Abstract
Small and dense LDL cholesterol (sdLDL-C) and apolipoprotein B (ApoB) have important roles in promoting the development of atherosclerosis and are highly correlated with the degree of atherosclerosis. Several studies have found differences in anterior and posterior circulation strokes and in the mechanisms of their atherosclerosis, but little research has been done on the relationship of sdLDL-C and ApoB to atherosclerotic stenosis in anterior and posterior circulation strokes. We analyzed the correlation between sdLDL-C and ApoB and the degree of arterial stenosis in patients with posterior circulation stroke. We included 230 anterior circulation stroke (ACS) patients and 170 posterior circulation stroke (PCS) patients. Blood specimens were collected at admission, serum ApoB and sdLDL-C concentrations were measured, and the degree of arterial stenosis was determined on the basis of vascular imaging. We analyzed the predictive value of ApoB and sdLDL-C for the degree of cerebral artery stenosis in patients with PCS. For patients with nonmild stenosis, sdLDL-C and ApoB levels were higher in the PCS group than in the ACS group (P < 0.05). SdLDL-C (P < 0.001) and ApoB (P < 0.05) were independent risk factors for increased intracranial artery stenosis in the posterior circulation group. Binary logistic regression analysis showed that sdLDL-C (P < 0.05) and ApoB (P < 0.05) were independent risk factors for non-mild stenosis of the intracranial arteries in patients with PCS after correction for confounders. In the posterior circulation group, there was an interaction between the effects of sdLDL and ApoB on intracranial artery stenosis, P < 0.05. Plotting the ROC curve showed that the AUC of the combined detection of sdLDL-C and ApoB was 0.791, which was better than that of the single index. We built nomogram model, the DCA curves, calibration curves, NRI index, and IDI index of both the modeling and validation groups indicated that the diagnostic efficacy and clinical benefit of the combined sdLDL-C and ApoB assay were greater than those of single-indicator assays for cerebral artery stenosis in posterior circulation stroke. Risk factors contributing to the increased degree of intracranial arterial stenosis in ACS and PCS vary somewhat. SdLDL-C and ApoB may be of value in clinical decision making as predictors of cerebral arterial stenosis in patients with PCS.
Similar content being viewed by others
Introduction
Acute cerebral infarction (ACI) is an ischemic cerebrovascular disease that poses a serious risk to human health, and the incidence of ACI has been increasing in developing countries in recent decades1,2,3. This seriously affects the quality of life of the population. Atherosclerosis is recognized as the pathological basis for the development of ACI4,5. Traditional risk factors for atherosclerosis include age, obesity, hypertensive disease, and low-density lipoprotein cholesterol6,7,8. However, these traditional factors only partially predict the risk of developing ACI.
Small and dense LDL cholesterol (sdLDL-C), as a subclass of LDL cholesterol (LDL-C) with small particle sizes, possesses greater oxidative susceptibility and greater atherogenic capacity9. While apolipoprotein B (ApoB), as one of the apolipoproteins of celiac microsomes (CM), LDL, and very low density lipoprotein (VLDL), the number of ApoB particles in the arterial lumen is a major determinant of its rate of entry into the intima, and is a better marker of atherosclerosis in comparison with other lipid indices10. Studies have demonstrated that serum sdLDL-C and ApoB are new independent risk factors for ACI11,12,13,14. Due to their high correlation with the degree of atherosclerosis15,16, the relationship with cerebrovascular disease has received increasing attention from researchers. Posterior circulation stroke (PCS), which accounts for about 20–30% of AIS cases, is more difficult to recognize compared to anterior circulation stroke (ACS), which is prone to delayed diagnosis17. And some studies have shown that there are differences between anterior and posterior circulation stroke and their risk factors for atherosclerosis, a study by Von Sarnowski B et al. found that men were more common in posterior circulation strokes compared to women and that cardiogenic embolism was more common in anterior circulation strokes18. Li Yan et al. found that the mechanisms of stroke in the posterior circulation were more complex than in the anterior circulation and that the posterior circulation arteries were more prone to occlusion in the presence of atherosclerotic stenosis19. In the past, due to technical limitations, more studies have focused on ACS, and fewer studies have been conducted on PCS. Therefore, in this study, we analyzed the correlation between related factors and the degree of intracranial artery stenosis in ACS and PCS, and further explored the predictive value of sdLDL-C and Apob on intracranial artery stenosis in PCS, with the aim of providing a certain theoretical basis for interventions on intracranial artery stenosis.
