis. (A) Expression level of CYP2E1 mRNA in the kidneys of O. cuniculus and L. yarkandensis. Each group consisted of six animals, with three technical replicates per animal. (B) (Top) CYP2E1 protein expression levels in O. cuniculus and L. yarkandensis kidneys. (Bottom) Optical density analysis of renal expression of the CYP2E1 protein in O. cuniculus and L. yarkandensis. Each group consisted of six animals, with three technical replicates per animal. **P < 0.01.
CYP2C expression in the kidneys of O. cuniculus and L. yarkandensis
CYP2C distribution in the kidneys of O. cuniculus and L. yarkandensis
We performed an IHC analysis of the distribution of CYP2C in the kidneys of O. cuniculus and L. yarkandensis. CYP2C was distributed on the basolateral plasma membrane of PCT cells (Fig. 3A and B), and the expression of CYP2C in PCT cells was significantly lower in L. yarkandensis than in O. cuniculus (Fig. 3C) (P < 0.001). CYP2C was distributed on the basolateral plasma membrane of PST cells (Fig. 3D and E), and the expression of CYP2C in PCT cells was lower in L. yarkandensis than in O. cuniculus (Fig. 3F) (P < 0.001). CYP2C was not detected in the OMCD, IMCD1, and IMCD2. The above results revealed that CYP2C was less distributed on the PCT and PST basolateral plasma membranes of L. yarkandensis than on those of O. cuniculus.
Distribution of CYP2C protein in the kidneys of O. cuniculus and L. yarkandensis. Paraffin section (6 μm) of the kidneys of O. cuniculus and L. yarkandensis. Sections were incubated with the anti-CYP2C antibody. Scale bar: 50 μm. CYP2C is located at PCT (A and B) and PST (D and E). The average optical density analysis revealed that the protein abundance of CYP2C significantly decreased in the PCT and PST of L. yarkandensis (C and F). Each group consisted of six animals, with three technical replicates per animal. ***P < 0.001.
CYP2C mRNA and protein expression in the kidneys of O. cuniculus and L. yarkandensis
The expression levels of CYP2C mRNA and protein in the kidneys of O. cuniculus and L. yarkandensis were determined via qRT‒PCR and western blotting. The expression level of CYP2C mRNA in kidneys was significantly lower in L. yarkandensis (P < 0.01) than in O. cuniculus (Fig. 4A). The expression of the CYP2C protein was detected at 50 kDa (top of Fig. 4B). The average optical density indicated that the protein expression of CYP2C in kidneys was significantly lower in L. yarkandensis than in O. cuniculus (bottom of Fig. 4B) (P < 0.01). CYP2C mRNA and protein expression levels in kidneys were significantly lower in L. yarkandensis than in O. cuniculus.
CYP2C mRNA and protein expression levels in the kidneys of O. cuniculus and L. yarkandensis. (A) Expression level of CYP2C mRNA in the kidneys of O. cuniculus and L. yarkandensis. Each group consisted of six animals, with three technical replicates per animal. (B) (Top) CYP2C protein expression in O. cuniculus and L. yarkandensis kidneys. (Bottom) Optical density analysis of the renal expression of CYP2C protein in O. cuniculus and L. yarkandensis. Each group consisted of six animals, with three technical replicates per animal. **P < 0.01.
CYP2J2 expression in the kidneys of O. cuniculus and L. yarkandensis
CYP2J2 distribution in the kidneys of O. cuniculus and L. yarkandensis
We performed an IHC analysis of the distribution of CYP2J2 in the kidneys of O. cuniculus and L. yarkandensis. CYP2J2 was distributed on the basolateral plasma membrane of PCT (Fig. 5A and B) and PST (Fig. 5D and E). The expression of CYP2J2 in PCT and PST was significantly lower in L. yarkandensis than in O. cuniculus (Fig. 5C and F) (P < 0.01, P < 0.05). CYP2J2 was not detected in the OMCD, IMCD1, and IMCD2. The results revealed that CYP2J2 was less distributed on the PCT and PST basolateral plasma membranes of L. yarkandensis than on those of O. cuniculus.
Distribution of CYP2J2 protein in the kidneys of O. cuniculus and L. yarkandensis. Paraffin Sect. (6 μm) of the kidneys of O. cuniculus and L. yarkandensis. Sections were incubated with the anti-CYP2J2 antibody. Scale bar: 50 μm. CYP2J2 is located at PCT (A and B) and PST (D and E). The average optical density analysis revealed that the protein abundance of CYP2J2 significantly decreased in PCT and PST of L. yarkandensis (C and F). Each group consisted of six animals, with three technical replicates per animal. **P < 0.01; *P < 0.05.
