Abstract
Very early onset inflammatory bowel disease (VEO-IBD) with interleukin-10 receptor-A (IL-10RA) defects is characterised by severe and unmanageable intestinal inflammation, perianal lesions, and a high mortality rate, with the onset of the disease occurring at a very early age. Currently, allogeneic hematopoietic stem cell transplantation (allo-HSCT) is one of the most effective treatments for VEO-IBD patients with IL-10 signaling deficiency. The objective of this study was to evaluate the clinical effectiveness of allo-HSCT in the treatment of children with VEO-IBD and IL-10RA deficiency, and to provide further clinical insights. A retrospective analysis and summary of the clinical data of seven patients with VEO-IBD and IL-10RA deficiency from January 2021 to December 2023 was performed. These patients subsequently underwent allo-HSCT after receiving a reduced-intensity conditioning regimen followed by a cyclosporine-based regimen for the prevention of graft versus host disease (GVHD). Hematopoietic reconstruction was performed on seven children with VEO-IBD combined with IL-10RA deficiency. Four patients developed grade I-II GVHD, while three patients developed grade III-IV GVHD after undergoing allo-HSCT. At a median follow-up of 518 days after allo-HSCT (range: 210–1072 days), six patients were alive, while one patient died 16 months after the procedure because of chronic GVHD and severe infections. The 3-year cumulative overall survival (OS) probability rate was 80.0% (95% CI: 44.7–100.0). All VEO-IBD patients demonstrated weight gain following HSCT, with substantial improvements observed in severe malnutrition and growth retardation associated with IL-10RA deficiency post-transplantation. Allo-HSCT is thus identified as the optimal curative therapy for VEO-IBD patients with IL10-RA deficiency. The importance of early multidisciplinary intervention and co-management of VEO-IBD is paramount in improving HSCT outcomes.
Similar content being viewed by others
Introduction
Inflammatory bowel disease (IBD) is a chronic inflammation of the gastrointestinal tract caused by a dysregulated immune response to commensal or pathogenic microorganisms. It includes ulcerative colitis (UC), Crohn’s disease (CD), and unclassified IBD (IBD-U)1,2. With the popularization of gastroenteroscopy and increasing awareness of IBD diseases, the number of cases of IBD among children has increased in recent decades3. Very early-onset inflammatory bowel disease (VEO-IBD) refers to IBD that occurs in children under the age of six, which accounts for 15% of all cases of IBD in children. In addition, IBD affects 1% of children under the age of two4,5. The cause of IBD is not fully understood, but it is believed to be the result of multiple factors, including genetics and the environment. In early-onset IBD, particularly within the first month after birth, genetic factors are thought to be the primary cause due to limited exposure to external environmental factors6. Among them, gene mutations in the interleukin-10 (IL-10) signaling pathway are closely related to VEO-IBD7,8,9. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is currently one of the most effective curative methods for VEO-IBD patients with IL-10 signaling deficiency.
VEO-IBD with IL-10 receptor-A (IL-10RA) defects is characterized by severe and unremitting intestinal inflammation, perianal lesions, and a high mortality rate, with an extremely early disease onset9. It frequently presents with perianal lesions, severe malnutrition, and growth retardation. Compared to traditional IBD, VEO-IBD mainly affects the colon and exhibits more severe clinical symptoms, for which the treatment of traditional IBD is less effective6,10. In recent years, it has been suggested that VEO-IBD is highly genetically related. One of most common single-gene defect causing VEO-IBD is believed to be IL-10 signaling defect9,11. IL-10RA serves as the ligand-binding subunit of IL-10R7. Recent studies have revealed that IL-10RA plays a crucial role in the immune response in the intestinal immune response, influencing the formation of the intestinal barrier and contributing to the pathogenesis of VEO-IBD12. This retrospective study analyzed the clinical data of seven patients with VEO-IBD combined with IL-10RA deficiency who underwent allo-HSCT. The aim of this study was to evaluate the clinical efficacy of allo-HSCT in treating children with VEO-IBD combined with IL-10RA deficiency and to provide more clinical experience.
