Abstract
We evaluated the incidence and risk factors for recurrence in patients with neovascular age-related macular degeneration (nAMD) who discontinued anti-vascular endothelial growth factor (VEGF) therapy under a modified treat-and-extend (TAE) protocol. A retrospective analysis of 68 patients was conducted. Therapy was discontinued after extending the treatment interval to ≥ 5 months and maintaining disease stability for 6 months. The modified TAE protocol included three phases: loading, observation, and TAE, with initial treatment intervals determined by the first recurrence interval. Recurrence rates were 22.2%, 42.2%, and 54.4% at 1-, 2-, and 3-year follow-ups, respectively. The median time to recurrence was 16 months, with patients receiving an average of 7.7 injections before discontinuation. Intraretinal cysts were significantly more prevalent in patients with recurrence. Rapid early response to treatment was associated with a lower risk of exudative recurrence. Vision loss of two or more lines occurred in five patients despite treatment resumption; all exhibited subretinal hemorrhages on baseline imaging. The modified TAE protocol allows for successful therapy discontinuation with fewer injections and reduced recurrence rates. Patients with a favorable early response to anti-VEGF therapy had a lower risk of recurrence.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is a leading cause of visual impairment and blindness among older individuals. Choroidal neovascularization (CNV) associated with AMD leads to fluid leakage, hemorrhage, fibrosis, and scarring, resulting in progressive visual dysfunction. Intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) agents have become the standard first-line therapy, significantly improving visual outcomes in patients with neovascular AMD1. Among various treatment regimens, the treat-and-extend (TAE) strategy—where injection intervals are adjusted based on disease activity—is widely adopted in clinical practice. Clinical trials have demonstrated that the TAE regimen maintains favorable visual outcomes while reducing the patient burden by decreasing injection frequency2.
However, the current TAE regimen lacks standardized guidelines regarding maximum treatment intervals or criteria for treatment cessation. Maximum treatment intervals are generally considered to be 12 or 16 weeks, requiring patients to receive maintenance injections at these intervals, even with stable disease activity. This practice imposes a considerable burden on older patients and healthcare systems3. Furthermore, frequent anti-VEGF injections may increase the risks of endophthalmitis and macular atrophy4. In a real-world study of aflibercept using a TAE regimen, patients received an average of 5.4 injections during the fourth year of treatment, with maximum treatment intervals extending to 16–20 weeks5,6. Conversely, in cases where disease inactivity was observed over at least three consecutive visits, monitoring without treatment was implemented, resulting in an average of 2.79 injections in year 4, with 37% of eyes requiring no injections during that period7.
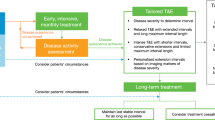
The current TAE regimen may lead to overtreatment in patients showing no recurrence following the initial loading dose. Monitoring patients for exudative activity post-loading, rather than initiating routine treatment, could help reduce overtreatment. Additionally, a recent study demonstrated that the interval between the initial loading phase and the first retreatment with ranibizumab was a strong predictor of subsequent treatment intervals8. Based on these findings, a modified TAE regimen has been proposed, incorporating an observation phase after the loading phase to establish the initial treatment interval for the TAE phase9. In this modified regimen, treatment is initiated after recurrence, with treatment intervals determined based on the disease recurrence interval. This approach may reduce overtreatment and lessen the treatment burden, emphasizing the importance of identifying patients who require retreatment shortly after the initial phase to avoid overtreatment. Previous studies have indicated that a modified TAE regimen for nAMD can achieve favorable functional outcomes over 12 months with fewer injections than the standard regimen9,10.
Since the timing of treatment cessation in clinical practice may vary depending on the clinical course, several studies have explored TAE regimen cessation and the incidence of recurrences following different predetermined disease-free intervals. These TAE algorithms with exit strategies have generally shown favorable outcomes with a manageable treatment burden11,12,13,14,15,16,17. However, the optimal treatment protocol remains a topic of ongoing debate. To address this issue, this study investigated the incidence and risk factors for recurrence in patients with nAMD treated with a modified TAE regimen following treatment discontinuation.
Results
Study population
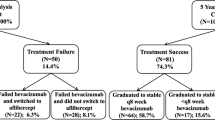
A total of 78 patients initially met the inclusion criteria and successfully discontinued TAE injection treatment. However, eight patients were excluded due to loss to follow-up within 36 months after the final injection. An additional two patients were excluded due to the presence of macular edema and vitreous hemorrhage associated with diabetic retinopathy, baseline foveal scarring, or atrophy.