CYP2J2 mRNA and protein expression in the kidneys of O. cuniculus and L. yarkandensis
The expression levels of CYP2J2 mRNA and protein in the kidneys of O. cuniculus and L. yarkandensis were determined via qRT‒PCR and western blotting. The expression level of CYP2J2 mRNA in kidneys was significantly lower in L. yarkandensis (P < 0.05) than in O. cuniculus (Fig. 6A). The expression of the CYP2J2 protein was detected in the kidney at 48–63 kDa (top of Fig. 6B). The protein expression of CYP2J2 was significantly lower in L. yarkandensis than in O. cuniculus (bottom of Fig. 6B) (P < 0.05). The results revealed that the CYP2J2 mRNA and protein expression levels in kidneys were significantly lower in L. yarkandensis than in O. cuniculus.
CYP2J2 mRNA and protein expression levels in the kidneys of O. cuniculus and L. yarkandensis. Expression levels of CYP2J2 mRNA in the kidneys of O. cuniculus and L. yarkandensis. Each group consisted of six animals, with three technical replicates per animal. (B) (Top) CYP2J2 protein expression levels in O. cuniculus and L. yarkandensis kidneys. (Bottom) Optical density analysis of renal expression of the CYP2J2 protein in O. cuniculus and L. yarkandensis. Each group consisted of six animals, with three technical replicates per animal. *P < 0.05.
CYP4A11 expression in the kidneys of O. cuniculus and L. yarkandensis
CYP4A11 distribution in the kidneys of O. cuniculus and L. yarkandensis
We performed an IHC analysis of the distribution of CYP4A11 in the kidneys of O. cuniculus and L. yarkandensis. CYP4A11 was distributed on the basolateral plasma membrane of PCT cells (Fig. 7A and B), and the expression of CYP4A11 in L. yarkandensis PCT decreased significantly compared with that in O. cuniculus (Fig. 7C) (P < 0.001). CYP4A11 was distributed on the basolateral plasma membrane of PST cells (Fig. 7D and E), and the expression of CYP4A11 in L. yarkandensis PCT significantly decreased compared with that in O. cuniculus (Fig. 7F) (P < 0.01). CYP4A11 was distributed on the basolateral plasma membrane of the OMCD main cells (Fig. 7G and H), and the expression levels of CYP4A11 in the OMCD in O. cuniculus and L. yarkandensis were not significantly different (Fig. 7I) (P > 0.05). CYP4A11 was distributed on the basolateral plasma membrane of IMCD1 principal cells (Fig. 7J and K), and a difference in the expression of CYP4A11 in IMCD1 between O. cuniculus and L. yarkandensis was not evident (Fig. 7L) (P > 0.05). CYP4A11 was not detected in the IMCD2. The results revealed that CYP4A11 was less distributed on the PCT and PST basolateral plasma membranes of L. yarkandensis than on those of O. cuniculus, and there was no significant difference in OMCD and IMCD1 in O. cuniculus and L. yarkandensis.
Distribution of CYP4A11 protein in the kidneys of O. cuniculus and L. yarkandensis. Paraffin section (6 μm) of the kidneys of O. cuniculus and L. yarkandensis. Sections were incubated with the anti-CYP4A11 antibody. Scale bar: 50 μm. CYP4A11 is located at PCT (A and B), PST (D and E), OMCD (G and H), and IMCD1 (J and K). The average optical density analysis revealed that the protein abundance of CYP4A11 significantly decreased in PCT and PST of L. yarkandensis (C and F), and there was no significant difference in OMCD and IMCD1 between O. cuniculus and L. yarkandensis (I and L). Each group consisted of six animals, with three technical replicates per animal. ***P < 0.001; **P < 0.01; ns, P > 0.05.
CYP4A11 mRNA and protein expression in the kidneys of O. cuniculus and L. yarkandensis
The expression levels of CYP4A11 mRNA and protein in the kidneys of O. cuniculus and L. yarkandensis were determined via qRT‒PCR and western blotting. The expression level of CYP4A11 mRNA was significantly lower in L. yarkandensis kidneys (P < 0.01) than in O. cuniculus kidneys (Fig. 8A). The protein expression of CYP4A11 in the kidney was measured, and a band at 59 kDa was detected (Fig. 8B, top). The average optical density indicated that the expression of the CYP4A11 protein was significantly lower in L. yarkandensis kidneys than in O. cuniculus kidneys (bottom of Fig. 8B) (P < 0.01). CYP4A11 mRNA and protein expression levels in kidneys were significantly lower in L. yarkandensis than in O. cuniculus.