Methods
Patients
A retrospective analysis was conducted on seven patients who were diagnosed with VEO-IBD with IL-10RA deficiency and treated with allo-HSCT. These patients were admitted to the hospital from January 2021 to December 2023. The median age of the patients at the time of HSCT was 49 months, with an age range from 4 months to 96 months. The diagnosis of VEO-IBD was confirmed on the basis of clinical manifestations, endoscopic evaluations, histopathology, and genome sequencing results. (Further details can be found in Table 1; Fig. 2, and Figure S1) Before and after HSCT, patients underwent detailed physical, laboratory, and endoscopic evaluations. Gene testing was conducted using the Illumina HiSeq2500 platform for whole-exome sequencing, achieving an average coverage of 100×. This study involving human participants was reviewed and approved by the Review Board and Ethics Committee of the Children’s Hospital affiliated to Zhengzhou University (NO. 2023-K-044). The procedures followed and described herein were in line with the Declaration of Helsinki as revised in 2013. The patient’s parents provided signed informed consent.
Treatment
Since VEO-IBD patients with IL-10RA mutations show severe clinical symptoms like malnutrition, pre-transplant infections, severe bowel disease, and/or post-surgery complications, all VEO-IBD patients underwent allogeneic HSCT following a reduced-intensity conditioning (RIC) regimen, as previous studies reported13,14. To reduce the incidence of transplantation failure and graft versus host disease (GVHD), we administered antithymocyte globulin (ATG)15,16. To prevent GVHD, a combined treatment regimen of cyclosporine and mycophenolate mofetil is used17. During the pancytopenic phase, all patients were kept in reverse isolation in rooms with a relative. Granulocyte colony-stimulating factor was administered to patients starting from day + 10 after HSCT until absolute neutrophil count (ANC) was >0.5 × 109/L for two consecutive days. After engraftment, all patients received cotrimoxazole for at least six months as prophylaxis against pneumocystis carinii infection. The supportive care and anti-infection treatment have been described in detail, nutritional therapy included partial parenteral and/or enteral nutritional support (amino acid-based formula)13,14,18.
Assessment and follow up
The study monitored patients from enrollment until December 31st, 2023, identifying events as either death or related to the progression of VEO-IBD. Overall survival rate (OS) was defined as the period from transplantation to the last follow-up or death. Clinical information and results, such as endoscopic evaluations, body weight and height were recorded. According to the World Health Organization child growth standards, the body mass index (BMI) for-age z scores before HSCT, six months after HSCT, and one year after HSCT were evaluated. Each patient’s BMI (kg/m2) was calculated. The BMI z-scores (BMI for age and sex) were used to distinguish between normal weight (z-score ≥ -1) and underweight (z-score < -1)19. The diagnosis and grading of acute or chronic GVHD were based on previously published criteria and scoring systems20.
Statistical analysis
SPSS 21.0 and GraphPad Prism 6.0 were employed for the statistical analysis. The continuous data are presented in the form of median (range). Categorical variable data are presented as frequencies and percentages. The categorical variables were compared using Pearson’s chi-square test. The cumulative OS was analyzed and depicted through Kaplan-Meier estimates. A p -value of less than 0.05 was considered statistically significant.
