Abstract
Endometriosis is a complex disorder characterized by genetic, immune, inflammatory, and multifactorial etiologies. Dietary fiber, a crucial component abundant in fruits, vegetables, and whole grains, is known for its diverse beneficial effects on weight control, inflammation, insulin resistance, lipid metabolism, and hormonal balance. However, the relationship between dietary fiber and endometriosis remains unclear. This study aimed to investigate the association between dietary fiber intake and endometriosis. This study utilized cross-sectional data obtained from the National Health and Nutrition Examination Survey, encompassing information from women aged 20–54 in the United States between1999 and 2006. After adjusting for relevant covariates, multivariable logistic regression analysis revealed a positive correlation between dietary fiber intake and the risk of endometriosis. Compared to individuals in the lowest quartile of dietary fiber intake (Q1: 0–7.8 g/day), the adjusted odds ratios (OR) for endometriosis were as follows: OR of 1.08 (95% CI 0.78–1.51, P = 0.639) for Q2 (7.9–11.9 g/day), OR of 0.98 (95% CI 0.69–1.39, P = 0.898) for Q3 (12.0–17.5 g/day), and OR of 1.73 (95% CI 1.13–2.63, P = 0.011) for Q4 (17.6–128.3 g/day). The trend test demonstrated a statistically significant positive trend in the risk of endometriosis with increasing dietary fiber intake, showing an OR of 1.15 (95% CI 1.01–1.31, P = 0.034). These findings suggest a positive association between endometriosis and dietary fiber intake. Further investigations are crucial to establish causality and elucidate the potential preventive benefits of dietary fiber intake in endometriosis.
Similar content being viewed by others
Introduction
Endometriosis, a prevalent inflammatory condition, is characterized by the presence of tissue resembling endometrial tissue outside the uterus on organs and tissues within the pelvic region1. Endometriosis is estimated to affect 10% of reproductive-age women2, which extrapolates to approximately 190 million women worldwide, given the WorldBank’s population estimates for 20173. Based on NHANES data (1999–2006), the weighted prevalence of endometriosis in the United States was 9.4%4. Endometriosis is more common in 40–60% of women who have dysmenorrhea, 21–47% of women who are infertile, and 71–87% of women who have pelvic pain5. Notably, women with endometriosis incur healthcare costs more than twice as high as those without the condition6. Its widespread prevalence has led to its classification as a public health issue7. Although endometriosis significantly affects quality of life and healthcare costs8, little is known about modifiable risk factors that can stop it from occurring. Due to effects on steroid hormones, inflammation, or food pollutants, dietary factors may play a part in the etiology of endometriosis.
Endometriosis is characterized by chronic inflammation and hormonal dysregulation, particularly involving estrogen metabolism1. Dietary fiber, an essential nutrient present in large quantities in fruits, vegetables, and whole grains, has been shown to benefit human health in a number of ways, including the management of inflammation, lipid metabolism, weight control, insulin resistance, and hormonal imbalances9,10,11,12. While dietary fiber is known for its anti-inflammatory properties and ability to modulate hormonal balance, it may also interact with these mechanisms in complex ways. For instance, high fiber intake can influence gut microbiota composition, potentially affecting estrogen metabolism and systemic inflammation in ways that are not yet fully understood13. Until now, there has been little research on the relationship between dietary fiber and endometriosis. To date, three studies have examined the associations between fruit and vegetable consumption and endometriosis risk, with differing findings. One case–control study reported lower fresh fruit and green vegetable intake in women with laparoscopically confirmed endometriosis14. Another population-based case–control study found no association with vegetable consumption but observed higher fruit intake among affected women15. A prospective cohort study suggested a non-linear inverse association between fruit consumption, particularly citrus fruits, and endometriosis risk, while certain vegetables (e.g., cruciferous) were linked to a higher risk16.
The above three studies focused on the relationship between vegetable and fruit intake and endometriosis. As far as we know, there are no studies that specifically focus on the relationship between dietary fiber intake and endometriosis. This cross-sectional study aims to explore this connection among American women aged 20 to 54, utilizing a substantial sample size (4453 participants) to address these information gaps. Our findings aim to provide new insights into endometriosis prevention strategies in the United States.