CYP4A11 mRNA and protein expression levels in the kidneys of O. cuniculus and L. yarkandensis. (A) Expression level of CYP4A11 mRNA in the kidneys of O. cuniculus and L. yarkandensis. Each group consisted of six animals, with three technical replicates per animal. (B) (Top) CYP4A11 protein expression in O. cuniculus and L. yarkandensis kidneys. (Bottom) Optical density analysis of renal expression of the CYP4A11 protein in O. cuniculus and L. yarkandensis. Each group consisted of six animals, with three technical replicates per animal. **P < 0.01.
CYP4F3 expression in the kidneys of O. cuniculus and L. yarkandensis
CYP4F3 distribution in the kidneys of O. cuniculus and L. yarkandensis
We performed an IHC analysis of the distribution of CYP4F3 in the kidneys of O. cuniculus and L. yarkandensis. CYP4F3 was distributed on the basolateral plasma membrane of PCT cells (Fig. 9A and B), and the expression of CYP4F3 in PCT was significantly lower in L. yarkandensis than in O. cuniculus (Fig. 9C) (P < 0.05). CYP4F3 was distributed on the basolateral plasma membrane of PST cells (Fig. 9D and E), and the expression of CYP4F3 in PCT was significantly lower in L. yarkandensis than in O. cuniculus (Fig. 9F) (P < 0.01). CYP4F3 was not detected in the OMCD, IMCD1, and IMCD2. The results revealed that CYP4F3 was less distributed on the PCT and PST basolateral plasma membranes of L. yarkandensis than on those of O. cuniculus.
Distribution of CYP4F3 protein in the kidneys of O. cuniculus and L. yarkandensis. Paraffin section (6 μm) of the kidneys of O. cuniculus and L. yarkandensis. Sections were incubated with the anti-CYP4F3 antibody. Scale bar: 50 μm. CYP4F3 is located at PCT (A and B), PST (D and E). The average optical density analysis revealed that the protein abundance of CYP4F3 significant decreased in the PCT and PST of L. yarkandensis (C and F). Each group consisted of six animals, with three technical replicates per animal. *, P < 0.05, **, P < 0.01.
CYP4F3 mRNA and protein expression in the kidneys of O. cuniculus and L. yarkandensis
The expression levels of CYP4F3 mRNA and protein in O. cuniculus and L. yarkandensis kidneys were determined via qRT‒PCR and western blotting. CYP4F3 mRNA expression in kidneys was lower in L. yarkandensis than in O. cuniculus (P < 0.001) (Fig. 10A). The protein expression of CYP4F3 in the kidney was measured, and a band at 60 kDa was detected (Fig. 10B, top). The average optical density indicated that the expression of the CYP4F3 protein in kidneys was significantly lower in L. yarkandensis than in O. cuniculus (P < 0.01) (Fig. 10B, bottom).
CYP4F3 mRNA and protein expression levels in the kidneys of O. cuniculus and L. yarkandensis. (A) Expression level of CYP4F3 mRNA in the kidneys of O. cuniculus and L. yarkandensis. Each group consisted of six animals, with three technical replicates per animal. (B) (Top) CYP4F3 protein expression in O. cuniculus and L. yarkandensis kidneys. (Bottom) Results of optical density analysis of renal expression of CYP4F3 protein in O. cuniculus and L. yarkandensis. Each group consisted of six animals, with three technical replicates per animal **, P < 0.01, ***, P < 0.001.
Contents of 19-HETE, 20-HETE, and EETs in the kidneys of O. cuniculus and L. yarkandensis
The contents of metabolites of the CYP450 enzymes in the kidneys of O. cuniculus and L. yarkandensis were detected via ELISA, and the results are shown in Fig. 11. The content of 19-HETE was greater in the kidney of L. yarkandensis than in O. cuniculus (P < 0.05) (Fig. 11A). The content of 20-HETE was lower in the kidney of L. yarkandensis than in O. cuniculus (P < 0.05) (Fig. 11B). The content of EETs was lower in the kidney of L. yarkandensis than in O. cuniculus (P < 0.01) (Fig. 11C).
Contents of 19-HETE, 20-HETE, and EETs in the kidneys of O. cuniculus and L. yarkandensis. Each group consisted of six animals, with three technical replicates per animal. (A) Contents of 19-HETE in the kidneys of O. cuniculus and L. yarkandensis (n = 6). (B) Contents of 20-HETE in the kidneys of O. cuniculus and L. yarkandensis (n = 6). (C) Contents of EETs in the kidneys of O. cuniculus and L. yarkandensis (n = 6). *, P < 0.05, **, P < 0.01.
