Abstract
Hypertension (HTN) is prevalent in non-small cell lung cancer (NSCLC) patients, yet the cardioprotective and survival benefits of β-adrenergic blockers during radiotherapy (RT) remain underexplored. We analyzed data from a Chinese clinical cohort of 750 patients with stage IIIA to IIIB NSCLC and HTN receiving RT between 2014 and 2018. The findings were further validated using data from the NHANES database. In Chinese clinical cohort, β-adrenergic blockers were associated with improved OS (β-adrenergic blockers: median overall survival (mOS) 17.64 months, 95% CI, 15.95–19.33; no β-adrenergic blockers: mOS 13.16 months, 95% CI, 12.62–13.70; p < 0.0001) and PFS (β-adrenergic blockers: median progression-free survival (mPFS) 7.50 months, 95% CI, 6.50–8.50; without β-adrenergic blockers: mPFS 4.91 months, 95% CI, 4.53–5.31; p < 0.0001). Simultaneously, in the NHANES database, the utilization of β-adrenergic blockers exhibited no discernible impact on OS within the entire tumor population, as evidenced by the Kaplan-Meier curve, which revealed no statistically significant difference between the two groups (p = 0.254). β-adrenergic blockers may improve OS and PFS in patients with HTN and NSCLC undergoing RT. β-adrenergic blockers show potential and warrant further investigation in the context of RT.
Similar content being viewed by others
Introduction
Cardiovascular disease and cancer are two of the most common causes of death worldwide1,2. Growing research evidence suggests a significant association between the two diseases, particularly among cancer patients with hypertension (HTN)3,4. The China Cardiovascular Health and Disease Report 2022 reveals that HTN prevalence has doubled over the past three decades, with approximately 245 million people suffering from HTN in China5. Of particular note, epidemiological statistics emphasize that HTN incidence among Chinese cancer patients is notably high, reaching up to 37% 6. The incidence of HTN among cancer patients is steadily increasing7. Therefore, proactive treatment, life extension, and enhancement of life quality are of paramount importance for this demographic.
A correlation exists between elevated blood pressure and cancer8,9. On one hand, heightened blood pressure increases the risk of cancer in patients10. For instance, males with HTN face a heightened risk of developing lung and kidney cancers, while females with HTN exhibit greater susceptibility to cervical and uterine body cancers11. Conversely, cancer treatment can also elevate the risk of HTN. In non-small cell lung cancer (NSCLC) patients, vascular endothelial growth factor (VEGF) inhibitors and tyrosine kinase inhibitors lower VEGF levels. This makes vasodilators less effective and increases blood pressure. Similarly, the use of alkylating drugs in lung cancer treatment may induce damage to the vascular endothelium, increased vascular intima thickness, and vascular remodeling, resulting in elevated blood pressure12. Moreover, advanced age, tobacco use, obesity, alcohol consumption, psychological stress, sedentary lifestyle, and exposure to air pollution are prevalent risk factors shared by both NSCLC and HTN.
Patients diagnosed with NSCLC and HTN may need to take blood pressure medications long-term. Currently, antihypertensive medications are predominantly categorized into five classes: angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), β-adrenergic blockers, calcium channel blockers (CCB), and diuretics13. β-adrenergic blockers are categorized as either selective or non-selective, with selective β-adrenergic blockers primarily targeting β-adrenergic receptors in cardiac tissue. They act to inhibit the sympathetic nervous system, leading to reductions in heart rate, myocardial contractility, stroke volume, myocardial oxygen consumption, and peripheral vascular resistance. Consequently, this mechanism has received attention and consideration14. One study revealed that among the aforementioned antihypertensive medications, only β-blockers demonstrated a reduction in the risk of developing prostate cancer15. Therefore, the clinical advantages of β-blocker therapy have been observed in both the treatment of HTN and clinical studies related to cancer. Some studies have shown that in patients with breast cancer16,17 and melanoma18 who have HTN, treatment with β-adrenergic blockers can reduce tumor metastasis, recurrence, and mortality. It is related to the inhibition of β-adrenergic signaling pathways by receptor blockers19. Activation of β-adrenergic signaling contributes to cancer development and progression. This involves processes like angiogenesis, DNA repair, immune response, and epithelial-mesenchymal transition20. β-adrenergic blockers impede the aforementioned signaling pathways, and beyond their cardiovascular benefits, they exert inhibitory effects on tumor metastasis and recurrence.