Results
Demographic features of patients
This study reviewed the clinical data of 7 VEO-IBD patients with IL-10RA deficiency. Table 1 showed the demographic information of the patients. Among these cases, three are male (42.9%). The median age of the patients at the onset of VEO-IBD was 2 months, with a range from 1 month to 36 months. It is noteworthy that none of the patients exhibited a family history of the disease, and their parents did not manifest any clinical symptoms. IL-10RA mutation in Cases 5 and 7 are homozygous mutations, but their parents are not consanguineous. The genetic mutations of the patients and their parents are shown in Table 1. The patients’ dominant feature was gastrointestinal involvement, with all experiencing intractable diarrhea and three also experiencing bloody stool. Multiple perianal complications were suffered by most patients (n = 5), including perianal abscess (n = 3) and perianal fistula (n = 3). Additionally, extraintestinal symptoms were manifested, including Oral ulcers (n = 4), respiratory infections (n = 2), skin rash (n = 2), and external auditory canal infection (n = 1). Based on the different clinical characteristics, endoscopy, and pathological features, all VEO-IBD patients were also idenitifed with Crohn’s disease (CD). According to pediatric CD activity index (PCDAI)21,22, there were 3 patients in the moderate disease activity phase, and 4 cases in the severe disease activity phase. Due to the inability to dilate or the ineffectiveness of dilation therapy for colorectal stenosis, two children underwent a protective colostomy prior to hematopoietic stem cell transplantation (see Table 1).
Patient characteristics after HSCT
There were no cases of primary implantation failure in any of the VEO-IBD patients. Furthermore, neutrophil recovery occurred within a median time of + 14 days (ranging from + 11 to + 17 days). Platelet recovery occurred within a median time of + 14 days (ranging from + 11 to + 30 days). In addition, four patients with VEO-IBD developed grade I–II GVHD after allo-HSCT, and three patients developed grade III–IV GVHD. The basic transplant characteristics of all patients are summarized in Table 2.
Outcome
The treatment plan encompassed the administration of mesalazine to all seven patients, followed by thalidomide and partial parenteral and/or enteral nutritional support for a duration of approximately three months. The treatment regimen was informed by prior experience in managing refractory paediatric IBD patients23,24. However, the intestinal symptoms and infections of the patients remained unresolved. Consequently, the administration of allo-HSCT was considered for these patients.
Table 3 displayed various grades of malnutrition before HSCT, and these grades showed significant improvements. The growth curve of BMI for age z-scores demonstrated that the nutritional status of all patients had improved compared to one year after HSCT. Among the patients, five (71.4%) were underweight prior to HSCT, yet only one (14.3%) exhibited a z score less than − 1 at one year post-HSCT (χ2 = 4.667, p = 0.031). (Fig. 1) Furthermore, the intestinal mucosa of VEO-IBD patients had greatly recovered after HSCT (Fig. 2).
Growth curve of body mass index (BMI) for age z-scores of seven patients before and after allo-HSCT. (A). World Health Organization (WHO) child growth curve of BMI for age z-scores for girls (0–5 years old). The curve displayed the malnutrition and improvement of patient 4 and patient 6 pre-HSCT and one year post-transplant. (B). WHO child growth curve of BMI for age z-scores for boys (0–5 years old). The curve displayed the malnutrition and improvement of patient 5 pre-HSCT and one year post-transplant. (C). WHO child growth curve of BMI for age z-scores for girls (5–19 years old). The curve displayed the malnutrition and improvement of patient 3 and patient 7 pre-HSCT and one year post-transplant. (D). WHO child growth curve of BMI for age z-scores for boys (5–19 years old). The curve displayed the malnutrition and improvement of patient 1 and patient 2 pre-HSCT and one year post-transplant.
Endoscopy evaluation images pre- and post-HSCT for the seven patients. (A, B) for patient 1, (C, D) for patient 2, (E, F) for patient 3, (G, H) for patient 4, (I, J) for patient 5, (K, L) for patient 6, M-N for patient 7). Prior to HSCT (A, C, E, G, I, K and M), the mucosa of the ascending colon, transverse colon, descending colon, sigmoid colon, and rectum exhibited the presence of polyps, haemorrhage, and ulcers, accompanied by the presence of erosions. After HSCT (B, D, F, H, J, L and N), mucosal oedema was observed in the same areas, but without ulceration, vesiculation, haemorrhage, or proliferative-like changes.