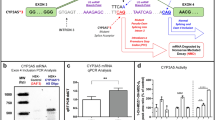
CYP450 expression in the kidneys of O. cuniculus and L. yarkandensis
As shown in Fig. 12, CYP2E1, CYP2C, CYP2J2, CYP4A11, and CYP4F3 are expressed in PCT and PST of the kidneys of O. cuniculus and L. yarkandensis. Additionally, CYP2E1 is also expressed in the OMCD and IMCD. Compared with that in O. cuniculus, the expression of CYP2E1 in the kidneys of L. yarkandensis is greater, whereas the expression of CYP2C, CYP2J2, CYP4A11, and CYP4F3 is lower. In the proximal tubules, the metabolite 19-HETE produced by CYP2E1 acts as a potent renal afferent arteriole dilator, which stimulates water reabsorption. The metabolites of AA metabolized by CYP4A and CYP4F are 20-HETE, whereas those metabolized by CYP2C and CYP2J are EETs. The high expression of CYP2E1 and the low expression of CYP2C, CYP2J2, CYP4A11, and CYP4F3 in the kidneys of L. yarkandensis may increase the reabsorption of water in the kidneys, which could help L. yarkandensis adapt to arid and water-deficient desert habitats.
The expression of CYP450s in the kidneys of O. cuniculus and L. yarkandensis. (A) Expression of CYP450s in the kidneys of O. cuniculus; (B) Expression of CYP450s in the kidneys of L. yarkandensis. CYP2E1 (green) is highly expressed in the convoluted and straight segments of the proximal tubules and the cells of the medullary collecting ducts, whereas CYP2C (red), CYP2J2 (pink), CYP4A11 (light blue), and CYP4F3 (dark blue) are expressed in the convoluted and straight segments of the proximal tubules. Phospholipids (PLs) are converted into free arachidonic acid (AA) by the action of phospholipase (PLA2), and AA is transformed into different metabolites by the cytochrome P450 enzyme. CYP2E1 and 19-HETE are marked in green; CYP4A, CYP4F and 20-HETE are marked in blue; and CYP2C, CYP2J and EETs are marked in red.
Discussion
Studies have shown that the CYP450 enzymes involved in the arachidonic acid pathway in the kidney are mainly CYP2E1, CYP2C, CYP2J2, CYP4A11, and CYP4F3, and the expression of these enzymes in the kidneys of L. yarkandensis and O. cuniculuswas detected for the first time. We examined the expression levels of the enzymes CYP2E1, CYP2C, CYP2J2, CYP4A11, and CYP4F3, which regulate renal water balance through the metabolites formed by the AA pathway. CYP2E1 is distributed mainly in the renal proximal tubules of mice18. This finding is generally consistent with our study, where immunohistochemical analysis revealed that CYP2E1 was distributed mainly in the cortical proximal tubular PCT and PST and in the medullary OMCD, IMCD1, and IMCD2. The immunohistochemical mean density revealed that the abundance of CYP2E1 in kidneys was greater in of L. yarkandensis than in of O. cuniculus. The highest expression of the CYP2E1 was localized in the proximal tubules of L. yarkandensis, suggesting that CYP2E1 may be involved in the in vivo synthesis of 19-HETE, especially in the proximal tubules.
The increase in CYP2E1 expression in dehydrated rats is consistent with the high CYP2E1 expression in the kidneys of L. yarkandensis of the water-deficient habitat. The metabolite of CYP2E1 is mainly 19-HETE, which can stimulate the activity of kidney Na-K-ATPase, thus promoting water reabsorption and improving the drought-resistant adaptability of Camelus bactrianus, a desert animal19. The Tarim Basin hare is smaller than domestic rabbits, and high expression of CYP2E1 can lead to thinness in animals20. 19-HETE can stimulate sodium transport in the proximal tubule and stimulate water reabsorption by the kidney. Therefore, high expression of CYP2E1 in L. yarkandensis can improve the renal reabsorption of water, thus improving water utilization efficiency and helping L. yarkandensis adapt to arid desert environments.
CYP2C has been found to be distributed in the proximal tubules of the rat kidney21. CYP4F3 and CYP4A11 are distributed in the proximal tubule2223;. Our results are consistent with the idea that CYP2C, CYP2J2, CYP4A11, and CYP4F3 are distributed on the basolateral plasma membrane of PCT and PST cells. The immunohistochemical mean density revealed that the abundance of CYP2C, CYP2J2, CYP4A11, and CYP4F3 in the PCT and PST was lower than that in the kidneys of O. cuniculus. These findings indicate that CYP2C, CYP2J2, CYP4A11, and CYP4F3 was localized in the proximal tubules of L. yarkandensis and O. cuniculus, and may be involved in the in vivo synthesis of EETs and 20-HETE.