Moreover, Chaudhary et al. reported that β-adrenergic blockers, besides improving tumor metastasis and overall survival (OS), also heightened tumor cell sensitivity to radiotherapy (RT)21. A retrospective study of NSCLC patients undergoing RT found that using β-adrenergic blockers was associated with improvements in distant metastasis-free survival (DMFS), disease-free survival (DFS), and OS22. Nevertheless, conflicting outcomes have been reported by other research groups. Shah et al. conducted a study involving 436 lung cancer patients, revealing no significant correlation between the utilization of β-adrenergic blockers and OS23. There is also no evidence indicating that β-blockers enhance survival outcomes for patients diagnosed with lung, breast, or colorectal cancer.
There are limited clinical studies of β-blockers in RT patients with NSCLC combined with HTN to explore the potential association between β-blockers and lung cancer survival outcomes. We hypothesized that a similar clinical advantage could be expected in NSCLC. Consequently, we conducted a retrospective analysis of NSCLC patients to assess the influence of β-blockers following RT. Concurrently, we utilized the National Health and Nutrition Examination Survey (NHANES) database to assess the effects of β-blockers on survival within the broader cancer population.
Materials and methods
Patient selection
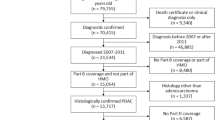
A retrospective study was conducted on 750 patients diagnosed with stage IIIA-IIIB NSCLC who underwent RT from January 2014 to December 2018 (Supplemental Fig. 1). The analysis included only patients with recently diagnosed and pathologically confirmed advanced NSCLC who were concurrently diagnosed with HTN or had a pre-existing diagnosis of HTN before undergoing tumor treatment. Patients with a history of coronary heart disease, diabetes mellitus, or renal insufficiency either before or during hospitalization were excluded from the study. Simultaneously, individuals with multiple primary lung cancers or a prior history of another malignancy were systematically excluded from the study.
The fundamental characteristics and clinical data of the patients were derived from electronic medical records, encompassing variables such as gender, age, Karnofsky Performance Status (KPS) score, pathological stage and type, smoking history, RT modality, HTN severity and classification, and the specific type of receptor blocker employed. Patients received a radiation dose of 60–70 Gy, delivered in conventional fractionation (1.8–2.0 Gy per fraction). The radiotherapy was administered using either Intensity-Modulated Radiotherapy or 3D Conformal Radiotherapy. All patients were positioned in a supine position with their arms at their sides, under a large-bore CT scanner. A thermoplastic mask was used to immobilize the patient, followed by laser alignment and enhanced CT scanning. Finally, the CT localization images were transferred to the target delineation system.
During the screening process, patients were strictly staged according to the 7th edition of the TNM classification system. This study received approval from the Ethics Committee and the Institutional of Cancer Hospital Affiliated to Shandong First Medical University (SDTHEC2024003169). Due to the retrospective design of the study and the absence of anticipated risks to the participants, patient consent was deemed unnecessary. This study ensures that all key information is detailed in accordance with the requirements of the STROBE guidelines24.
The NHANES (https://www.cdc.gov/nchs/data-linkage/mortality-public.htm) is a comprehensive research program conducted by the National Center for Health Statistics (NCHS) to assess the health status of adults and children in the United States.This database can be used to assess survival outcomes, allowing for the analysis of the relationship between diseases, medication use, and survival outcomes25. On the other hand, adhering to the identical exclusion criteria, essential data of 568 cancer patients in the NHANES database spanning from 2014 to 2018 were gathered. This information encompassed gender, age, ethnicity, smoking and alcohol consumption history, average systolic blood pressure (SBP), average diastolic blood pressure (DBP), cardiovascular and cerebrovascular disease history, as well as the utilization of β-blockers, among other parameters.