Materials and methods
Participants
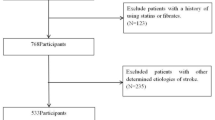
Patients with acute cerebral infarction who were hospitalized in the Department of Neurology of Qianfoshan Hospital in Shandong Province from August 1, 2021 to March 1, 2024 were selected for the study. Based on the exclusion criteria, we finally selected 400 ACI patients from 723 ACI patients (Fig. 1). This study was approved by the Ethics Review Committee of the First Affiliated Hospital of Shandong First Medical University [Reference No.(S531)]. All methods were carried out in accordance with relevant guidelines and regulations. We confirmed that informed consent was obtained from all subjects and/or their legal guardian(s).Inclusion criteria: (1) meeting the criteria of the Chinese Guidelines for Diagnosis and Treatment of Acute Ischemic Stroke 2018 and confirmed by imaging; (2) onset to admission time ≤ 72 h; (3) first onset or non-first onset without residual neurological deficits; (4) age ≥ 18 years old; and (5) complete clinical data such as imaging, personal history, and laboratory test indexes. Exclusion criteria: (1) Combination of immune system diseases, serious infections, malignant tumors; (2) Previous history of transient ischemic attack (TIA), cerebral hemorrhage, and head trauma; (3) Combination of cardiovascular diseases, such as heart failure, coronary artery disease, and myocardial infarction; (4) Previous cerebral artery stenting; (5) Patients with stroke of cardiac origin, coagulopathies, altered blood composition, vasculitis of various causes, vascular malformations, and stroke of unknown origin. The patients were divided into the ACS group (230 cases) and the PCS group (170 cases) according to the site of the vessel responsible for the acute lesion and the degree of stenosis. In the ACS group, there were 135 cases of mild stenosis and 95 cases of non-mild stenosis of the intracranial arteries (including 48 cases of moderate stenosis and 47 cases of severe stenosis and occlusion), and in the PCS group, there were 95 cases of mild stenosis and 75 cases of non-mild stenosis of the intracranial arteries (including 42 cases of moderate stenosis and 33 cases of severe stenosis and occlusion).
Data collection
General information of the patients was collected, including age, gender, history of smoking, alcohol consumption, history of hypertension, diabetes mellitus, and hyperlipidemia. Early morning fasting venous blood was drawn from the patients within 24 h of admission and tested for sdLDL-C, ApoB, ApoA1, triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), homocysteine, and lipoprotein-associated phospholipase A2(Lp-PLA2). The patients underwent CT angiography (CTA), whole-brain digital subtraction angiography (DSA), or cranial magnetic resonance angiography (MRA). The stenosis rate and grading of intracranial arteries were calculated according to the 2017 “Guidelines for the Management of Symptomatic Intracranial and Extracranial Atherosclerotic Large Artery Stenosis-Scientific Statement of the Chinese Stroke Association”. The stenosis rate was calculated as follows: 1- diameter of the narrowest part of the diseased vessel/diameter of the normal part of the proximal part of the diseased vessel; and the stenosis degree of the intracranial arteries was graded as follows: 0–49% was considered as mild stenosis, 50-69% was considered as moderate stenosis, and 70-99% was considered as Severe stenosis. The intracranial arteries included the intracranial segments of bilateral vertebral arteries, basilar arteries, bilateral anterior, middle and posterior cerebral arteries and intracranial segments of bilateral internal carotid arteries.
Statistical analysis
All data were statistically analyzed using SPSS 27.0 statistical software and R.4.3.3. The count data were expressed as frequency and percentage (%), and the χ2 test was used for comparison between groups; the measurement data were tested for normality by Shapiro-Wilk (S-W test), and obeyed the normal distribution or approximate normal distribution expressed as mean ± standard deviation (X ± SD). The comparison between two groups was performed by two independent samples t-test, and one-way analysis of variance (ANOVA) was used for the comparison between groups, and the non-normal distribution was performed by median and quartiles. The median and quartiles were used for non-normal distribution [M(P25, P75)], and the non-parametric rank sum test was used for comparison between groups. We used univariate and multivariate ordered logistic regression to analyze the factors affecting the degree of intracranial artery stenosis in the ACI and PCS groups, respectively. Binary logistic regression was used to analyze the correlation between sdLDL-C and ApoB on non-mild stenosis of intracranial arteries in the PCS group. The sdLDL-C and ApoB were divided into 4 groups by quartiles and then exploring the interaction between them at different levels. R language is used to construct the nomogram, and receiver operating characteristic curves (ROC Curvers), calibration plots, and decision curve analysis (DCA) are used to evaluate the reliability and predictive ability of the prediction model. Net reclassification indices (NRI) and integrated discriminatory improvement indices (IDI) were used for different comparison of prediction models. All P-values were two-tailed with a significance level of 0.05.