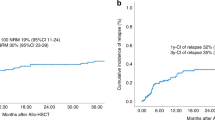
At a median follow-up of 518 days after allo-HSCT (range: 210–1072 days), six out of seven patients were still alive (Table 2), the 3-year cumulative OS probability was 80.0% (95% CI: 44.7–100.0) (Fig. 3). On the 12th day after transplantation, the Case 6 exhibited symptoms of a widespread rash across the body, severe diarrhoea, and a diagnosis of acute GVHD. Eight months after the transplant, the child developed mild to moderate chronic GVHD in the lungs. According to the treatment guideline for GVHD25, glucocorticoids and immunosuppressants were administered to this patient. However, due to the prolonged use of these medications for chronic GVHD, the patient ultimately died from a pulmonary fungal infection complicated by respiratory failure 16 months after the transplant.
Discussion
Symptoms of VEO-IBD patients with IL-10RA mutations typically include refractory colitis, perianal fistulae, and recurrent infections. However, there have also been reports of neonatal Crohn’s disease patients presenting with oral ulcers as their first symptom26. The clinical manifestations of VEO-IBD patients with IL-10RA defects in our dataset were similar to those previously reported27, Most patients presented with predominantly diarrhoea with perianal lesions, and some had extra-intestinal manifestations, such as oral infections, rashes, and external ear infections. Some of these children had received repeated medication and surgery before transplantation, but their condition were still poor. Conversely, some patients with VEO-IBD exhibited no perianal lesions and a potentially less severe phenotype at the onset of the disease, which may be attributed to parasite-driven selection pressure influencing the genetic variation of IL10RA in East Asia28.
When treating VEO-IBD, traditional methods may not be as effective due to haematopoietic defects caused by mutated genes. In such cases, allogeneic bone marrow stem cell or umbilical cord blood stem cell transplantation is preferred as a therapeutic option for children with VEO-IBD29. Previous study had reported that stem cell transplantation therapy can cure twenty types of monogenic mutation in VEO-IBD cases10. A clinical study was conducted to analyze 102 Chinese children with IBD who had IL-10 deficiency and underwent hematopoietic stem cell transplantation. The results showed that children who received transplantation (64.2%) had a higher overall survival rate compared to those who did not receive it (47.5%). The survival rate of transplanted children was influenced by factors such as post-HSCT lung infection and pre-HSCT enterostomy14. In another Indian cohort study, four VEO-IBD patients with IL-10R defects were received allo-HSCT treatment, the OS was 50% and the surviving patients did not experience a recurrence of any IBD features30. In the systematic review by Niusha et al.31, the data of 190 IL-10RA-deficient VEO-IBD patients reported in the literature was summarised. Of these patients, 39 underwent HSCT, resulting in a 3-year overall survival rate of 80%. Furthermore, the analysis revealed that HSCT led to complete remission in 78.9% of patients, thus substantiating the notion that HSCT constitutes a viable therapeutic modality for IL-10RA-deficient VEO-IBD patients. In our clinical data, a 3-year cumulative overall survival (OS) rate of 80.0% for children diagnosed with VEO-IBD who were treated with allo-HSCT. This finding is higher than the rates documented in previous studies13,14,30, and is analogous to the findings reported by Niusha et al.31. On one hand, this may be due to the fact that four of the children in this group were relatively old at the time of transplantation, and the nutritional and intestinal status of the patients were not poor. This reduced transplantation-related complications compared to those with low body weight and poor intestinal status. On the other hand, we carefully evaluated the nutritional status of the children with VEO-IBD and their co-infections before transplantation. We also provided them with supportive and symptomatic treatments. Pre-HSCT stabilization in conjunction with pediatric gastroenterologists is essential. The evaluation of nutritional status and co-infections of VEO-IBD children before transplantation was given great attention. Additionally, two out of seven patients underwent protective enterostomy, which not only improved their survival but also significantly increased their chances of receiving stem cell transplantation.