Materials and methods
Data source
The National Health and Nutrition Examination Survey (NHANES) and the National Center for Health Statistics (NCHS) provided the data for this investigation. The investigation covered 4 consecutive 2-year NHANES cycles from 1999 to 2006. A nationally representative stratified sample gathered through interviews and physical examinations was used by NHANES. Ethical approval for the study was granted by the NCHS Ethics Review Committee. In accordance with ethical research guidelines, all study participants provided written informed consent.
Participants and definition
Endometriosis determination relied on participants’ responses to a specific question in the reproductive health questionnaire: "Has a doctor or other health professional ever told you that you had endometriosis?" Participants answering affirmatively were categorized as patients. Since the questionnaire only addressed the relevant questions to individuals aged 20–54, our study population was limited to participants within this age range.
Dietary fiber assessment
Assessment of dietary fiber consumption was conducted through the NHANES dietary survey, a component of the "What We Eat in America" survey. This survey was carried out at the Mobile Examination Center (MEC) using a 24-h recall method administered by skilled interviewers. The NHANES computer-assisted dietary interview (CADI) system documented participants’ food and beverage intake within the 24 h preceding the interview18.
Participants were randomized, in accordance with the study design, to data collecting sessions that took place in the morning, afternoon, or evening. Nutritional intake and dietary fiber were assessed using the University of Texas Food Intake Analysis System and the US Department of Agriculture Survey Nutrients Database. It is important to note that pharmaceuticals or dietary supplements were excluded from nutritional calculations. Two 24-h dietary recall interviews were conducted, with a second interview completed 3–10 days later over the phone. The initial in-person interview at the MEC was chosen for analysis, given the widespread use of the 24-h recall method in large-scale surveys19.
Measurements
Our study considered a comprehensive set of covariates, drawing from variables identified in the literature20,21,22. Age, marital status, race/ethnicity, education level, family income, smoking status, physical activity, BMI, alcohol consumption, use of birth control pills, high blood pressure, diabetes, coronary heart disease, chronic bronchitis, caloric consumption, total fat intake, total cholesterol intake, and usage of nutritional supplements were among these variables. Racial and ethnic categories included non-Hispanic white, non-Hispanic black, Mexican American, and other races. Marital status categories were defined as living with a partner or living alone. Educational attainment was stratified into fewer than nine years, nine to twelve years, and more than twelve years. Family income, assessed using the Poverty Income Ratio (PIR), categorized income into low, medium, and high based on ranges from 1.3 to 3.5, as per the US government’s Agriculture report23. Smoking status was dichotomized into smokers and never smokers (defined as those who had smoked fewer than 100 cigarettes). Alcohol drinking status was determined by the survey question, “In any 1 year, have you had at least 12 drinks of any type of alcoholic beverage?” Participants who answered “yes” were defined as alcohol drinkers. Physical activity levels were categorized into three groups: unable to perform physical activity, moderate (defined as at least 10 min of movement within the previous 30 days resulting in only light perspiration or a mild to moderate increase in respiration or heart rate), and vigorous (at least 10 min of activity within the last 30 days resulting in profuse sweating or an increased heart rate). The determination of previous disease (high blood pressure, diabetes, coronary heart disease and chronic bronchitis) was based on the inquiry in the questionnaire of whether the doctor had been informed of the condition in the past.
Participants underwent a dietary recall interview before the Mobile Examination Center (MEC) interview to collect 24-h nutritional data, including calorie intake and macronutrients. Additionally, information on medications, including dietary supplements, taken in the previous month was collected.
Statistical analyses
This secondary analysis of publicly available datasets utilized descriptive statistics to characterize continuous variables (mean/SD or median/IQR) and proportions (%) for categorical variables. Group differences were assessed using Kruskal–Wallis tests and one-way analyses of variance. Logistic regression models were employed to investigate the relationship between dietary fiber intake and endometriosis across three models. Model 1 adjusted for sociodemographic (age, race/ethnicity, education level, family income and marital status). Model 2 was adjusted for Model 1 plus BMI, smoking status, vigorous activity, moderate activity, alcohol drinker, birth control pills taken, high blood pressure, diabetes, coronary heart disease and chronic bronchitis, and Model 3 was adjusted for Model 2 plus calorie consumption, total fat consumption, total cholesterol consumption and dietary supplements taken. These models aimed to comprehensively account for potential confounding factors and refine the understanding of the relationship between dietary fiber intake and endometriosis.