Results
For all ACI patients and patients with mild stenosis of intracranial arteries, there was no difference in general data between the ACS and PCS groups (P > 0.05, Tables 1 and 2). For patients with non-mild stenosis of the intracranial arteries, The levels of sdLDL-C, ApoB, ApoA1/ApoB, LDL-C, TC, and Lp-PLA2 were higher in the PCS group than in the ACS group (P<0.05, Table 3).
Using the degree of intracranial artery stenosis as the dependent variable (mild stenosis = 0, moderate stenosis = 1, and severe stenosis or occlusion = 2), univariate ordinal logistic regression analyses were performed first. Independent variables with P<0.2 in the univariate analyses were selected for multiple covariance tests, and those with VIF<5 (diabetes mellitus, hyperlipidemia, history of alcohol consumption, sdLDL-C, TG, ApoA1/ApoB, and Lp-PLA2) were included in multifactor logistic regression. The results showed that history of diabetes mellitus was an independent risk factor for increased intracranial arterial stenosis in the ACS group (no history of diabetes mellitus vs. history of diabetes mellitus, OR = 0.499, P < 0.05, Table 4); sdLDL-C (OR = 5.573, P < 0.001) and ApoB (OR = 16.643, P < 0.05) were independent risk factors for increased intracranial arterial stenosis in the PCS group(Table 5).
Comparing the general data between the mild stenosis group and the non-mild stenosis group of PCS patients, the differences in sdLDL-C, ApoB, LDL-C, TC, TG, Lp-PLA2, diabetes mellitus, and hyperlipidemia were statistically significant between the two groups (P < 0.05). Binary logistic regression analysis was performed with the degree of intracranial artery stenosis as the dependent variable (mild stenosis = 0, non-mild stenosis = 1) and sdLDL-C and ApoB as independent variables. Model 1 was not corrected for any confounders; model 2 was corrected for age and sex on the basis of model 1; and model 3 was corrected for TG, Lp-PLA2, diabetes, and hyperlipidemia on the basis of model 2. The final model results showed that sdLDL-C (OR = 4.191, P < 0.05) and ApoB (OR = 48.091, P < 0.05) were independent risk factors for non-mild stenosis of intracranial arteries in patients undergoing PCS (Table 6). In the post-circulation group, we further stratified sdLDL and ApoB into subgroups by interquartile range. SdLDL ≤ 0.854mmol/L and ApoB ≤ 0.790 g/L were assigned a value of 0, sdLDL ≤ 1.236 mmol/L and ApoB ≤ 0.930 g/L were assigned a value of 1, and sdLDL ≤ 1.569 mmol/L and ApoB ≤ 1.080 g/L were assigned a value of 2, and sdLDL > 1.569 mmol/L and ApoB > 1.080 g/L were assigned a value of 3. Repeated measures ANOVA found that sdLDL at subgroup 2 level interacted with ApoB at subgroup 2 and 3 levels, and that sdLDL at subgroup 3 level interacted with ApoB at subgroup 3 level, P<0.05(Table 7).