Statistical analysis
The primary clinical endpoints included OS and PFS. OS is defined as the duration from randomization to death resulting from any cause during a clinical trial. PFS measures the time from the start of a clinical trial until tumor progression or patient death from any cause.
Initially, descriptive statistics and frequency tables are employed to encapsulate the diverse data. Chi-square tests and Fisher tests were utilized to scrutinize group distinctions among clinical single centers and diverse baseline information in the database. The Kaplan-Meier method was employed to illustrate the distribution of OS time and PFS time, with a comparison of median survival and PFS based on drug utilization. Multivariate Cox proportional hazard models were employed to assess the impacts of pertinent covariates on OS and PFS. All computations were conducted using SPSS (IBM SPSS Statistics 23, Armonk, USA) and R (R 4.2.3 Foundation for Statistical Computing, Vienna, Austria). A p-value below 0.05 was deemed statistically significant.
Results
Patient characteristics
Among patients undergoing RT for NSCLC with HTN, 750 individuals met the criteria for inclusion in the analysis. Analytical procedures were conducted based on the utilization of antihypertensive medications among the enrolled patients, and a frequency distribution table was generated. Among this cohort, 278 (37%) individuals opted for the administration of β-blockers, whereas 472 (63%) refrained from their usage. Similarly, 240 (32%) patients chose ACEIs, while 510 (68%) opted not to. Excluding 547 (73%) patients, 203 (27%) individuals were prescribed antihypertensive ARBs. Diuretic antihypertensive medications were prescribed for 309 (41%) patients and omitted for 441 (59%) patients. Additionally, 172 (2%) patients were administered CCB, while the remaining 578 (77%) were not (Supplemental Table S1). The distribution table shows that diuretics were the most commonly prescribed antihypertensive drugs, making up 41% of the total. The use of β-adrenergic blockers ranks second, accounting for 37% of the total patients. Furthermore, the distribution of people using antihypertensive drugs reveals that the number of individuals who do not use these drugs exceeds half of the total population. This finding further highlights the lack of awareness among people regarding HTN control and their failure to promptly manage and regulate their elevated blood pressure.
A total of 750 patients were included in this study according to the exclusion criteria, and the baseline characteristics of each cohort were listed in Table 1. The participants in this study primarily consisted of individuals with advanced NSCLC in stages IIIA and IIIB. Patients were required to undergo RT five days a week, with a total prescribed dose of 95% of the planned target volume. Out of these, 368 (49%) underwent 3D-conformal radiotherapy, while 382 (51%) received intensity-modulated radiotherapy. This study classified HTN and found that patients are mainly in grades II and III, corresponding to medium to high risk. Furthermore, the utilization of β-adrenergic blockers exhibited a gradual increase as KPS scores declined. The Chi-square test revealed a statistically significant difference in KPS between the two groups (p < 0.050). Among patients with a KPS less than 80, 55% used β-adrenergic blockers, which was 10% higher than those with a KPS greater than 80 (Table 1). No differences were observed in age, T stage, sequence of occurrence, pathological tumor type, smoking status, type of RT, HTN grade, and group (p > 0.050).
To further investigate the impact of β-adrenergic blockers on total tumor population, we also verified the information obtained from the NHANES database. The study included basic information from 568 individuals in the NHANES database. The differences in baseline characteristics between the two groups were compared using a Chi-square test, as shown in Supplemental Table S2. The results indicated significant differences in age (p = 0.001) and DBP (p = 0.007) between the two groups. However, no statistical differences were observed in other factors between the groups (p > 0.050).