Potential modifiers of the association between dietary fiber intake and endometriosis were explored, incorporating variables such as family income (low vs. medium or high), marital status (living with a partner vs. living alone), smoking status, and dietary supplements taken. Multivariate logistic regression assessed heterogeneity among subgroups, and interactions between subgroups and dietary fiber intake were scrutinized through likelihood ratio testing.
To ensure the robustness of the findings, sensitivity analyses were conducted by excluding participants with extreme energy intake, defined as those consuming fewer than 500 or more than 5000 kcal per day. This meticulous approach aimed to evaluate the consistency and reliability of results under different conditions, enhancing the validity of study outcomes.
Sample size determination relied on available data, and no a priori statistical power assessments were carried out. Statistical analyses were performed using R 3.3.2, a statistical software program developed by The R Foundation, Shanghai, China (accessed on 10 January 2023), along with Free Statistics Software 1.524. A two-tailed analysis was employed for hypothesis testing, with a significance level of 0.05 considered for determining statistical significance. This widely accepted threshold reflects a standard level of confidence in interpreting the results and drawing meaningful conclusions from the conducted analyses.
Results
Study population
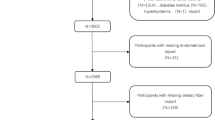
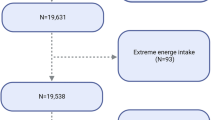
The original dataset comprised 5557 female participants. After excluding individuals with missing endometriosis-related information and those falling outside the age range of 20 to 54 years, our final study population consisted of 4453 women. Among them, 337 were diagnosed with endometriosis, while 4116 were not. Exclusions were applied to 991 pregnant women, and 113 lacking information on dietary fiber intake. Figure 1 provides a detailed illustration of the inclusion and exclusion process.
Baseline characteristics
Table 1 outlines the fundamental characteristics of the 4453 participants in the study. Among the sample, 337 individuals (7.6%) received a diagnosis of endometriosis, and 2820 (63.3%) were categorized as overweight. A total of 1741 participants (39.1%) re-ported being smokers, while 2099 (47.1%) acknowledged the use of dietary supplements. Regarding age distribution, 2378 participants (53.4%) were below 40 years old, 1424 (32%) were between 40 and 50 years old, and 651 (14.6%) were above 50 years old. Notably, dietary fiber intake appeared to be higher in individuals who used dietary supplements, engaged in exercise, lived with a partner, had a higher income, were older than 50 years, had a BMI < 25, and had an education level < 9 years or > 12 years. These baseline characteristics offer an overview of the diversity within the study population and establish the foundation for subsequent analyses exploring the association between these factors and endometriosis.
Relationship between dietary fiber intake and endometriosis
Table 2 presents the relationships between dietary fiber intake and endometriosis. The univariate analysis revealed significant associations, indicating that dietary fiber intake, age, family income, race, education level, use of birth control pills, high blood pressure, chronic bronchitis, and dietary supplements taken were correlated with the presence of endometriosis. These findings highlight a complex interplay between dietary factors, demographic variables, and lifestyle choices influencing the occurrence of endometriosis. Subsequent multivariate analyses will further dissect these associations to elucidate the independent contributions of each factor to the risk of endometriosis.