To further assess the predictive value of sdLDL-C and ApoB for non-mild stenosis of intracranial arteries in PCS, We first plotted ROC curves (Fig. 2), the AUC for sdLDL-C was 0.754 [95% CI (0.679,0.828)] with 1.377 cutoff value, 64.90% sensitivity, 81.2% specificity, and 0.461 Youden index; ApoB had an AUC of 0.770 [95% CI (0.699,0.841)] with 1.045 cutoff value, 54.10% sensitivity, 87.50% specificity, and 0.416 Youden index; The combination of sdLDL-C and ApoB had the highest AUC of 0.791 [95% CI (0.722,0.860)] with 77.00% sensitivity and 71.90% specificity, suggesting that the combination of the two tests has a high predictive value for non-mild stenosis of the intracranial arteries in PCS (Table 8). Then, we randomly split 170 PCS patients into a modeling group (n = 104) and a validation group (n = 66) according to a ratio of 7:3, and plotted nomogram model of the combined predictors of severe stenosis or occlusion of the intracranial arteries in PCS patients(Fig. 3). We plotted predictive calibration curves, and DCA curves. The calibration curve of both modeling and validation groups suggests that the predictive calibration curve of the joint prediction model of the two is in the best agreement with the standard curve (Fig. 4). DCA curves showed that the joint prediction model outperforms the single prediction model at risk thresholds of 0.25–0.46 and 0.66–0.88 for the modeling group and the joint prediction model outperformed the single prediction model at risk thresholds of 0.17–0.30 and 0.77–0.98 in the validation group (Fig. 5).
Nomogram for predicting intracranial artery stenosis in PCS patiens. The nomogram model scores each variable, with the horizontal line following each variable corresponding to the corresponding score; the higher the sum of the scores for multiple variables, the higher the risk that the patient will have ≥ 50% stenosis of the intracranial arteries.
DCA curves for predictive modeling of non-mild stenosis in patients with PCS. (A: modeling group; B: validation group). None means no intervention for all and All means intervention for all. The model has real value when the model curve is above the solid line representing both All and None. The joint prediction model outperforms the single prediction model at risk thresholds of 0.25–0.46 and 0.66–0.88 for the modeling group. In the validation group, the joint prediction model outperformed the single prediction model at risk thresholds of 0.17–0.30 and 0.77–0.98.
We utilized NRI and IDI to compare the predictive efficacy of the combination of both sdLDL-C and ApoB versus single-indicator testing for non-mild stenosis in PCS. The combined sdLDL-C and ApoB assay showed an improvement in NRI and IDI compared with sdLDL-C (NRIm= 0.545, Pm < 0.05; IDIm= 0.063, Pm< 0.05; NRIv= 0.279, Pv < 0.05; IDIv= 0.084, Pv< 0.05) and ApoB (NRIm= 0.079, Pm < 0.05; IDIm= 0.012, Pm< 0.05; NRIv= 0.122, Pv < 0.05; IDIv= 0.024, Pv< 0.05) in both the modeling and validation groups, indicating that the combined detection of sdLDL-C and ApoB has better predictive efficacy compared with a single test indicator (Table 9).
Discussion
The posterior circulation consists of the vertebral, basilar and posterior cerebral arteries originating from the subclavian artery. Compared with ACS, the incidence of PCS is low, but its prognosis is poor, with high rates of disability and mortality. Atherosclerosis is a common disease of the posterior circulation arteries, and in situ thrombosis often leads to complete occlusion of the vessels, especially the basilar arteries, with a very high mortality rate20. It has been noted that there are significant anatomical differences between the anterior and posterior circulations, and unlike the carotid system, the vertebrobasilar artery has a specific geometry, high frequency of variations, and a vertebrobasilar junction angle of more than 90°, which all increase the risk factors for localized atherosclerosis in the posterior circulation21,22. Serological markers have been of interest to researchers and are widely used in the clinic due to their simplicity and less invasiveness compared with imaging. Therefore, the study of risk factors for cerebral artery stenosis in PCS is clinically important for early assessment of cerebral infarction.