The optimal pre-conditioning regimen for HSCT is still a matter of debate. The use of myeloablative conditioning (MAC) regimens prior to HSCT is linked to a high incidence of transplant-related morbidity and mortality, as well as some long-term complications. However, a study of 21 VEO-IBD patients who received MAC regimens showed a sustained chimerism of over 95% and no recurrence of any IBD features, with an overall survival rate of 64% 30. Given the severe clinical symptoms experienced by VEO-IBD patients, such as malnutrition, pretransplant infections, severe bowel disease, or postsurgery status, RIC regimens appear to be the preferred choice12,14,32,33. In this study, we aimed for a balanced approach in selecting the preconditioning regimen. It has been demonstrated that ATG is a more optimal treatment option for paediatric HSCT, offering superior OS while reducing the risk of relapse and infectious complications16. Therefore, we have selected RIC regimens in combination with the use of ATG, and our results of this study indicate that no any cases of primary implantation failure. But in cases of cord blood transplantation, the lack of a complete match may have resulted in more severe complications of GVHD. However, even within RIC, there is scope to fine-tune the doses of chemotherapeutic agents and radiation. For example, adjusting the dosage of chemotherapy drugs like busulfan could potentially lower the risk of GVHD while still achieving sufficient immune suppression34. Besides traditional chemotherapeutic agents, drugs that specifically target certain pathways involved in GVHD pathogenesis could be explored, such as vedolizumab that involved in T-cell activation or migration, could be added to the conditioning regimen, which targets the α4β7 integrin and has shown promise in reducing the incidence of intestinal acute GVHD35. In the current study, it is clear that due to the small number of cases in the current study, more cases need to be included to evaluate the advantages and disadvantages of RIC versus MAC regimens.
IL-10RA is crucial for maintaining a healthy immune response in the intestine and for forming and preserving the intestinal barrier36. VEO-IBD patients with IL-10RA defects are often associated with low body weight and malnutrition. Nutrition therapy is an important aspect of the multidisciplinary management of IBD. It can prevent and cure malnutrition, promote growth and development in children, and prevent osteoporosis. Therefore, it is an indispensable clinical treatment measure for children with IBD at all stages37,38. Improving the nutritional status of children with VEO-IBD through enteral and/or parenteral routes, depending on their condition, is effective in reducing the incidence of complications related to transplantation39. The endoscopic mucosal changes observed in VEO-IBD with IL-10RA defect are classically characterized by skip lesions, with varying degrees of inflammation (including polyps, erythema, friability, erosions, and ulcers) next to areas of normal-seeming mucosa13,40, however, the damage to these mucosa has been improved to a certain extent after transplantation, and similar results had also been reported in other types of monogenic VEO-IBD41. Based on our experience, the body weight and intestinal mucosa of all patients in this study improved after HSCT with sensitive nutritional treatment and support care. Collaboration within a multidisciplinary team, including gastroenterologists, hematologists, nutritionists, gastrointestinal surgeons, and nurses, was crucial for the success of HSCT.
In summary, this is a retrospective case study with a small sample size. Further expansion is needed to explore the impact of clinical characteristics of pre-transplant patients on prognosis. The study provides clinical characteristics and efficacy of a single center, indicating that allo-HSCT is the optimal curative therapy for VEO-IBD patients with IL10R deficiency. However, not all VEO-IBD patients are eligible for HSCT42. Further large-scale studies are needed to develop a scoring system to guide decision making. In clinical practice, it is necessary to screen children with a family history of suspected genetic disorders, or chronic gastrointestinal symptoms and perianal lesions in children under six years of age. Early multidisciplinary intervention and co-management of VEO-IBD are key factors in improving HSCT outcomes.
Data availability
The datasets generated and/or analysed during the current study are available in the ClinVAR: Available at: https://www.ncbi.nlm.nih.gov/clinvar. Results from the analysis of this data for the current study are available from the corresponding author on reasonable request.