In comparison to individuals with lower dietary fiber consumption (Q1 < 7.8 g/day), and with adjustments for age, marital status, race/ethnicity, education level, family income, smoking status, physical activity, BMI, alcohol consumption, use of birth control pills, high blood pressure, diabetes, coronary heart disease, chronic bronchitis, caloric consumption, total fat intake, total cholesterol intake, and usage of nutritional supplements, the adjusted odds ratios (OR) for endometriosis in the Q2 (7.9–11.9 g/day) and Q3 (12.0–17.5 g/day) groups were 1.08 (95% CI 0.78–1.51, P = 0.639) and 0.98 (95% CI 0.69–1.39, P = 0.898), respectively. The highest quartile, Q4 (17.6–128.3 g/day), showed a significant increase with an OR of 1.73 (95% CI 1.13–2.63, P = 0.011) (Table 3). A significant positive trend across increasing fiber intake suggests an association between higher dietary fiber consumption and an increased risk of endometriosis (OR = 1.15; 95% CI 1.01–1.31, P = 0.034) (Table 3).
Stratified analyses based on additional variables
In a meticulous examination of various subgroups, we conducted stratified analyses to assess potential modifications in the relationship between dietary fiber intake and endometriosis (refer to Fig. 2). Remarkably, no significant interactions were detected in any subgroups, irrespective of stratification by age, race, BMI, marital status, family income, smoking status, education level, or dietary supplements taken. These results suggest that the observed association between dietary fiber intake and endometriosis holds consistently across diverse demographic and lifestyle factors, reinforcing the robustness of the findings.
The relationship between dietary fiber intake and endometriosis according to basic features. Except for the stratification component itself, each stratification factor was adjusted for all other variables (age, marital status, race/ethnicity, education level, family income, BMI, calorie consumption, vigorous activity, moderate activity, dietary supplements taken and smoking status).
Sensitivity analysis
The sensitivity analysis involved 4375 individuals after excluding those with daily caloric intakes below 500 kcal or above 5000 kcal. The adjusted odds ratios (OR) for endometriosis compared to the lowest fiber intake group (Q1 < 7.8 g/day) were 1.11 (95% CI 0.79–1.54, P = 0.546) for Q2 (7.9–11.9 g/day), 1.04 (95% CI 0.73–1.49, P = 0.811) for Q3 (12.0–17.5 g/day), and 1.74 (95% CI 1.14–2.63, P = 0.010) for Q4 (17.6–128.3 g/day). These findings demonstrate a statistically significant positive trend (OR = 1.16; 95% CI 1.02–1.32, P = 0.024) in endometriosis risk with increasing dietary fiber intake, reinforcing the relationship’s robustness against dietary energy variations Detailed results of this analysis are provided in the Supplementary Materials.
Moreover, we have expanded our sensitivity analysis to include a stratified approach by several potential modifiers, such as age, BMI, family income, race/ethnicity, and smoking status. Each subgroup was further analyzed across quartiles of dietary fiber intake to comprehensively assess potential variations in the association between dietary fiber intake and the risk of endometriosis. These findings from the sensitivity analysis underscore the complexity of the relationship between dietary fiber intake and endometriosis risk, suggesting that individual characteristics such as age, BMI, income, and race might influence this association Detailed results of the stratified analysis are provided in the Supplementary Materials.
Discussion
In this extensive cross-sectional study involving American adults, a noteworthy and previously unexplored positive correlation between endometriosis and dietary fiber intake was identified. After stratified analysis, these associations were more significant among patients < 40 years of age, middle-income families, 9–12 years of education level, living alone, and not eligible for dietary supplements taken. The sensitivity analysis confirmed the robustness of our findings, with the highest quartile of dietary fiber intake (Q4) consistently associated with a significantly increased risk of endometriosis. This association may reflect a non-linear relationship influenced by extremes in dietary intake, gut microbiota composition, estrogen metabolism, or unmeasured dietary patterns. Future longitudinal or interventional studies are needed to further explore these mechanisms and validate the findings.