Despite the differences between ACS and PCS in terms of clinical manifestations and prognosis, the conclusions regarding the risk factors for both are controversial. A study by Yun L, et al.23 found that TG, HDL-C, and diabetes mellitus differed between ACS and PCS, with diabetes mellitus being more common in PCS. However, a recent study showed that the difference in general clinical data comparing anterior and posterior circulation stroke was not statistically significant24, which is consistent with the results of the present study. Previous studies comparing atherosclerosis in the anterior and posterior intracranial circulation are scarce. He Jianfeng et al. showed differences in the distribution of plaque in the anterior and posterior circulation, with plaque in the posterior circulation being more dispersed, suggesting to some extent that there are differences in atherosclerosis in the anterior and posterior cerebral arteries25. In this study, we found that in patients with mild stenosis of cerebral arteries, there was no statistically significant difference between the general data of the ACS and PCS groups, but in patients with non-mild stenosis, the levels of sdLDL-C, ApoB, ApoA1/ApoB, LDL-C, TC, and Lp-PLA2 were higher in the PCS group than in the ACS group. For this reason we hypothesized that the risk factors for anterior and posterior circulation cerebral atherosclerosis may be different. To verify this speculation, this study further performed univariate and multivariate ordered logistic regression analyses, which showed that ApoB and sdLDL-C were independent risk factors for the degree of cerebral arterial stenosis in PCS. However, sdLDL-C and ApoB did not show a significant correlation with the degree of cerebral artery stenosis in patients with ACS. It has been shown26,27 that there is some variability in the risk factors for cerebral atherosclerosis in the anterior and posterior circulations, and that atherosclerosis in the posterior circulation intracranial arteries is more closely associated with metabolic risk factors. For this reason, we believe that, on the one hand, compared with the anterior circulation, the posterior circulation vessels possess less sympathetic innervation28, which explains to some extent the presence of positive remodeling of the vessels in the posterior circulation. On the other hand, complex plaque and hemodynamic factors are also involved in the development of stroke, with the anterior circulation being more susceptible to hemodynamic alterations29,30,31. These make the posterior circulation vessels more resistant to arterial stenosis, and therefore atherogenic lipoproteins such as sdLDL possess a stronger correlation with the degree of arterial stenosis in posterior circulation cerebral infarction.
SdLDL-C, a subclass of LDL-C, is more likely to cross the vascular endothelium and be deposited because of its small particle size. On the other hand, oxidative stress plays an important role in atherosclerosis, and sdLDL-C possesses a stronger oxidative susceptibility, with the sdLDL molecule having a lower affinity for apoB-100 on the surface of the LDL receptor. Those make it difficult for the receptor to recognize and remove sdLDL, which is then taken up by phagocytes, forming foam cells and promoting the development of atherosclerosis9,32. ApoB is the key structural protein component found in all major atherogenic lipoproteins, with each very low density lipoprotein (VLDL), LDL, intermediate density lipoprotein (IDL), and Lp(a) particle containing only one molecule of ApoB, so that the level of ApoB responds to the total amount of atherogenic lipoproteins. ApoB has also been implicated in the inflammatory process of atherosclerosis itself, and may serve as a superior predictor of atherosclerotic cardiovascular disease (ASCVD)33,34,35. It has been found that sdLDL-C is closely related to the degree of vasculopathy in patients with ACI36,37,38, and is an independent risk factor affecting the degree of intracranial artery stenosis in patients with ACI39. Scholars at home and abroad have found that ApoB not only has a high predictive value for the occurrence of ACI40,41, but also is closely related to the degree of atherosclerosis in patients with ACI42,43,44. In the study by Wang Shi et al., ApoB and ApoA1 were significantly correlated with positive remodeling of posterior circulation vessels, but the correlation was not significant in the anterior circulation45. Vascular remodeling is a compensatory change in vessel size under pathological or physiological conditions, characterized by alterations in vessel wall structure and function, which significantly contributes to the progression of cardiovascular disease46. For this reason, we further investigated the predictive value of ApoB and sdLDL-C for non-mild stenosis of PCS intracranial arteries. This study further found that sdLDL and ApoB are independent risk factors for non-mild stenosis of PCS intracranial arteries, and that they have an interactive effect on intracranial artery stenosis, suggesting that the two can interact to influence the development of intracranial artery stenosis. The suggestion is that we should balance the control of the levels of both so as to maximize patient benefit. To this end, we included sdLDL and ApoB together in the prediction model. Compared with single-indicator testing, the combined testing of sdLDL-C and ApoB has higher predictive efficacy for non-mild stenosis in posterior circulation cerebral infarction.
Limitations
This study has several limitations. First, the sample size of this study was limited and all of them were from the neurology department of the same hospital, which is prone to selection bias. Second, the accuracy of MRA in observing the degree of intracranial artery stenosis is not as good as CTA, which is prone to group bias. Finally, this study was retrospective and lacked follow-up and observation of patients. In future studies, it is necessary to further expand the sample size and select CTA and DSA, which are more accurate in grouping the degree of stenosis, to further investigate the relationship between sdLDL-C and ApoB levels and the degree of intracranial artery stenosis in patients with PCS.