Abbreviations
- VEO-IBD:
-
Very early onset inflammatory bowel disease
- IL-10RA:
-
Interleukin-10 receptor-A
- allo-HSCT:
-
Allogeneic haematopoietic stem cell transplantation
- GVHD:
-
Graft versus host disease
- OS:
-
Overall survival
- UC:
-
Ulcerative colitis
- CD:
-
Crohn’s disease
- IBD-U:
-
Unclassified inflammatory bowel disease
- RIC:
-
Reduced-intensity conditioning
- ATG:
-
Antithymocyte globulin
- ANC:
-
Absolute neutrophil count
- BMI:
-
Body mass index
- PCDAI:
-
Pediatric Crohn’s disease activity index
- MAC:
-
Myeloablative conditioning
References
Banerjee, R. et al. Challenges in the diagnosis and management of inflammatory bowel disease in resource-limited settings in Asia. Lancet Gastroenterol. Hepatol. 5, 1076–1088. https://doi.org/10.1016/S2468-1253(20)30299-5 (2020).
Rosen, M. J., Dhawan, A., ,Saeed, S. A. & Inflammatory bowel disease in children and adolescents. JAMA Pediatr. 169, 1053–1060. https://doi.org/10.1001/jamapediatrics.2015.1982 (2015).
Lee, R. B. & Gasparetto, M. Novel pharmacological developments in the management of paediatric inflammatory bowel disease: time for guideline update - A narrative review. J. Paediatr. Child. Health https://doi.org/10.1111/jpc.16519 (2023).
Xiao, Y. et al. Comprehensive mutation screening for 10 genes in Chinese patients suffering very early onset inflammatory bowel disease. World J. Gastroenterol. 22, 5578–5588. https://doi.org/10.3748/wjg.v22.i24.5578 (2016).
Snapper, B. S Very-Early-onset inflammatory bowel disease. Gastroenterol. Hepatol. (N. Y.) 11, 554–556 (2015).
Kelsen, J. R., Russo, P., ,Sullivan, K. E. & Early-onset inflammatory bowel disease. Immunol. Allergy Clin. North Am. 39, 63–79. https://doi.org/10.1016/j.iac.2018.08.008 (2019).
Sandy, N. S. Al elevated IgA and IL-10 levels in very-early-onset inflammatory bowel disease secondary to IL-10 receptor deficiency. Rev. Paul Pediatr. 40, e2020434. https://doi.org/10.1590/1984-0462/2022/40/2020434 (2021).
Nameirakpam, J. et al. Genetics on early onset inflammatory bowel disease: an update. Genes Dis. 7, 93–106. https://doi.org/10.1016/j.gendis.2019.10.003 (2020).
Glocker, E. O. et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N. Engl. J. Med. 361, 2033–2045. https://doi.org/10.1056/NEJMoa0907206 (2009).
Nambu, R. A systematic review of monogenic inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 20: e653–e663. https://doi.org/10.1016/j.cgh.2021.03.021 (2022).
Collen, L. V. Al clinical phenotypes and outcomes in monogenic versus non-monogenic very early onset inflammatory bowel disease. J. Crohns Colitis 16, 1380–1396. https://doi.org/10.1093/ecco-jcc/jjac045 (2022).
Sasahara, Y. et al. Primary immunodeficiencies associated with early-onset inflammatory bowel disease in Southeast and East Asia. Front. Immunol. 12, 786538. https://doi.org/10.3389/fimmu.2021.786538 (2021).
Peng, K. et al. Umbilical cord blood transplantation corrects very early-onset inflammatory bowel disease in Chinese patients with IL10RA-associated immune deficiency. Inflamm. Bowel Dis. 24, 1416–1427. https://doi.org/10.1093/ibd/izy028 (2018).
Ye, Z. et al. Clinical outcome of infantile-onset inflammatory bowel disease in 102 patients with interleukin-10 signalling deficiency. Aliment. Pharmacol. Ther. 55, 1414–1422. https://doi.org/10.1111/apt.16837 (2022).