Dietary fiber, a vital component abundant in fruits, vegetables, and whole grains, manifests multiple beneficial effects on human health. These benefits encompass regulation of body weight, modulation of inflammation, improvement of insulin sensitivity, lipid metabolism, and hormonal regulation10,11,12,13. Prior research indicates that a high-fiber diet lowers serum estrogen levels in premenopausal women25,26. Observational studies have posited that plant-based and fiber-rich diets promote estrogen excretion and diminish levels of bioavailable estrogen, potentially reducing endometriosis risk27,28. Dietary fiber exerts a profound influence on human health by modulating gut microbial ecology and host physiology29. Certain gut bacteria, by fermenting dietary fiber, generate short-chain fatty acids, which confer protection against endometriosis30. Epidemiological evidence robustly links a high intake of dietary fiber to a decreased risk of several chronic inflammatory diseases31, noting that the beneficial effects vary depending on the types of fibers and their dietary sources32,33,34. For instance, insoluble fibers and those derived from cereals are strongly inversely associated with the risk of coronary heart disease32, whereas the most significant benefits for conditions like Crohn’s disease and diverticulitis are noted with fruit fibers 33,34. Not all fibers exert equivalent effects. Both in vitro and in vivo studies have demonstrated that structural variances in dietary fibers can trigger distinct anti-inflammatory responses35,36,37. For example, apple-derived pectin, compared to inulin, distinctly influences gut microbiome composition and significantly promotes Eubacterium eligens35. Recent experimental findings have further clarified the role of dietary fiber in influencing gut microbiota composition and its involvement in the pathogenesis of inflammation by impairing intestinal barrier function and enhancing permeability38,39,40. These insights collectively provide compelling human evidence supporting diverse fiber-gut-microbiome interactions pertinent to chronic systemic inflammation 41. The study suggests that high dietary fiber intake may mitigate the risk of chronic inflammatory diseases such as cardiovascular disease and inflammatory bowel disease, partly by alleviating chronic systemic inflammation induced by gut microbiota dysbiosis41.
Few human research has examined the relationship between endometriosis and food, with inconsistent results for the dietary components that were examined. To our knowledge, there are currently few studies that specifically look at the relationship between dietary fiber intake and endometriosis. There are currently three ancillary research investigating the relationship between consumption of fruits and vegetables and endometriosis. Women with endometriosis were found to consume more fruit in a population-based case–control research conducted in Washington state, USA, but there was no link with vegetable consumption15. On the other hand, those who had endometriosis confirmed laparoscopically showed a significantly lower consumption of fresh fruit and green vegetables14. Trabert et al.15 suggested that the elevated risk of endometriosis observed in their study could potentially be attributed to pesticide exposure through fruit consumption. Furthermore, a prospective cohort study16 that included information from 70,835 premenopausal women gathered as part of the Nurses’ Health Study II cohort between 1991 and 2013 revealed a non-linear inverse relationship between the risk of endometriosis confirmed by laparoscopy and increased fruit consumption. With citrus fruits, this inverse connection was particularly noticeable. In contrast, there was a higher incidence of endometriosis linked to the consumption of corn, cruciferous vegetables, and peas/lima beans. Harris et al.16 explain that beta-cryptoflavin in fruits may reduce the risk of endometriosis, while consumption of some vegetables increases the risk of endometriosis, possibly because gastrointestinal symptoms make endometriosis-related pain worse.
Although our study’s findings differ from previous research, there may be more complex mechanisms influencing the relationship between dietary fiber and endometriosis. Firstly, phytoestrogens are plant-derived compounds with estrogenic properties, primarily falling into two groups: isoflavones and lignans, abundantly present in the human diet. While initially recognized for their mild estrogenic activity, many of these compounds exhibit various other biological activities that could potentially impact disease risk42,43. In a particular study44, cereal fiber demonstrated a more pronounced positive association with endometrial cancer risk in postmenopausal women, although the trend was not distinctly clear. Similarly, a different study45 found a link between eating cereal fiber and a lower risk of endometrial cancer.
Secondly, polysaccharides such as cellulose, pectin, and beta-glucans are easily broken down by the microbiota in the colon but resist digestion by enzymes in the small intestine. The gut microbiota can modify the kinds or content of hormones in circulation and increase their levels through deconjugation by means of processes including hydroxylation/dehydroxylation and methylation/demethylation46. The types and quantities of circulating steroid hormones may be influenced by the makeup of the gut microbiota, potentially increasing the risk of endometriosis at high levels. Additionally, a small number of investigations employing animal models indicate that the consumption of fiber and/or the metabolites it produces may be detrimental to the health of the host in some circumstances, such as colitis47 and/or colon cancer48. While the majority of the research on the health effects of fiber is positive, there may be some situations in which generalizations should be made with care. It is crucial to emphasize that tolerance to dietary fiber varies among individuals and frequently gets better with time as the gastrointestinal tract and microbiota adjust to higher dietary fiber intake49.