Survival outcomes with beta blocker use
In the Chinese single-center cohort, the Kaplan-Meier curve illustrates OS and PFS in patients treated with and without β-blockers, as depicted in Fig. 1. The x-axis of the survival curve represents follow-up time in months, and the y-axis represents the survival rate. The blue curve represents the Yes group, and the orange curve represents the No group. The median OS was 17.64 months with β-blockers and 13.16 months without β-blockers. The data clearly indicates that the utilization of β-blockers extends the median survival time, leading to a significant benefit in terms of OS as illustrated in Fig. 1a (p < 0.0001). Similarly, the median PFS with β-blockers was 7.50 months, whereas the median PFS without β-blockers was 4.91 months. This suggests a substantial advantage associated with the use of β-blockers, as depicted in Fig. 1b (p < 0.0001). Compared with no β-adrenergic blockers, patients using β-adrenergic blockers had a relative increase in median OS of 4.48 months and a relative increase in median PFS of 2.59 months. Consequently, these findings indicate that the use of β-adrenergic blockers has benefits in terms of overall survival time as well as disease progression and metastasis.
Survival outcomes in association with β-adrenergic blockers use in a Chinese cohort. The Kaplan-Meier curves for OS (a) and PFS (b) show data for patients using β-adrenergic blockers (Yes) and those not using them (No). The x-axis indicates follow-up time in months, and the y-axis shows survival rates, with 1.00 representing 100%. The blue curve represents the Yes group, and the orange curve represents the No group. For OS (a), the median survival time was 17.64 months for Yes and 13.16 months for No (p < 0.0001). For PFS (b), the median survival time was 7.50 months for Yes and 4.91 months for No (p < 0.0001). These results indicate that beta-blocker use is associated with longer OS and PFS.
Upon adjusting for gender, age, KPS score, clinical stage, sequence of occurrence, pathological type, smoking status, type of RT, HTN stage and group, and specific antihypertensive drug use, the utilization of β-blockers demonstrated a significant association with improved OS (β-blockers vs. no β-blockers: HR, 0.71; 95% CI, 0.33–0.91; p = 0.010) (Supplemental Table 3). Similarly, a significant correlation was identified with PFS (β-blockers vs. no β-blockers: HR, 0.64; 95% CI, 0.39–0.96; p = 0.030) (Table 2). β-adrenergic blocker use reduced the risk of death by 29% and the risk of progression or metastasis by 36%. In additions, Lower KPS scores did not reduce in the risk of death (KPS < 80 vs. KPS ≥ 80: HR, 0.76; 95% CI, 0.51–1.23; p = 0.230) or disease progression (KPS < 80 vs. KPS ≥ 80: HR, 0.64; 95% CI, 0.23–1.04; p = 0.060), as these observations did not attain statistical significance. Likewise, there was no association between the middle-risk (OS: p = 0.850; PFS: p = 0.450), high-risk (OS: p = 0.630; PFS: p = 0.330), and very high-risk groups (OS: p = 0.420; PFS: p = 0.220) for overall survival or disease progression compared with the low-risk group for hypertension. The results of this study insufficiently support the notion that ACEIs, ARBs, CCB, and diuretics influence the survival and PFS of NSCLC patients undergoing RT.
Simultaneously, within the NHANES database, the Kaplan-Meier curve did not indicate a significant influence of β-blockers on OS in the tumor population. (HR, 1.228; 95% CI, 0.84–1.79; p = 0.254) (Fig. 2). In a multivariate Cox regression model, mean DBP and the use of β-blockers were also not identified as independent risk factors for survival in the tumor population (β-blockers vs. no β-blockers: HR, 1.05; 95% CI, 0.74–1.51; p = 0.780). However, age was found to be statistically significant (Age ≥ 65 vs. Age < 65: HR, 3.27; 95% CI, 2.23–4.78; p < 0.001) as shown in Table 3. Although findings from the NHANES database indicate that β-adrenergic blockers do not show statistically significant effects on OS in tumor patients, survival benefits were observed in more than 700 clinical samples. Therefore, β-adrenergic blockers warrant further research and exploration in lung cancer patients undergoing RT.
Survival outcomes in association with β-adrenergic blockers use in NHANES database. The figure shows the overall survival curves for patients using β-adrenergic blockers (Yes) and those not using them (No) in the NHANES database. The x-axis represents follow-up time, and the y-axis shows survival rate. The blue curve represents the Yes group, and the orange curve represents the No group. The difference between the two groups was not statistically significant (p = 0.254).