Baseline characteristics and treatment course of patients
At baseline, the mean age of the patients was 71.2 ± 8.7 years, with 60.3% of the cohort being men. The mean follow-up period before treatment cessation was 17.7 ± 6.6 months, and the mean number of injections administered before treatment cessation was 7.7 ± 2.0. Table 1 summarizes the demographic and baseline characteristics of the study participants.
Significant differences in baseline characteristics were observed between the groups with (n = 37) and without (n = 31) exudative recurrence (Table 1). The proportion of rapid responders was substantially higher in the non-recurrence group compared to the recurrence group (71.0% vs. 37.8%; P = 0.006). Baseline intraretinal cysts (IRC) were more prevalent in eyes with recurrence compared to those without recurrence (75.7% vs. 25.8%; P = 0.008). Although the number of type 3 macular neovascularization cases in the recurrence group was twice that in the non-recurrence group, the difference between the groups was not statistically significant. No notable differences were observed between the two groups in terms of age, sex, mean baseline best-corrected visual acuity (BCVA), mean central subfoveal thickness, pigment epithelial detachment (PED) height, or mean number of injections before treatment cessation.
Time and proportion of recurrence
Figure 1 displays the Kaplan–Meier survival curves for the time to and proportion of overall recurrence. Exudative recurrence of nAMD following treatment discontinuation was observed in 15 of 68 patients (22.2%) at 12 months, 29 patients (42.6%) at 24 months, and 37 patients (54.4%) at 36 months of follow-up. The mean time to recurrence after the final injection was 15.8 ± 7.5 months (median: 16 months; range: 7–34 months). Cox regression analysis results (Table 2) revealed that a higher proportion of rapid responders was significantly associated with the absence of recurrence (odds ratio: 5.81; P = 0.006).
Changes in BCVA
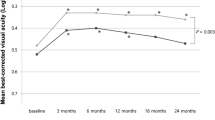
The BCVA tended to be worse at both baseline and the final visit in the non-recurrence group, although the difference was not statistically significant. In the recurrence group, the mean BCVA at recurrence and the 3-year follow-up were 0.36 ± 0.28 logMAR and 0.39 ± 0.27 logMAR, respectively, with no statistically significant difference. Five patients experienced a loss of more than 15 letters after treatment discontinuation. Among these, four developed foveal fibrosis, and one exhibited geographic atrophy involving the fovea at the 3-year follow-up. Notably, three of these patients experienced significant subretinal hemorrhages involving the fovea after treatment cessation. Additionally, all five patients displayed evidence of subretinal hemorrhage in the baseline color fundus photographs (Fig. 2).
Discussion
We investigated the incidence and risk factors for recurrence following the cessation of the TAE regimen in patients with nAMD, where the treatment interval had been extended to ≥ 24 weeks. Recurrence rates were 22.2% and 54.4% at 12 and 36 months of follow-up, respectively. Despite recurrence, BCVA was maintained for 24 months after the resumption of anti-VEGF therapy, consistent with findings from earlier studies. Significant factors associated with recurrence included the presence of baseline intraretinal cysts and a rapid responder status.
Anti-VEGF therapy is highly effective in managing nAMD; however, the need for monthly or bimonthly injections imposes a considerable burden regarding treatment frequency, time commitment, and financial costs. TAE regimens aim to treat patients just before the onset of disease activity or fluid accumulation by adjusting treatment intervals based on exudative recurrence. This approach contrasts with the PRN regimen, where treatment is administered only in response to clinical worsening18. The TAE regimen has shown efficacy comparable to fixed dosing while significantly reducing treatment burden19. Approximately 33% of patients had injection intervals of 8–12 weeks, while 22% had intervals exceeding 12 weeks20. However, the standard TAE regimen may lead to overtreatment, particularly in patients without signs of recurrence after the initial loading dose, as recurrence intervals vary substantially among individuals. Mantel et al. reported favorable outcomes with a modified TAE regimen that included an observation phase following the induction phase9. In this approach, patients were observed without injections until exudative activity reappeared after the induction phase, thereby minimizing overtreatment and enabling a more individualized treatment plan. Recent studies have demonstrated that this modified TAE regimen achieves comparable visual outcomes with fewer injections compared to the bimonthly regimen after three initial monthly doses from the VIEW study or the standard TAE regimen9,10.