Thirdly, it has been demonstrated that excessive consumption of certain rapidly fermenting fibers can lead to the accumulation of metabolites known as short-chain fatty acids (SCFAs), which may be detrimental to the mucosa of the intestines. This phenomenon could compromise the integrity of the intestinal barrier, induce mucosal inflammation, and heighten the sensitivity of the viscera50. Another study indicated that the ingestion of short-chain oligofructose increased intestinal permeability, although this effect was confined to the colon51. A study from France revealed that mice fed either oligofructose or lactose exhibited heightened numbers of mucosal mast cells in the proximal colon compared to the control group, accompanied by increased ab-dominal sensitivity. The activation of mast cells triggers the release of pro-inflammatory cytokines, such as IL-10 and IL-33, into the local environment. This series of events leads to elevated levels of inflammation in the body, which may be associated with endometriosis.
Our study has some strengths. It is the first research explicitly examining the as-sociation between dietary fiber consumption and endometriosis. We identified a positive correlation between dietary fiber intake and endometriosis, and these results remained robust after conducting multiple regression and sensitivity analyses. However, our study is not without limitations. Firstly, one important restriction is the dearth of data on the relationship between endometriosis risk and other fiber subtypes, like soluble fiber, cellulose, and lignin. Secondly, the 24-h dietary recall interviews introduces measurement errors that may impact the results. Additionally, the cross-sectional design of our study precludes the ability to establish causality or assess the temporal relationship between dietary fiber intake and endometriosis risk. Moreover, the diversity and composition of gut microbiota could potentially modify the effects of dietary fiber on endometriosis risk. Dietary fiber serves as a substrate for microbial fermentation, generating metabolites such as SCFAs that influence systemic inflammation, immune modulation, and estrogen metabolism—critical pathways in endometriosis development. The absence of microbiome data in this study limits our ability to account for these potential interactions. Future research incorporating microbiome profiling could provide valuable insights into the complex relationship between dietary fiber intake, gut microbiota, and endometriosis.
Conclusion
In summary, our findings highlight a positive association between dietary fiber intake and endometriosis risk. This study underscores the need for further exploration of underlying mechanisms and the development of targeted preventive measures.
Data availability
The datasets used in this study are publicly available and can be accessed through the National Health and Nutrition Examination Survey (NHANES) repository at https://wwwn.cdc.gov/nchs/nhanes/default.aspx (accessed on March 5, 2024). Detailed information about the survey design, data collection, and variables is available on this website.
References
Zondervan, K. T., Becker, C. M. & Missmer, S. A. Endometriosis. N Engl J Med 382, 1244–1256 (2020).
Shafrir, A. L., Farland, L. V., Shah, D. K., Harris, H. R., Kvaskoff, M., Zondervan, K., Missmer, S. A.: Risk for and consequences of endometriosis: A critical epidemiologic review.
Population ages 15–64 (% of population) https://data.worldbank.org/indicator/SP.POP.1564.TO.ZS
Hall, M. S., Talge, N. M. & Upson, K. Urinary cadmium and endometriosis prevalence in a US nationally representative sample: results from NHANES 1999–2006. Hum Reprod 38, 1835–1842 (2023).
Giampaolino, P. et al. Dioxin and endometriosis: A new possible relation based on epigenetic theory. Gynecol Endocrinol 36, 279–284 (2020).
Soliman AM, Surrey ES, Bonafede M, Nelson JK, Vora JB, Agarwal SK: Health care utilization and costs associated with endometriosis among women with medicaid insurance.
Agarwal, S. K. et al. Clinical diagnosis of endometriosis: A call to action. Am J Obstet Gynecol 220, 354 (2019).
Simoens, S. et al. Endometriosis cost assessment (the EndoCost study): A cost-of-illness study protocol. Gynecol Obstet Invest 71, 170–176 (2011).