Discussion
To date, clinical studies on the impact of β-adrenergic blockers on the survival of NSCLC patients undergoing RT and concurrent HTN are still limited. In our study, we found the following: (1) Among the target population using β-adrenergic blockers, the mOS was extended (17.64 months with beta-blockers vs. 13.16 months without). (2) The use of β-adrenergic blockers also prolonged the mPFS (7.50 months with beta-blockers vs. 4.91 months without). (3) After adjusting for other factors, the use of beta-blockers was significantly associated with improved OS and PFS (OS: HR, 0.71, 95% CI, 0.33–0.91, p = 0.010; PFS: HR, 0.64, 95% CI, 0.39–0.96, p = 0.030).
Recent clinical studies have shown that the use of β-adrenergic blockers is associated with reduced tumor progression and lower mortality rates in patients with bladder cancer26 and breast cancer27,28. Similarly, our study found that β-adrenergic blockers can improve survival outcomes in patients with NSCLC and may play a positive role during RT. This effect may be attributed to the inhibition of the adrenergic signaling pathway by β-adrenergic blockers, which reduces tumor metastasis and improves patient prognosis. Studies have found that during physiological and pathological stress, the sympathetic nervous system releases large amounts of adrenaline and norepinephrine, which bind to specific adrenergic receptors, including α1, α2, β1, β2, and β3. It is important to note that adrenergic receptors are found in vulnerable sites such as the lungs, brain, breast, and prostate, where tumor development and metastasis can occur29. The activation of β2-adrenergic receptors triggers a chain reaction: It activates adenylate cyclase through G proteins, converting ATP to cAMP. cAMP then activates protein kinase A, which phosphorylates downstream proteins. This ultimately boosts the expression of cyclins like Cyclin D1 and anti-apoptotic proteins like Bcl-2, promoting cell growth and preventing cell death30,31,32. Selective β2-adrenergic receptors bind to β1-AR and β2-AR, blocking adrenaline and noradrenaline from binding and inhibiting downstream signaling33. With the cAMP-PKA pathway inhibited, the expression of Cyclin D1 and Bcl-2 is reduced, thereby suppressing tumor cell proliferation and survival30. β-adrenergic blockers may influence patient survival by inhibiting the adrenergic signaling pathway.
In our study, patients using β-adrenergic receptors had better OS and PFS after RT than those not using them. This might be due to β-adrenergic receptors reducing cardiac toxicity from RT. In the Lung-ART study34, adjuvant radiotherapy for stage IIIA-N2 NSCLC did not improve OS or DFS but increased cardiopulmonary toxicity. Several investigations consistently indicate the presence of specific adverse effects on the myocardium, valves, pericardium, and vasculature during thoracic RT. Haseltine et al. observed that thoracic RT, while effective in eliminating tumor lesions, concurrently induces coronary atherosclerosis, resulting in heightened lumen pressure and increased blood pressure35. In addition, Jiang et al. found that RT can activate the renin-angiotensin-aldosterone system, which further promotes the formation of myocardial fibrosis and leads to the occurrence of cardiovascular diseases36. Building upon prior research, the utilization of β-adrenergic blockers in this investigation is grounded in their capacity to diminish heart rate, attenuate myocardial contraction, and suppress sympathetic nerve activity. β-adrenergic blockers may also have a potential radiosensitizing effect. Additionally, RT causes damage to tumor cells, activating DNA repair mechanisms. β-adrenergic blockers can inhibit the DNA repair capacity of tumor cells, reducing non-homologous end joining and homologous recombination repair, thereby decreasing the radiation resistance of tumor cells. β-adrenergic receptors may demonstrate their potential value in RT37. Similarly, the 2023 European Society of Hypertension Guidelines reaffirm the status of β-adrenergic blockers as the primary treatment, either as a standalone therapy or in combination38. This multifaceted positive impact may help improve the OS and prognosis of NSCLC patients undergoing RT, and is worth further exploration in the field of RT.