Conclusion
This demonstration suggests that there are some differences in the risk factors contributing to the increased degree of intracranial arterial stenosis in patients with anterior and posterior circulation cerebral infarction. Clinical prevention and treatment strategies should be adjusted to the vascular lesions of anterior and posterior circulation cerebral infarction. And the combination of sdLDL-C and ApoB as novel serum markers is highly valuable in predicting non-mild stenosis of intracranial arteries in patients with PCS. However, further studies are needed to determine whether the two may be potential therapeutic targets for ACI and atherosclerosis.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Campbell, B. C. V. et al. Ischaemic stroke. Nat. Rev. Dis. Prim. 5 (1), 70. https://doi.org/10.1038/s41572-019-0118-8 (2019).
Pu, L. et al. Projected global trends in ischemic stroke incidence, deaths and disability-adjusted life years from 2020 to 2030. Stroke 54 (5), 1330–1339. https://doi.org/10.1161/STROKEAHA.122.040073 (2023).
Bhatta, D. N. & Bommer, W. Trends in California cardiovascular disease mortality: Sex-race/ethnicity disparity and income inequality. Mayo Clinic Proc. 99 (11), 1756–1770 https://doi.org/10.1016/j.mayocp.2024.02.018 (2024).
Zhang, J. et al. Exploring the role of myeloperoxidase in the atherosclerotic process in hypoxic mice based on the MAPK signaling pathway. Biochem. Pharmacol. 225, 116275. https://doi.org/10.1016/j.bcp.2024.116275 (2024).
Prochilo, G. et al. Recent translational research models of intracranial atherosclerotic disease. Stroke 55 (6), 1707–1719. https://doi.org/10.1161/STROKEAHA.124.044520 (2024).
Khan, M. et al. Risk factors for stroke in the young (18–45 years): A case-control analysis of INTERSTROKE data from 32 countries. Neuroepidemiology 57 (5), 275–283. https://doi.org/10.1159/000530675 (2023).
Li, N. Platelets as an inter-player between hyperlipidaemia and atherosclerosis. J. Intern. Med. 296 (1), 39–52. https://doi.org/10.1111/joim.13794 (2024).
Libby, P. The changing landscape of atherosclerosis. Nature 592 (7855), 524–533. https://doi.org/10.1038/s41586-021-03392-8 (2021).
Jin, X., Yang, S., Lu, J. & Wu, M. Small, dense low-density lipoprotein–cholesterol and atherosclerosis: Relationship and therapeutic strategies. Front. Cardiovasc. Med. 8, 804214. https://doi.org/10.3389/fcvm.2021.804214 (2022).
Sniderman, A. D. et al. Apolipoprotein B particles and cardiovascular disease: A narrative review. JAMA Cardiol. 4 (12), 1287–1295. https://doi.org/10.1001/jamacardio.2019.3780 (2019).
Duran, E. K. et al. Triglyceride-Rich lipoprotein cholesterol, small dense LDL cholesterol, and incident cardiovascular disease. J. Am. Coll. Cardiol. 75 (17), 2122–2135. https://doi.org/10.1016/j.jacc.2020.02.059 (2020).
Chen, Y. et al. Elevated SdLDL level and LDLR rs688 C > T mutation are independent risk factors for ischemic stroke. Med. Clin. 159 (10), 465–469. https://doi.org/10.1016/j.medcli.2022.01.016 (2022).
Kriemler, L. et al. Discordance between LDL-C and Apolipoprotein B is associated with large-artery-atherosclerosis ischemic stroke in patients ≤ 70 years of age. Eur. Stroke J. 9 (2), 494–500. https://doi.org/10.1177/23969873231221619 (2024).
Balling, M. et al. Small dense low-density lipoprotein cholesterol and ischemic stroke. Ann. Neurol. 93 (5), 952–964. https://doi.org/10.1002/ana.26598 (2023).
Yao, T. et al. Small dense low-density lipoprotein cholesterol is strongly associated with NIHSS score and intracranial arterial calcification in acute ischemic stroke subjects. Sci. Rep. 10 (1), 7645. https://doi.org/10.1038/s41598-020-64715-9 (2020).
Duan, R. et al. Estimation of the LDL subclasses in ischemic stroke as a risk factor in a Chinese population. BMC Neurol. 20 (1), 414. https://doi.org/10.1186/s12883-020-01989-6 (2020).