Reynolds, A. N., Akerman, A. P. & Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med 17, e1003053 (2020).
Rock, C. L. et al. Effects of a high-fiber, low-fat diet intervention on serum concentrations of reproductive steroid hormones in women with a history of breast cancer. J Clin Oncol 22, 2379–2387 (2004).
Wayne, S. J., Neuhouser, M. L., Ulrich, C. M., Koprowski, C., Baumgartner, K. B., Baumgartner, R. N., Ballard-Barbash, R. Dietary fiber is associated with serum sex hormones and insulin-related peptides in postmenopausal breast cancer survivors.
Chandalia, M. et al. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med 342, 1392–1398 (2000).
Sonnenburg, E. D., Sonnenburg, J. L. Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates.
Parazzini, F. et al. Selected food intake and risk of endometriosis. Hum Reprod 19, 1755–1759 (2004).
Trabert, B., Peters, U., De Roos, A. J., Scholes, D. & Holt, V. L. Diet and risk of endometriosis in a population-based case-control study. Br J Nutr 105, 459–467 (2011).
Harris, H. R., Eke, A. C., Chavarro, J. E. & Missmer, S. A. Fruit and vegetable consumption and risk of endometriosis. Hum Reprod 33, 715–727 (2018).
Raper, N., Perloff, B., Ingwersen, L., Steinfeldt, L. & Anand, J. An overview of USDA’s dietary intake data system. J. Food Compos. Anal. 17, 545–555 (2004).
Foster, E. et al. Validity and reliability of an online self-report 24-h dietary recall method (Intake24): A doubly labelled water study and repeated-measures analysis. J Nutr Sci 8, e29 (2019).
Liu, H. A.-O., Wang, L., Chen, C., Dong, Z. A.-O., Yu, S. Association between Dietary Niacin Intake and Migraine among American Adults: National Health and Nutrition Examination Survey. LID https://doi.org/10.3390/nu14153052.
Xiao, Q. et al. L-shaped association of serum 25-hydroxyvitamin D concentrations with cardiovascular and all-cause mortality in individuals with osteoarthritis: Results from the NHANES database prospective cohort study. BMC Med 20, 308 (2022).
Zhang, Y. Y., Qiu, H. B., Tian, J. W.: Association between vitamin D and hyperuricemia among adults in the United States.
What We Eat in America: Data Tables https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables/
Yang, Q. et al. Association between preadmission metformin use and outcomes in intensive care unit patients with sepsis and type 2 diabetes: A cohort study. Front Med (Lausanne) 8, 640785 (2021).
Rose, D. P., Goldman, M., Connolly, J. M. & Strong, L. E. High-fiber diet reduces serum estrogen concentrations in premenopausal women. Am. J. Clin. Nutr. 54, 520–525 (1991).
Bagga, D. et al. Effects of a very low fat, high fiber diet on serum hormones and menstrual function implications for breast cancer prevention. Cancer 76, 2491–2496 (1995).
Kaneda, N., Nagata, C., Kabuto, M., & Shimizu, H.: Fat and fiber intakes in relation to serum estrogen concentration in premenopausal Japanese women.
Armstrong, B. K., Brown, J. B., Clarke, H. T., Crooke, D. K., Hähnel, R., Masarei, J. R., & Ratajczak, T.: Diet and reproductive hormones: A study of vegetarian and nonvegetarian postmenopausal women.
Makki, K., Deehan, E. C., Walter, J. & Backhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 23, 705–715 (2018).
Chadchan, S. B., Popli, P., Ambati, C. R., Tycksen, E., Han, S. J., Bulun, S. E., Putluri, N., Biest, S. W., Kommagani, R.: Gut microbiota-derived short-chain fatty acids protect against the progression of endometriosis. Life Sci. Alliance 2021, 4.
Reynolds, A. et al. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 393, 434–445 (2019).
Threapleton, D. E. et al. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ: Br. Med. J. 347, f6879 (2013).
Ananthakrishnan, A. N. et al. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology 145, 970–977 (2013).