The clinical cohort identified a survival benefit associated with β-adrenergic blockers. However, analyses using the NHANES database failed to demonstrate an improvement in survival rates for the general cancer population. This lack of observed benefit in the broader cancer population may be due to the fact that the NHANES database includes a wide range of cancer types, treatments, and comorbidities. This diverse scope may dilute the potential benefits of β-adrenergic blockers25. In the clinical cohort, we focused on NSCLC patients with HTN undergoing RT, with strict standardization of selection and treatment. The NHANES study, based on cross-sectional data, lacks detailed records of treatment and long-term follow-up despite its large sample size39. Additionally, the database may not fully control for comorbidities or lifestyle confounders. The clinical cohort strictly excluded patients with certain conditions (such as diabetes and coronary heart disease) to eliminate these factors as potential confounders. These factors may lead to different outcomes in the two cohorts.
Due to the limitations of our study, there is a lack of consensus with the findings of other relevant studies. Firstly, because this study was initially retrospective, we could not truly assess the interaction of medications taken by all hospitalized patients during treatment. Nonetheless, employing a multiple regression model revealed a relatively uniform distribution of known confounding variables among the patient cohort. This study may be subject to potential biases and unmeasured confounding factors that could affect the results. Future studies will incorporate prospective research and randomized controlled trials to further validate our findings. Secondly, with the progress of immunotherapy in studies like IMpower010 40and PACIFIC41,42, NSCLC treatment has entered a new phase43. Research shows that combining β-adrenergic blockers with immunotherapy can enhance cytotoxic T-cell function, improving anti-tumor effects37. The data collection period for our study predates the widespread clinical use of immunotherapy, so our findings do not include data related to immunotherapy. Thirdly, in a study conducted by Barron TI, there was a decrease in breast cancer-specific mortality among propranolol users, and no significant difference was observed in breast cancer-specific mortality among atenolol users. In our analysis, the sample size within each group was insufficient to adequately address the distinction between selective and non-selective β-blocker medications and to definitively ascertain which class of drugs exerted an impact on survival. The lack of data on the type, dosage, and duration of β-adrenergic blockers use is a limitation of this study, which we hope to address with more detailed information in future research. Conversely, results from single-center clinical studies suggest that β-adrenergic blockers enhance OS and PFS in lung cancer patients undergoing RT. The disparity in these two outcomes may be pertinent to patients undergoing RT in clinical investigations. There exists an interaction between β-adrenergic blockers and RT. Nevertheless, the mechanism and principles of action remain unclear and necessitate further exploration and research. The aim of this study was to determine β-adrenergic blockers enhance OS and PFS in patients with NSCLC complicated by HTN, thereby counteracting the current unfavorable trend and ameliorating both the quality of life and prognosis for these patients.
Conclusion
In summary, β-adrenergic blockers prolonged OS and PFS in HTN patients with NSCLC undergoing RT. Leveraging substantial expertise in β-blocker usage for other medical conditions, the application of these discoveries could potentially be promptly integrated. Given the elevated prevalence of HTN among patients with NSCLC, it is essential to investigate the influence of adrenergic control on tumor prognosis.
Data availability
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Abbreviations
- NSCLC:
-
non-small cell lung cancer
- RT:
-
radiotherapy
- HTN:
-
hypertensive
- OS:
-
overall survival
- PFS:
-
progression-free survival
- mOS:
-
median overall survival
- mPFS:
-
median progression-free survival
- VEGF:
-
vascular endothelial growth factor
- ACEIs:
-
angiotensin-converting enzyme inhibitors
- ARBs:
-
angiotensin receptor blockers
- CCB:
-
calcium channel blockers
- DMFS:
-
distant metastasis-free survival
- DFS:
-
disease-free survival
- KPS:
-
Karnofsky Performance Status
- SBP:
-
systolic blood pressure
- DBP:
-
diastolic blood pressure
- cAMP:
-
cyclic adenosine monophosphate
- CREB:
-
cAMP-response element binding protein
References
Kocarnik, J. M. et al. Cancer incidence, mortality, years of life lost, years lived with disability, and Disability-Adjusted life years for 29 Cancer groups from 2010 to 2019. JAMA Oncol. 8 https://doi.org/10.1001/jamaoncol.2021.6987 (2022).