De Marchis, G. M. et al. Posterior versus anterior circulation strokes: Comparison of clinical, radiological and outcome characteristics. J. Neurol. Neurosurg. Psychiatry 82 (1), 33–37. https://doi.org/10.1136/jnnp.2010.211151 (2011).
von Sarnowski, B. et al. Posterior versus anterior circulation stroke in young adults: A comparative study of stroke aetiologies and risk factors in stroke among young Fabry patients (sifap1). Cerebrovasc. Dis. (Basel Switzerland) 43 (3–4), 152–160. https://doi.org/10.1159/000454840 (2017).
Li, Y., Cai, Y., Zhao, M. & Sun, J. Risk factors between intracranial-extracranial atherosclerosis and anterior-posterior circulation stroke in ischaemic stroke. Neurol. Res. 39 (1), 30–35. https://doi.org/10.1080/01616412.2016.1250856 (2017).
Hoyer, C. & Szabo, K. Pitfalls in the diagnosis of posterior circulation stroke in the emergency setting. Front. Neurol. 12, 682827. https://doi.org/10.3389/fneur.2021.682827 (2021).
Li, J. et al. Vertebrobasilar junction angle over 90°: A potential imaging marker associated with vertebrobasilar atherosclerosis. Front. NeuroSci. 15, 789852. https://doi.org/10.3389/fnins.2021.789852 (2022).
Sparaco, M., Ciolli, L. & Zini, A. Posterior circulation ischaemic stroke-a review part I: Anatomy, aetiology and clinical presentations. Neurol. Sci.: Off. J. Italian Neurol. Soc. Italian Soc. Clin. Neurophysiol. 40 (10), 1995–2006. https://doi.org/10.1007/s10072-019-03977-2 (2019).
Luo, Y., Li, Z., Zhang, J., Li, J. & Lu, Z. Dyslipidaemia was correlated to the posterior circulation infarction in non-diabetic populations. Lipids Health Dis. 17 (1), 150. https://doi.org/10.1186/s12944-018-0799-0 (2018).
Zhao, Y. et al. Prevalence and risk factors comparison of anterior and posterior intracranial arterial stenosis. Evidence-based Complement. Altern. Medicine: eCAM. 2022, 7710374. https://doi.org/10.1155/2022/7710374 (2022).
Markus, H. S., van der Worp, H. B. & Rothwell, P. M. Posterior circulation ischaemic stroke and transient ischaemic attack: Diagnosis, investigation, and secondary prevention. Lancet Neurol. 12 (10), 989–998. https://doi.org/10.1016/S1474-4422(13)70211-4 (2013).
Kim, J. S. et al. Risk factors and stroke mechanisms in atherosclerotic stroke: Intracranial compared with extracranial and anterior compared with posterior circulation disease. Stroke 43 (12), 3313–3318. https://doi.org/10.1161/STROKEAHA.112.658500 (2012).
Zheng, L. et al. Patterns and implications of intracranial atherosclerosis in anterior and posterior circulation identified by High-Resolution vessel wall imaging. Cerebrovasc. Dis. (Basel Switzerland) 53 (4), 403–410. https://doi.org/10.1159/000534822 (2024).
Edvinsson, L., Owman, C. & Sjöberg, N. O. Autonomic nerves, mast cells, and amine receptors in human brain vessels. A histochemical and pharmacological study. Brain Res. 115 (3), 377–393. https://doi.org/10.1016/0006-8993(76)90356-5 (1976).
Wong, K. S., Caplan, L. R. & Kim, J. S. Stroke mechanisms. Front. Neurol. Neurosci. 40, 58–71. https://doi.org/10.1159/000448302 (2016).
Bai, X. et al. Distribution and regional variation of wall shear stress in the curved middle cerebral artery using four-dimensional flow magnetic resonance imaging. Quant. Imaging Med. Surg. 12 (12), 5462–5473. https://doi.org/10.21037/qims-22-67 (2022).
Zhou, L. et al. Plaque features and vascular geometry in Basilar artery atherosclerosis. Medicine 99 (18), e19742. https://doi.org/10.1097/MD.0000000000019742 (2020).
Krauss, R. M. Small dense low-density lipoprotein particles: Clinically relevant? Curr. Opin. Lipidol. 33 (3), 160–166. https://doi.org/10.1097/MOL.0000000000000824 (2022).