Mohty, M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia 21, 1387–1394. https://doi.org/10.1038/sj.leu.2404683 (2007).
Kang, H. M. et al. Efficacy of low dose antithymocyte globulin on overall survival, relapse rate, and infectious complications following allogeneic peripheral blood stem cell transplantation for leukemia in children. Bone Marrow Transpl. 56, 890–899. https://doi.org/10.1038/s41409-020-01121-9 (2021).
Ruutu, T. et al. Prophylaxis and treatment of GVHD: EBMT-ELN working group recommendations for a standardized practice. Bone Marrow Transpl. 49, 168–173. https://doi.org/10.1038/bmt.2013.107 (2014).
Zheng, C. et al. Phenotypic characterization of very Early-onset inflammatory bowel disease with interleukin-10 signaling deficiency: based on a large cohort study. Inflamm. Bowel Dis. 25, 756–766. https://doi.org/10.1093/ibd/izy289 (2019).
Borghi, E. et al. Construction of the world health organization child growth standards: selection of methods for attained growth curves. Stat. Med. 25, 247–265. https://doi.org/10.1002/sim.2227 (2006).
Jagasia, M. H. et al. In chronic graft-versus-Host disease: I. The 2014 diagnosis and staging working group report. Biol. Blood Marrow Transpl. 21, 389–401e381. https://doi.org/10.1016/j.bbmt.2014.12.001 (2015).
Ruemmele, F. M. et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J. Crohns Colitis 8, 1179–1207. https://doi.org/10.1016/j.crohns.2014.04.005 (2014).
Hyams, J. S. Et al development and validation of a pediatric Crohn’s disease activity index. J. Pediatr. Gastroenterol. Nutr. 12, 439–447 (1991).
Zheng, C. F. et al. Treatment of pediatric refractory Crohn’s disease with thalidomide. World J. Gastroenterol. 17, 1286–1291. https://doi.org/10.3748/wjg.v17.i10.1286 (2011).
Huang, Z. et al. Mutations in Interleukin-10 receptor and clinical phenotypes in patients with very early onset inflammatory bowel disease: A Chinese VEO-IBD collaboration group survey. Inflamm. Bowel Dis. 23, 578–590. https://doi.org/10.1097/MIB.0000000000001058 (2017).
Saad, A. et al. Hematopoietic cell transplantation, version 2.2020, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 18, 599–634. https://doi.org/10.6004/jnccn.2020.0021 (2020).
Lv, H. et al. Neonatal Crohn’s disease with oral ulcer as the first symptom caused by a compound heterozygote mutation in IL-10RA: a case report. Hereditas 156, 38. https://doi.org/10.1186/s41065-019-0114-8 (2019).
Ye, Z. et al. Phenotype and management of Infantile-onset inflammatory bowel disease: experience from a tertiary care center in China. Inflamm. Bowel Dis. 23, 2154–2164. https://doi.org/10.1097/MIB.0000000000001269 (2017).
Aschenbrenner, D. et al. Pathogenic interleukin-10 receptor alpha variants in humans - balancing natural selection and clinical implications. J. Clin. Immunol. 43, 495–511. https://doi.org/10.1007/s10875-022-01366-7 (2023).
Kotlarz, D. et al. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: implications for diagnosis and therapy. Gastroenterology 143, 347–355. https://doi.org/10.1053/j.gastro.2012.04.045 (2012).
Meena, S. et al. Hematopoietic stem cell transplantation in children with very early onset inflammatory bowel disease secondary to monogenic disorders of immune-dysregulation. Indian J. Hematol. Blood Transfus. 39, 183–190. https://doi.org/10.1007/s12288-022-01586-2 (2023).
Sharifinejad, N. et al. The clinical, molecular, and therapeutic features of patients with IL10/IL10R deficiency: a systematic review. Clin. Exp. Immunol. 208, 281–291. https://doi.org/10.1093/cei/uxac040 (2022).