Treatment cessation after stabilization through continuous therapy may effectively reduce the treatment burden on patients and improve medical resource allocation. Prolonged anti-VEGF injections for nAMD are associated with various risks, including infectious endophthalmitis and complications such as retinal pigment epithelium tears and geographic atrophy21,22. Recent long-term real-world studies have reported successful treatment cessation in 14.8–26.0% of patients following continuous treatment13,23,24. Additionally, a 2023 study from Korea found that anti-VEGF injections were discontinued in 66% of nAMD cases, with 67% of these patients requiring retreatment25. Despite these findings, the optimal timing for treatment cessation after the TAE regimen for nAMD remains unclear, and outcomes following the discontinuation of anti-VEGF therapy have shown variable results. The decision regarding when and under what conditions treatment should be suspended varies considerably among physicians. Hirata et al.17 discontinued the TAE regimen after extending the treatment interval to ≥ 12 weeks, reporting recurrence rates of 33% at 1 year and 48% at 2 years. Adrean et al.16 proposed an exit strategy where treatment was suspended if no CNV activity was detected at 12-week intervals for two consecutive visits, resulting in a recurrence rate of 16% at 1 year. Similarly, Munk et al.26 discontinued treatment after three consecutive injections at 16-week intervals, observing a recurrence rate of 15% within 9 months. Other studies have reported recurrence rates ranging from 38 to 53% within 1 year11,12,15,27,28.
In this study, an “exit strategy” was employed, wherein treatment was suspended only after a 24-week disease-free interval. This approach resulted in a recurrence rate of 22.2% at 1 year. The lower recurrence rate observed, compared to previous reports, may be attributable not only to differences in treatment discontinuation criteria but also to the use of a modified TAE regimen during the initial treatment phase. Patients underwent treatment for an average duration of 17.7 ± 6.6 months and received an average of 7.7 ± 2.0 injections before discontinuing TAE. Consequently, it is likely that treatment for patients in this analysis was suspended earlier than in previous studies. The key focus of our study is to identify patient criteria that enable safe treatment cessation while maintaining favorable outcomes. Our modified T&E regimen determines treatment intervals based on the duration of the first CNV recurrence interval and may have allowed patients with a lower propensity for recurrence to meet the exit criteria more efficiently. A study conducted in Korea and Japan reported recurrence rates of 85% and 74%, respectively, over 24 months following three monthly loading injections and a PRN regimen29,30. These rates are considerably higher than those observed in this study using the modified TAE regimen. The lower 2-year recurrence rate in this study is attributed to the treatment protocol, which mandated a minimum of six injections: the initial three loading doses followed by additional injections at 3, 4, and 5 months.
Among previously reported predictive factors, patients with a favorable early response to treatment demonstrated a significantly lower risk of recurrence. In this study, 71% of rapid responders in the non-recurrence group discontinued TAE treatment without recurrence following loading injections, compared to 57.1% in the recurrence group. This finding aligns with a recent report suggesting that the interval between ranibizumab loading doses and the first retreatment is a reliable predictor of subsequent intervals8. Additionally, early response to anti-VEGF therapy has been correlated with greater final visual acuity gains in several studies31. Patients with IRC at baseline were found to have a significantly higher risk of recurrence, whereas another study identified baseline PED as a predictive factor for recurrence12. IRC at baseline has also been reported as a poor prognostic factor in terms of BCVA gain in AMD treatment32. Previous studies have suggested that fewer injections and shorter treatment duration before cessation are associated with a higher risk of recurrence, implying that premature treatment suspension may lead to poorer outcomes17. However, in this study, no significant differences in visual acuity, treatment duration, or the number of injections were observed between the recurrence and non-recurrence groups. Cho et al. reported that younger age, man sex, and polypoidal choroidal vasculopathy (PCV) were associated with retreatment in a real-world nAMD cohort25. However, no significant associations were found between demographic factors, AMD subtypes, and the risk of nAMD reactivation in this study.