The high number of CYP2J gene copies in C. bactrianusmay play a vital role in the metabolism of exogenous and endogenous substances, which contributes to adaptation to harsh desert environments15. The primary metabolites of CYP2C and CYP2J2 are EETs. EETs inhibit sodium transport via Na-K-ATPase activity in the proximal tubule, and Na+/H+exchange and ENaC activity in the collecting duct have a renal tubular natriuresis effect2425;. The CYP4A and CYP4F subgroups converted AA to 20-HETE. Quigley et al. demonstrated that 20-HETE inhibited sodium transport and reabsorption in isolated rabbit proximal tubules by inhibiting the Na-K-ATPase and Na-K-2Cl cotransporter and subsequently reabsorbing water in the kidney26. The low expression levels of CYP2C, CYP2J2, CYP4A11, and CYP4F3 in the kidneys of L. yarkandensis living in saline areas reduce the formation of the AA metabolite EETs, and 20-HETE promotes the reabsorption of water and sodium in their kidneys, which helps them adapt to the desert environment of drought and water shortage.
Other enzymes and signaling pathways, such as the renin-angiotensin system, vasopressin-regulated water reabsorption, aldosterone-regulated sodium reabsorption, also play critical roles in the regulation of renal water reabsorption. Our findings demonstrate that the expression patterns of cytochrome P450 enzymes in L. yarkandensis kidneys differ significantly from those in O. cuniculus. These changes in enzyme expression influence renal function and may either enhance or mitigate increases in blood pressure, thereby promoting renal water reabsorption—a vital adaptation for the survival of L. yarkandensis in arid desert environments.
Materials and methods
Animals and tissues
The experimental animals were adult male rabbits that were approximately 8 months old. All experimental animals used in this research were raised and used in accordance with the ARRIVE Guidelines. The experimental animals and procedures employed in this study were authorized by the Research Department of Tarim University, Xinjiang Uyghur Autonomous Region, with approval number 2,022,015. The care and utilization of the experimental animals adhered strictly to the local animal welfare laws, guidelines, and policies. Six L. yarkandensis were sourced from Shaya County in the Aksu Region of the northwest Tarim Basin. Six adult O. cuniculus individuals were obtained from the animal laboratory station of Tarim University. The animals were assessed as adults if their skull length was greater than 75.50 mm. All the animals selected for the experiment were in robust health.
Animal husbandry and diet Prior to the commencement of the study, L. yarkandensis were acclimated for one week with a diet consisting of grass indigenous to the Tarim Basin and fresh water from the same area to mimic their natural habitat. O. cuniculus rabbits were maintained under standard husbandry conditions. Both species of rabbits were housed individually in spacious, well-ventilated cages with constant temperature and humidity to ensure their comfort. They had ad libitum access to food and water, and their intake was monitored daily to assess their health and adaptation to the diet.
All animals used in the study were of similar age, and were screened for health status prior to inclusion in the experiments. Following the acclimation period, the rabbits were anaesthetized with 3% sodium pentobarbital (5 mL/kg) for kidney dissection. The left kidney tissue was collected and divided into small pieces, which were then placed in cryotubes containing RNA preservation solution for subsequent protein and RNA analysis. The right kidney tissue was dissected into small pieces (2 × 2 × 2 mm) and fixed in 4% paraformaldehyde solution for paraffin embedding, enabling histological analysis.
Primer design and associated nucleotide sequences
According to the gene sequence information included in NCBI, real-time PCR primers were designed via Primer Premier 5.0. Shanghai Biotech Co. synthesized the primers (Table 1).
Tissue total RNA extraction
First, 80 mg of tissue was placed in a 2 mL enzyme-free tube, and at 4 °C, 1 mL of TRIzol reagent was added. The tissue was cut on ice, homogenized for 30 s, and allowed to stand at 25 °C for 5 min. Then, at 4 °C, it was subjected to centrifugation at 12,000 rpm for 10 min, and 800 µL of the supernatant was removed and placed in a 1.5 mL enzyme-free tube. Then, 400 µL of chloroform (precooled to 4 °C) was added, vortexed with an oscillator for 10 s, and allowed to stand at room temperature for 10 min. High-speed centrifugation was performed at 4 °C (12,000 rpm) for 15 min. Then, 600 µL of the supernatant was placed in a new 1.5-mL enzyme-free tube, and 600 µL of isopropanol (precooled to 4 °C) was added. The solution was mixed well and allowed to stand at room temperature for 10 min.
The mixture was subsequently centrifuged with a low-temperature high-speed centrifuge at 4 °C (12,000 rpm) for 15 min, after which the supernatant was discarded, leaving the precipitate. The precipitate was washed with 500 µL of 75% ethanol (precooled to 4 °C), inverted five times, and centrifuged at 4 °C and 8,000 rpm for 5 min. The supernatant was discarded. The precipitate was dried naturally, 40 µL of enzyme-free water was added to dissolve the RNA precipitate, and it was stored in a − 80 °C refrigerator.
cDNA synthesis (reverse transcription)
The RNA was reverse transcribed into cDNA via EasyScript® One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech, China).