Moser, L. M. Al treatment of inborn errors of immunity patients with inflammatory bowel disease phenotype by allogeneic stem cell transplantation. Br. J. Haematol. 200, 595–607. https://doi.org/10.1111/bjh.18497 (2023).
Arai, K. & Very Early-onset inflammatory bowel disease. A challenging field for pediatric gastroenterologists. Pediatr. Gastroenterol. Hepatol. Nutr. 23, 411–422. https://doi.org/10.5223/pghn.2020.23.5.411 (2020).
Krivoy, N. et al. Busulfan use in hematopoietic stem cell transplantation: pharmacology, dose adjustment, safety and efficacy in adults and children. Curr. Drug Saf. 3, 60–66. https://doi.org/10.2174/157488608783333899 (2008).
Chen, Y. B. Al Vedolizumab for the prevention of intestinal acute GVHD after allogeneic hematopoietic stem cell transplantation: a randomized phase 3 trial. Nat. Med. 30, 2277–2287. https://doi.org/10.1038/s41591-024-03016-4 (2024).
Nemati, S. et al. Very early onset inflammatory bowel disease: investigation of the IL-10 signaling pathway in Iranian children. Eur. J. Med. Genet. 60, 643–649. https://doi.org/10.1016/j.ejmg.2017.08.016 (2017).
Kelsen, J. R. et al. North American society for pediatric gastroenterology, hepatology, and nutrition position paper on the evaluation and management for patients with very early-onset inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 70, 389–403. https://doi.org/10.1097/MPG.0000000000002567 (2020).
Ashton, J. J., Ennis, S. & ,Beattie, R. M. Early-onset paediatric inflammatory bowel disease. Lancet Child Adolesc. Health 1, 147–158. https://doi.org/10.1016/S2352-4642(17)30017-2 (2017).
Krawiec, P., Pawlowska-Kamieniak, A. & ,Pac-Kozuchowska, E. Interleukin 10 and Interleukin 10 receptor in paediatric inflammatory bowel disease: from bench to bedside lesson. J. Inflamm. (London) 18, 13. https://doi.org/10.1186/s12950-021-00279-3 (2021).
Feuerstein, J. D., Cheifetz, A. S. & Crohn disease epidemiology, diagnosis, and management. Mayo Clin. Proc. 92 1088–1103. https://doi.org/10.1016/j.mayocp.2017.04.010 (2017).
Morita, M. et al. Intestinal outcome of bone marrow transplantation for monogenic inflammatory bowel disease. Pediatr. Int. 64, e14750. https://doi.org/10.1111/ped.14750 (2022).
Tesch, V. K. et al. Long-term outcome of LRBA deficiency in 76 patients after various treatment modalities as evaluated by the immune deficiency and dysregulation activity (IDDA) score. J. Allergy Clin. Immunol. 145, 1452–1463. https://doi.org/10.1016/j.jaci.2019.12.896 (2020).
Funding
This work was supported by the Henan Medical Science and Technique Foundation (SBGJ202303048), the Henan Provincial Science and Technology Research Project (222102310431), and Open Project of National Children’s Regional Medical Center (NRMC0109).
Author information
Authors and Affiliations
Contributions
YW, DL, HG recollected the medical history of the patient. YW wrote the manuscript drafts. WL carried out writing-review and editing. YW and YM substantially contributed to the literature review and the writing of this manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by Children’s Hospital Affiliated to Zhengzhou University Review Board and Ethics Committee. The procedures followed and described here were in accordance with the Declaration of Helsinki as revised in 2013. Signed informed consent was obtained from the patient’s parents.
Consent for publication
Parents gave the informed consent to publication and signed informed consent regarding publishing their child’s data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Y., Liu, D., Gao, H. et al. Treatment of IL-10RA deficiency of pediatric patients with very early onset inflammatory bowel disease by allogeneic haematopoietic stem cell transplantation. Sci Rep 15, 9606 (2025). https://doi.org/10.1038/s41598-025-92979-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-92979-6