Our study demonstrated that CNV recurrence after treatment cessation is generally well controlled, with vision maintained over three years, consistent with findings from previous studies11,12,16,17. Among the 37 cases with recurrences, five experienced a loss of more than 15 letters despite resuming treatment. Notably, all five cases exhibited evidence of subretinal hemorrhage in the baseline color fundus photographs. These findings suggest that while treatment cessation can be relatively safe with continuous monitoring and prompt retreatment, baseline subretinal hemorrhage is a significant risk factor for vision loss. Therefore, subretinal hemorrhage at the time of initial diagnosis should be considered a key predictor of poor outcomes following treatment discontinuation, and treatment cessation should be approached with greater caution in patients with such risk factors. Further research is warranted to identify high-risk patients prior to treatment cessation to minimize the risk of irreversible vision loss.
Our study has several limitations. These include its retrospective design and relatively small sample size. Additionally, the study population may have been biased toward patients with favorable outcomes, as those with poor prognoses were more likely to be lost to follow-up due to disqualification from government insurance coverage, which made treatment prohibitively expensive. This includes cases in which patients developed fibrosis, atrophy, or failed to meet the minimal BCVA criteria. Furthermore, patients unable to continue the TAE regimen after the initial three monthly injections were excluded because they were disqualified from insurance coverage upon review. This selection bias may limit the generalizability of our findings to the broader nAMD population. Lastly, we acknowledge the concern that the type of anti-VEGF agent used may influence recurrence risk. However, in our study, switching between ranibizumab and aflibercept was relatively common during the T&E phase at the discretion of physicians, preventing us from including drug type as a variable in the regression analysis. Further studies with larger cohorts are necessary to establish an optimal strategy for treatment cessation that achieves favorable outcomes with fewer injections and clinic visits.
In conclusion, we investigated the incidence and clinical course of recurrence following the cessation of a modified TAE treatment in patients with nAMD. Our results demonstrated that while over 50% of the patients experienced reactivation within three years of cessation, overall visual acuity was well maintained with prompt resumption of treatment. Recurrence was more frequent in patients with baseline IRC and less frequent in those with a rapid response following initial treatment. For patients with nAMD and subretinal hemorrhage at the time of initial diagnosis, it is important to consider the potential for vision loss after treatment discontinuation.
Methods
This was a single-center, retrospective study. The study design was approved by the Institutional Review Board of Kyung Hee University Medical Center (No. 2025-01-015). Informed consent was obtained from all subjects and/or their legal guardian and all the study procedures adhered to the principles of the Declaration of Helsinki. Medical records of treatment-naïve patients with nAMD who received an initial loading dose of three-monthly intravitreal injections of ranibizumab or aflibercept under a modified TAE regimen between January 2015 and December 2021 at Kyung Hee University Hospital were reviewed. We included patients with nAMD who met the predefined criteria and were able to discontinue TAE injections. Additionally, all included eyes required a minimum follow-up period of 36 months after treatment discontinuation to calculate the cumulative recurrence rate.
At baseline, each participant underwent a complete ophthalmologic examination, including BCVA measurement, intraocular pressure measurement, fundoscopy, color fundus photography, spectral-domain optical coherence tomography (SD-OCT; Spectralis HRA + OCT, Heidelberg Engineering), and fluorescein and indocyanine green angiography (FA, ICGA; HRA-2, Heidelberg Engineering). The subtypes of nAMD included in this study were type 1, type 2, type 3, and PCV, classified based on OCT and ICGA findings.
Treatment protocol
The modified TAE regimen included an observation phase during which patients were monitored monthly for signs of exudative activity following an initial loading dose of three-monthly injections. Recurrence was defined as the presence of a new retinal hemorrhage, intraretinal and/or subretinal fluid, or enlargement of PED observed on SD-OCT. In the subsequent TAE phase, the initial treatment interval was determined based on the interval at which the first signs of recurrence occurred. If no recurrence was observed for 12 weeks or more after the initial three injections, the first TAE interval was set to 12 weeks. The treatment interval was then extended by 2–4 weeks at a time, up to a maximum of 20 weeks. In cases of recurrence, the treatment interval was reduced by 2–4 weeks until a dry macula was achieved. Continuous injections were considered suspended if the treatment interval reached ≥ 24 weeks. Rapid responders were defined as patients who could successfully discontinue TAE treatment without recurrence after the initial three injections. These patients received a minimum of six injections, including the three loading doses and additional injections at 12, 16, and 20 weeks during the TAE phase. After suspending anti-VEGF treatments, lesion status was monitored using OCT, fundus examinations, and BCVA measurements at each follow-up visit, typically every 3–4 months. The first follow-up visit after treatment suspension was at 3 months. In cases of recurrence, anti-VEGF injections were resumed.