The following reaction system (20 µL system) was prepared in a 1.5 mL centrifuge tube after DEPC water treatment and sterilization (Table 2). The mixture was gently mixed, incubated at 42 °C for 30 min, inactivated at 90 °C for 10 min, and stored at − 20 °C.
Quantitative real-time polymerase chain reaction (qRT‒PCR)
The expression of the CYP450 family gene mRNA in L. yarkandensis and O. cuniculus kidneys was analysed via qRT‒PCR. For the 20 µL reaction system, we used 10 µL of SYBR Premix ExTaq (2), 2 µL of cDNA, 1.0 µmol/L (1 µL) of upstream and downstream primers, and 6.4 µL of RNase-free H2O. The PCR conditions were as follows: 94 °C predenaturation for 30 s; 40 cycles of denaturation at 94 °C for 5 s, annealing at 60 °C for 15 s, and extension at 72 °C for 10 s; and three replicates for each sample tested. The relative expression of target genes was calculated via the 2–∆∆Ct method, with GAPDH used as the reference control.
Western blotting analysis
Total kidney protein was extracted from L. yarkandensis and O. cuniculus. We placed 100 mg of renal tissue into 1 mL of lysis buffer containing 0.04 mol/L Tris-HCl (pH 7.4), 0.82% NaCl, 1.5% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, and 1 mM PMSF. Tissues were homogenized and kept on ice for 30 min, and the supernatant was centrifuged at 12,000 rpm at 4 °C for 20 min. The sample was heated for 5 min at 95 °C after the total protein concentration was determined using a BCA protein assay kit and the protein concentration was mixed and adjusted with 6 sample buffers. SDS‒polyacrylamide gel electrophoresis was subsequently performed (Sup. Figure 1). After being transferred to a polyvinylidene fluoride membrane, the tissues were blocked with TBST containing 5% skim milk for 2 h at room temperature. They were incubated overnight at 4 °C with antibodies against CYP2E1, CYP2C, CYP2J2, CYP4F3, and CYP4A11 (dilution ratios of 1:1500, 1:1000, 1:800, 1:800, and 1:800, respectively) or an anti-GAPDH antibody (1:5,000) after being washed with TBST. The PVDF membranes were washed with TBST and incubated with peroxidase-labelled anti-rabbit or anti-mouse secondary antibodies for 1 h at room temperature. The PVDF membranes were washed again with TBST and placed into the ECL assay kit for 2 min. Bands were observed via Tanon 5200 scan images (Sup. Figures 2–6), and the bands were analysed via Quantity One 4.6.2 software. GAPDH protein was used as the control protein using GraphPad Prism software.
Immunohistochemistry (IHC)
The renal tissue was fixed with 4% paraformaldehyde after conventional dehydration and transparent treatment, and embedded in paraffin. Paraffin-embedded kidney tissue was cut into 6 μm thick sections for immunohistochemical staining. The sections were dewaxed and washed with xylene three times for 10 min, followed by a series of ethanol washes and rehydration from 100 to 70% for 5 min. The sections were then soaked in distilled water. The slides were immersed in 0.01 mol/L citrate buffer at pH 6.0, heated to boiling, cooled, and washed with 0.1 mol/L PBS (pH 7.4). The sections were immersed in 3% methanol hydrogen peroxide at room temperature and washed with 0.1 mol/L PBS (pH 7.4). The slides were incubated with 15% goat serum at 25 °C for 2 h. The sectioned kidney tissue was incubated overnight at 4 °C with antibodies against CYP2E1, CYP2C, CYP2J2, CYP4F3, and CYP4A11 (dilution ratios of 1:300, 1:200, 1:200, 1:100, and 1:100, respectively). As a control, the primary antibody was replaced with PBS in the tissue sections. The sections were rinsed with 0.1 mol/L PBS (pH 7.4), incubated with 50 µL of HRP-labelled sheep anti-rabbit secondary antibody for 30 min at room temperature, washed with 0.1 mol/L (pH 7.4) PBS, incubated for 2 min, and then washed with distilled water for 10 min. After counterstaining with haematoxylin for 90 s and washing with running water for 5 min, the xylene was dehydrated, made transparent, and sealed with neutral gum. The stained sections were observed and imaged via a microscope.