Bajaj, A. et al. Lipids, apolipoproteins, and risk of atherosclerotic cardiovascular disease in persons with CKD. Am. J. Kidn. Dis.: Off. J. Natl. Kidn. Found. 73 (6), 827–836. https://doi.org/10.1053/j.ajkd.2018.11.010 (2019).
Tian, J., Chen, H., Liu, P., Wang, C. & Chen, Y. Fasting apolipoprotein B48 is associated with large artery atherosclerotic stroke: A case-control study. Sci. Rep. 9 (1), 3729. https://doi.org/10.1038/s41598-019-40385-0 (2019).
Kounatidis, D. et al. ApoB100 and atherosclerosis: What’s new in the 21st century?? Metabolites 14 (2), 123. https://doi.org/10.3390/metabo14020123 (2024).
Zhou, P. et al. Association between carotid intima media thickness and small dense low-density lipoprotein cholesterol in acute ischaemic stroke. Lipids Health Dis. 19 (1), 177. https://doi.org/10.1186/s12944-020-01353-0 (2020).
Ma, X. et al. Association of sdLDL-C with incident carotid plaques with stable and vulnerable morphology: A prospective cohort study. Stroke 55 (3), 576–585. https://doi.org/10.1161/STROKEAHA.123.045601 (2024).
Liu, F. et al. Relationship between small dense low-density lipoprotein cholesterol with carotid plaque in Chinese individuals with abnormal carotid artery intima-media thickness. BMC Cardiovasc. Disord. 21 (1), 216. https://doi.org/10.1186/s12872-021-02023-4 (2021).
Zhou, P. et al. Association of small dense Low-Density lipoprotein cholesterol with stroke risk, severity and prognosis. J. Atheroscler. Thromb. 27 (12), 1310–1324. https://doi.org/10.5551/jat.53132 (2020).
Johannesen, C. D. L., Mortensen, M. B., Langsted, A. & Nordestgaard, B. G. ApoB and non-HDL cholesterol versus LDL cholesterol for ischemic stroke risk. Ann. Neurol. 92 (3), 379–389. https://doi.org/10.1002/ana.26425 (2022).
Yuan, S., Tang, B., Zheng, J. & Larsson, S. C. Circulating lipoprotein lipids, apolipoproteins and ischemic stroke. Ann. Neurol. 88 (6), 1229–1236. https://doi.org/10.1002/ana.25916 (2020).
Sun, Y. et al. Apolipoprotein B/AI ratio as an independent risk factor for intracranial atherosclerotic stenosis. Aging 11 (17), 6851–6862. https://doi.org/10.18632/aging.102216 (2019).
Rehman, M. B. & Tudrej, B. V. Lipoprotein(a) and risk-weighted apolipoprotein B: a novel metric for atherogenic risk. Lipids Health Dis. 23 (1), 316. https://doi.org/10.1186/s12944-024-02307-6 (2024).
Van Tuyen, N. et al. Differential distribution of plasma apoA-I and ApoB levels and clinical significance of ApoB/apoA-I ratio in ischemic stroke subtypes. Front. Neurol. 15, 1398830. https://doi.org/10.3389/fneur.2024.1398830 (2024).
Wang, S. et al. Plasma ApoB/AI: an effective indicator for intracranial vascular positive remodeling. J. Neurol. Sci. 436, 120226. https://doi.org/10.1016/j.jns.2022.120226 (2022).
Xie, M. et al. The crosstalks between vascular endothelial cells, vascular smooth muscle cells, and adventitial fibroblasts in vascular remodeling. Life Sci. 361, 123319. https://doi.org/10.1016/j.lfs.2024.123319 (2025).
Author information
Authors and Affiliations
Contributions
ZY Sun and JZ Liu wrote the main manuscript text. ZY Sun and JZ Liu collected data. ZY Sun prepared all tables and pictures. ZY Sun analyzed the data. All authors reviewed the manuscript. JZ LIU, ZH Si and AH Wang provided technical guidance.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, Z., Liu, J., Wang, A. et al. Correlation of sdLDL-C and Apob with the degree of cerebral artery stenosis in posterior circulation stroke. Sci Rep 15, 8343 (2025). https://doi.org/10.1038/s41598-025-93074-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-93074-6