Outcomes measures
The primary outcome measure was the incidence of reactivation after treatment discontinuation. Secondary outcome measures included changes in BCVA, total treatment period, number of injections, last treatment interval, OCT parameters before cessation, and the risk and timing of recurrence.
Statistical analysis
All statistical analyses were performed using SPSS software (Version 20.0 for Windows, IBM, Chicago, Illinois). For continuous variables, significance was tested using the Mann–Whitney U test, and changes in BCVA were assessed using Wilcoxon’s signed-rank test. Nominal variables were analyzed using the chi-squared test. Correlations between various parameters were assessed using Spearman’s rank correlation coefficients. Cox proportional hazards regression analysis was applied to estimate hazard ratios for recurrence, using both univariate and multivariate analyses and including variables. Variables selection was guided by univariate analysis results and clinical considerations. Statistical significance was defined as P < 0.05. The proportion and time to lesion reactivation were further analyzed using Kaplan–Meier survival analysis. Nested Cox proportional hazards models were used to examine the effects of prior treatment, age, visual acuity at treatment suspension, lesion type, and treatment duration before suspension, with a nesting variable for patients within practices. Paired t-tests were used to evaluate changes in vision at designated follow-up time points.
Data availability
The full raw data from this study cannot be publicly shared due to the potential risk of identifying or exposing sensitive patient information. However, selected datasets used in the study may be available upon request from the corresponding author, subject to meeting the criteria for access to confidential data.
References
Heier, J. S. et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 119, 2537–2548 (2012).
Berg, K., Pedersen, T. R., Sandvik, L. & Bragadóttir, R. Comparison of Ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology 122, 146–152 (2015).
Kim, S., Park, S. J., Byun, S. J., Park, K. H. & Suh, H. S. Incremental economic burden associated with exudative age-related macular degeneration: A population-based study. BMC Health Serv. Res. 19, 828 (2019).
Daien, V. et al. Incidence and outcomes of infectious and noninfectious endophthalmitis after intravitreal injections for age-related macular degeneration. Ophthalmology 125, 66–74 (2018).
Ishibashi, K. et al. Four-year outcomes of intravitreal aflibercept treatment for neovascular age-related macular degeneration using a treat-and-extend regimen in Japanese patients. Ther. Adv. Ophthalmol. 13, 2515841420984586 (2021).
Tsunekawa, Y., Kataoka, K., Asai, K., Ito, Y. & Terasaki, H. Four-year outcome of aflibercept administration using a treat-and-extend regimen in eyes with recurrent neovascular age-related macular degeneration. Jpn. J. Ophthalmol. 65, 69–76 (2021).
Lukic, M. et al. Four-year outcomes of aflibercept treatment for neovascular age-related macular degeneration: Results from real-life setting. Eur. J. Ophthalmol. 31, 1940–1944 (2021).
Mantel, I., Deli, A., Iglesias, K. & Ambresin, A. Prospective study evaluating the predictability of need for retreatment with intravitreal Ranibizumab for age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 251, 697–704 (2013).
Mantel, I., Niderprim, S. A., Gianniou, C., Deli, A. & Ambresin, A. Reducing the clinical burden of ranibizumab treatment for neovascular age-related macular degeneration using an individually planned regimen. Br. J. Ophthalmol. 98, 1192–1196 (2014).
Ohnaka, M. et al. A modified treat-and-extend regimen of aflibercept for treatment-naïve patients with neovascular age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 255, 657–664 (2017).
Matsubara, H. et al. Effects of suspension of anti-vascular endothelial growth factor treatment for neovascular age-related macular degeneration in clinical setting. Graefes Arch. Clin. Exp. Ophthalmol. 260, 1867–1876 (2022).
Aslanis, S., Amrén, U., Lindberg, C. & Epstein, D. Recurrent neovascular age-related macular degeneration after discontinuation of vascular endothelial growth factor inhibitors managed in a treat-and-extend regimen. Ophthalmol. Retina. 6, 15–20 (2022).
Kinoshita, T., Mori, J., Hatanaka, A., Shimizu, M. & Imaizumi, H. Visual outcome and treatment frequency of anti-VEGF therapy using the treat-and-extend and treatment cessation regimen for exudative age-related macular degeneration and pachychoroid neovasculopathy. Clin. Ophthalmol. 15, 4405–4418 (2021).