Ma, W., Nguyen, L. H., Song, M., Jovani, M., Liu, P.-H., Cao, Y., Tam, I., Wu, K., Giovannucci, E. L., Strate, L. L., Chan, A. T. Intake of dietary fiber, fruits, and vegetables and risk of diverticulitis. Off. J. Am. College Gastroenterol. ACG 2019, 114.
Chung, W. S. F. et al. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol. 14, 3 (2016).
Baxter, N. T., Schmidt, A. W., Venkataraman, A., Kim, K. S., Waldron, C., Schmidt, T. A.-O.: Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. LID - https://doi.org/10.1128/mBio.02566-18 LID - e02566–18.
Deehan, E. C., Yang, C., Perez-Muñoz, M. E., Nguyen, N. K., Cheng, C. C., Triador, L., Zhang, Z., Bakal, J. A., Walter, J. Precision microbiome modulation with discrete dietary fiber structures directs short-chain fatty acid production.
Llewellyn, S. R. et al. Interactions between diet and the intestinal microbiota alter intestinal permeability and colitis severity in mice. Gastroenterology 154, 1037-1046.e1032 (2018).
Schroeder, B. O. et al. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe 23, 27-40.e27 (2018).
Desai, M. S. et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167, 1339-1353.e1321 (2016).
Ma, W. et al. Dietary fiber intake, the gut microbiome, and chronic systemic inflammation in a cohort of adult men. Genome Med 13, 102 (2021).
Setchell, K. D. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr 68, 1333S-1346S (1998).
Adlercreutz, H. & Mazur, W. Phyto-oestrogens and Western diseases. Ann Med 29, 95–120 (1997).
Cui, X., Rosner, B., Willett, W. C. & Hankinson, S. E. Dietary fat, fiber, and carbohydrate intake in relation to risk of endometrial cancer. Cancer Epidemiol Biomarkers Prev 20, 978–989 (2011).
Chen, K., Zhao, Q., Li, X., Zhao, J., Li, P., Lin, S., Wang, H., Zang, J., Xiao, Y., Xu, W., et al. Dietary fiber intake and endometrial cancer risk: A systematic review and meta-analysis. Nutrients 2018, 10.
Hullar, M. A., Burnett-Hartman, A. N. & Lampe, J. W. Gut microbes, diet, and cancer. Cancer Treat Res 159, 377–399 (2014).
Miles, J. P. et al. Supplementation of low- and high-fat diets with fermentable fiber exacerbates severity of DSS-induced acute colitis. Inflamm Bowel Dis 23, 1133–1143 (2017).
Belcheva, A. et al. Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells. Cell 158, 288–299 (2014).
Mego, M., Accarino, A., Tzortzis, G., Vulevic, J., Gibson, G., Guarner, F., Azpiroz, F.A.-O.X. Colonic gas homeostasis: Mechanisms of adaptation following HOST-G904 galactooligosaccharide use in humans. LID - https://doi.org/10.1111/nmo.13080.
Gibson, P. R., Halmos, E. P. & Muir, J. G. Review article: FODMAPS, prebiotics and gut health-the FODMAP hypothesis revisited. Aliment Pharmacol Ther 52, 233–246 (2020).
Schepens, M. A. et al. Dietary calcium decreases but short-chain fructo-oligosaccharides increase colonic permeability in rats. Br J Nutr 104, 1780–1786 (2010).
Kamphuis, J. B. J. et al. Lactose and fructo-oligosaccharides increase visceral sensitivity in mice via glycation processes, increasing mast cell density in colonic mucosa. Gastroenterology 158, 652–663 (2020).
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
B.X. and Y.H. wrote the main manuscript text. J.H. and L.F. prepared the analysis and methodology. Y.W. and X.L. contributed to data curation and formal analysis. Y.W. and L.F. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
Since the secondary analysis did not require extra institutional review board approval, ethical review and approval were waived for this investigation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, Y., Liang, F., Wei, Y. et al. Unveiling the correlation between dietary fiber intake and endometriosis: a cross-sectional analysis of NHANES data. Sci Rep 15, 9202 (2025). https://doi.org/10.1038/s41598-025-92978-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-92978-7