Enzyme-linked immunosorbent assay (ELISA)
Five clean 2 mL centrifuge tubes were used in the first step, and the standard was diluted in a gradient. In the second step, blank wells (no sample or enzyme label reagent in the blank control well, and the other steps were the same as those in the other wells), standard wells, and sample wells were set up. Then, 50 µL of the standard was added to the coated plate, 40 µL of sample dilution was added to the sample well, and 10 µL of the sample was added after heating (the final dilution was five times). The sample was added to the bottom of the hole of the microplate while attempting not to touch the hole wall, gently shaken, and mixed. In the third step, the plate was warmed to 37 °C for 30 min. In the fourth step, the 30× concentrated washing solution was diluted with distilled water for 30× backup use. The fifth step involved carefully removing the sealing plate film, discarding the liquid, shaking it dry, filling each well with the washing wave, allowing it to stand for 30 s, repeating it five times, and beating it dry. In the sixth step, 50 µL of the reagent was added to each well. The seventh step involved warming the plate to 37 °C for 30 min. The eighth step included removing the sealing plate film, discarding the liquid, shaking it dry, filling each well with the washing wave, allowing it to stand for 30 s, repeating it five times, and beating it dry. The ninth step involved colour development. For each well, colour developer A was first added (50 µL), and then colour developer B was added (50 µL). We gently shook the entire mixture at 37 °C and avoided light colour display for 10 min. The tenth step included adding 50 µL of termination solution to each well. The reaction was terminated (blue turned yellow). The eleventh step involved adjusting the blank well to zero and measuring each well’s absorbance (OD) at a wavelength of 450 nm. The termination was performed within 15 min after adding the termination solution. In the final step, the standard curve was determined from the concentration and absorbance of the blank and standard wells, and the sample concentration was calculated from the standard curve.
Statistical analyses
The collected images were analysed for optical density via IpWin32 software. The experimental data were processed via the statistical analysis software GraphPad Prism, and the significance of the means in each group was analysed via a t test. * represents the comparison of differences between O. cuniculus and L. yarkandensis. Statistical significance was defined as follows: p < 0.05 was considered significant and marked with *; p < 0.01 was considered highly significant and marked with **; p < 0.001 was considered extremely significant and marked with ***; and p > 0.05 was considered not significant and marked as ns.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Kumar, B., Cheng, J., Ge, D., Xia, L. & Yang, Q. Phylogeography and ecological niche modeling unravel the evolutionary history of the Yarkand Hare, Lepus Yarkandensis (Mammalia: Leporidae), through the Quaternary. Bmc Evol. Biol. 19, 113 (2019).
Zhang, J. et al. Higher expression levels of Aquaporin family of proteins in the kidneys of Arid-Desert living Lepus Yarkandensis. Front. Physiol. 10, 1172 (2019).
Zhang, J. et al. Higher expression levels of Aquaporin (Aqp)1 and Aqp5 in the lungs of Arid-Desert living Lepus Yarkandensis. J. Anim. Physiol. Anim. Nutr. 104, 1186–1195 (2020).
Zhang, J. et al. Distribution of Aquaporins and sodium transporters in the Gastrointestinal tract of a desert Hare, Lepus Yarkandensis. Sci. Rep. 9, 16639 (2019).
Shan, W., Tursun, M., Zhou, S., Zhang, Y. & Dai, H. Complete mitochondrial genome sequence of Lepus Yarkandensis Gunther, 1875 (Lagomorpha, Leporidae): Characterization and Phylogenetic Analysis. Zookeys 1012, 135–150 (2021).
Harris, K. L. et al. Ancestral sequence reconstruction of a cytochrome P450 family involved in chemical defense reveals the functional evolution of a promiscuous, Xenobiotic-Metabolizing enzyme in vertebrates. Mol. Biol. Evol. 39, 6 (2022).
Singh, A., Panwar, R., Mittal, P., Hassan, M. I. & Singh, I. K. Plant cytochrome P450s: role in stress tolerance and potential applications for human welfare. Int. J. Biol. Macromol. 184, 874–886 (2021).
Elshenawy, O. H., Shoieb, S. M., Mohamed, A. & El-Kadi, A. O. S. Clinical implications of 20-Hydroxyeicosatetraenoic acid in the kidney, liver, lung and brain: an emerging therapeutic target. Pharmaceutics 9, 9 (2017).
Laniado-Schwartzman, M. & Abraham, N. G. The renal cytochrome P-450 arachidonic acid system. Pediatr. Nephrol. 6, 490–498 (1992).
Laethem, R. M., Balazy, M., Falck, J. R., Laethem, C. L. & Koop, D. R. Formation of 19(S)-, 19(R)-, and 18(R)-Hydroxyeicosatetraenoic acids by Alcohol-Inducible cytochrome P450 2E1. J. Biol. Chem. 268, 12912–12918 (1993).