Jaggi, D. et al. Aflibercept for age-related macular degeneration: 4-year outcomes of a ‘treat-and-extend’ regimen with exit-strategy. Br. J. Ophthalmol. 106, 246–250 (2022).
Nguyen, V. et al. Outcomes of suspending VEGF inhibitors for neovascular age-related macular degeneration when lesions have been inactive for 3 months. Ophthalmol. Retina 3, 623–628 (2019).
Adrean, S. D., Chaili, S., Grant, S. & Pirouz, A. Recurrence rate of choroidal neovascularization in neovascular age-related macular degeneration managed with a treat-extend-stop protocol. Ophthalmol. Retina. 2, 225–230 (2018).
Hirata, Y. et al. Recurrence of neovascular age-related macular degeneration after cessation of treat and extend regimen. Sci. Rep. 12, 14768 (2022).
Lalwani, G. A. et al. A variable-dosing regimen with intravitreal Ranibizumab for neovascular age-related macular degeneration: Year 2 of the pronto study. Am. J. Ophthalmol. 148, 43–58 (2009).
Rosenberg, D. et al. Efficacy, safety, and treatment burden of treat-and-extend versus alternative anti-VEGF regimens for nAMD: A systematic review and meta-analysis. Eye 37, 6–16 (2023).
Urbano, C. A. et al. Real-World treatment patterns in a population with neovascular AMD treated with Anti-VEGF agents. Ophthalmic Surg. Lasers Imaging Retina 52, 190–198 (2021).
Bailey, C. et al. Intralesional macular atrophy in anti-vascular endothelial growth factor therapy for age-related macular degeneration in the IVAN trial. Ophthalmology 126, 75–86 (2019).
Gillies, M. C. et al. Long-term outcomes of treatment of neovascular age-related macular degeneration: Data from an observational study. Ophthalmology 122, 1837–1845 (2015).
Traine, P. G., Pfister, I. B., Zandi, S., Spindler, J. & Garweg, J. G. Long-term outcome of intravitreal aflibercept treatment for neovascular age-related macular degeneration using a treat-and-extend regimen. Ophthalmol. Retina 3, 393–399 (2019).
Maguire, M. G. et al. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: The comparison of age-related macular degeneration treatments trials. Ophthalmology 123, 1751–1761 (2016).
Cho, S. C., Park, K. H., Park, S. J., Joo, K. & Woo, S. J. Discontinuation of treatment and retreatment of neovascular age-related macular degeneration in the real-world: Bundang AMD cohort study report 5. Front. Med. 10, 1204026 (2023).
Munk, M. R. et al. The impact of the vitreomacular interface in neovascular age-related macular degeneration in a treat-and-extend regimen with exit strategy. Ophthalmol. Retina 2, 288–294 (2018).
Garweg, J. G. et al. Continued anti-VEGF treatment does not prevent recurrences in eyes with stable neovascular age-related macular degeneration using a treat-and-extend regimen: A retrospective case series. Eye 36, 862–868 (2022).
Arendt, P. et al. Exit strategy in a treat-and-extend regimen for exudative age-related macular degeneration. Retina 39, 27–33 (2019).
Kuroda, Y. et al. Factors associated with recurrence of age-related macular degeneration after Anti-Vascular endothelial growth factor treatment: A retrospective cohort study. Ophthalmology 122, 2303–2310 (2015).
Cho, H. J. et al. Neovascular age-related macular degeneration without exudative recurrence over 24 months after initial remission. Sci. Rep. 12, 15662 (2022).
Ying, G. S. et al. Association of baseline characteristics and early vision response with 2-year vision outcomes in the comparison of AMD treatments trials (CATT). Ophthalmology 122, 2523–2531 (2015).
Ashraf, M., Souka, A. & Adelman, R. A. Age-related macular degeneration: Using morphological predictors to modify current treatment protocols. Acta Ophthalmol. 96, 120–133 (2018).
Author information
Authors and Affiliations
Contributions
K.K. formulated the original idea and contributed to the study design. S.Y.Y. contributed to the study design and data analysis. J.L. and J.C. analyzed data and reviewed images.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, J., Choi, J., Yu, SY. et al. Recurrence of neovascular age-related macular degeneration after discontinuation of modified treat and extend treatment. Sci Rep 15, 8952 (2025). https://doi.org/10.1038/s41598-025-92832-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-92832-w