Imig, J. D., Jankiewicz, W. K. & Khan, A. H. Epoxy fatty acids: from salt regulation to kidney and cardiovascular therapeutics: 2019 Lewis K. Dahl memorial lecture. Hypertens. (Dallas Tex. 1979). 76, 3–15 (2020).
Gong, Y. et al. Cytochrome P450 oxidase 2J Inhibition suppresses choroidal neovascularization in mice. Metabolism 134, 155266 (2022).
Zhu, Y. et al. Cyp2J2-Produced epoxyeicosatrienoic acids attenuate Ischemia / Reperfusion-Induced acute kidney injury by activating the Sirt1-Foxo3a pathway. Life Sci. (1973). 246, 117327–117328 (2020).
Kamel, K. S., Schreiber, M. & Halperin, M. L. Renal potassium physiology: integration of the renal response to dietary potassium depletion. Kidney Int. 93, 41–53 (2018).
Hasi, S., Yao, J., Yu, S. & Tian, Y. Diversity and distribution of cyp gene family in Bactrian camel. Funct. Integr. Genomics. 18, 23–29 (2018).
Cheng, S., Wu, T., Li, Y., Huang, J. & Cai, T. Romidepsin (Fk228) in a mouse model of Lipopolysaccharide-Induced acute kidney injury is associated with Down-Regulation of the Cyp2E1 gene. Med. Sci. Monit. 26, e918528 (2020).
Roman, R. J. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 82, 131–185 (2002).
Vieira, I., Pasanen, M., Raunio, H. & Cresteil, T. Expression of Cyp2E1 in human lung and kidney during development and in Full-Term placenta: A differential methylation of the gene is involved in the regulation process. Pharmacol. Toxicol. 83, 183–187 (1998).
Hotchkiss, J. A., Kim, H., Hahn, F. F., Novak, R. F. & Dahl, A. R. Pyridine induction of Sprague-Dawley rat renal cytochrome P4502E1: immunohistochemical localization and quantitation. Toxicol. Lett. 78, 1–7 (1995).
Dang, T. & Yun, J. W. Cytochrome P450 2E1 (Cyp2E1) positively regulates lipid catabolism and induces Browning in 3T3-L1 white adipocytes. Life Sci. 278, 119648 (2021).
Wang, H. et al. Cloning, expression, and characterization of three new mouse cytochrome P450 enzymes and partial characterization of their fatty acid oxidation activities. Mol. Pharmacol. 65, 1148–1158 (2004).
Cui, X. & Strobel, H. W. Cloning and characterization of the rat cytochrome P450 4F5 (Cyp4F5) gene. Gene 297, 179–187 (2002).
Stec, D. E., Deng, A. Y., Rapp, J. P. & Roman, R. J. Cytochrome P4504a Genotype Cosegregates with Hypertension in Dahl S Rats. Hypertension (Dallas, Tex. 27, 564–568 (1996). (1979).
Imig, J. D., Jankiewicz, W. K. & Khan, M. A. H. Epoxy fatty acids: from salt regulation to kidney and cardiovascular therapeutics. Hypertens. (Dallas Tex. 1979). 76, 3–15 (2020).
Yu, Z. et al. Increased Cyp2J expression and epoxyeicosatrienoic acid formation in spontaneously hypertensive rat kidney. Mol. Pharmacol. 57, 1011–1020 (2000).
Kirchheimer, C., Mendez, C. F., Acquier, A. & Nowicki, S. Role of 20-Hete in D1/D2 dopamine receptor synergism resulting in the Inhibition of Na+-K+-ATPase activity in the proximal tubule. Am. J. Physiol. Renal. Physiol. 292, F1435–F1442 (2007).
Acknowledgements
The author wishes to express gratitude to Figdraw (https://www.figdraw.com/) for providing the drawing platform when creating Fig. 12.
Funding
This study was funded by the National Natural Science Foundation of China (32160112), Xinjiang Production & Construction Corps Key Laboratory of Protection and Utilization of Biological Resources in Tarim Basin (BRFW2402) and the Tarim University Graduate Research Innovation Project (TDGRI202307).
Author information
Authors and Affiliations
Contributions
JZ designed and wrote the manuscript and did data analysis, Quantitative RT-PCR, western blotting and immunohistochemical studies. YL wrote the manuscript and did data analysis, Quantitative RT-PCR, western blotting and immunohistochemical studies. DS did sample collection and staining. BC did sample collection and processing. GL designed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, J., Li, Y., Shao, D. et al. Comparative study of cytochrome P450 expression in the kidneys of Lepus yarkandensis and Oryctolagus cuniculus. Sci Rep 15, 6954 (2025). https://doi.org/10.1038/s41598-025-91603-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-91603-x