Raisi-Estabragh, Z. et al. Incident cardiovascular events and imaging phenotypes in UK biobank participants with past cancer. Heart 109, 1007–1015. https://doi.org/10.1136/heartjnl-2022-321888 (2023).
Kidoguchi, S. et al. A. New concept of Onco-Hypertension and future perspectives. Hypertension 77(1), 16–27 (2021 Jan). https://doi.org/10.1161/HYPERTENSIONAHA.120.16044
Sahni, G. & Onco-Hypertension Changing paradigm of treating hypertension in patients with Cancer. J. Clin. Oncol. 41, 958–963. https://doi.org/10.1200/JCO.22.01875 (2023).
Wang, Z., Ma, L., Liu, M., Fan, J. & Hu, S. Summary of the 2022 report on cardiovascular health and diseases in China. Chin. Med. J. 136, 2899–2908. https://doi.org/10.1097/cm9.0000000000002927 (2023).
Collaboration, N. C. D. R. F. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 398, 957–980. https://doi.org/10.1016/S0140-6736(21)01330-1 (2021).
Nagasawa, H. et al. Association of cancer with the risk of developing hypertension. Eur. Heart J. Qual. Care Clin. Outcomes. 10, 228–234. https://doi.org/10.1093/ehjqcco/qcad036 (2024).
Kaneko, H. et al. Medication-Naive blood pressure and incident cancers: analysis of 2 nationwide Population-Based databases. Am. J. Hypertens. 35, 731–739. https://doi.org/10.1093/ajh/hpac054 (2022).
Florido, R. et al. Cardiovascular disease risk among Cancer survivors: the atherosclerosis risk in communities (ARIC) study. J. Am. Coll. Cardiol. 80, 22–32. https://doi.org/10.1016/j.jacc.2022.04.042 (2022).
Kim, C. S. et al. Association of hypertension and blood pressure with kidney Cancer risk: A nationwide Population-Based cohort study. Hypertension 75, 1439–1446. https://doi.org/10.1161/HYPERTENSIONAHA.120.14820 (2020).
Stocks, T. et al. Blood pressure and risk of Cancer incidence and mortality in the metabolic syndrome and Cancer project. Hypertension 59, 802–810. https://doi.org/10.1161/hypertensionaha.111.189258 (2012).
Cohen, J. B., Geara, A. S., Hogan, J. J. & Townsend, R. R. Hypertension in Cancer patients and survivors. JACC: CardioOncology. 1, 238–251. https://doi.org/10.1016/j.jaccao.2019.11.009 (2019).
Laurent, S. Antihypertensive drugs. Pharmacol. Res. 124, 116–125. https://doi.org/10.1016/j.phrs.2017.07.026 (2017).
Farzam, K., Beta, J. A. & Blockers StatPearls [Internet] StatPearls Publishing (2023 Aug 22).
Perron, L., Bairati, I., Harel, F. & F, M. Antihypertensive drug use and the risk of prostate cancer. Cancer Causes Control. 15, 535–541. https://doi.org/10.1023/B:CACO.0000036152.58271.5e (2004).
Hiller, J. G. et al. Preoperative β-Blockade with propranolol reduces biomarkers of metastasis in breast cancer: A phase II randomized trial. Clin. Cancer Res. 26, 1803–1811. https://doi.org/10.1158/1078-0432.Ccr-19-2641 (2020).
Zheng, G., Chattopadhyay, S., Sundquist, J., Sundquist, K. & Ji, J. Antihypertensive drug targets and breast cancer risk: a two-sample Mendelian randomization study. Eur. J. Epidemiol. 39, 535–548. https://doi.org/10.1007/s10654-024-01103-x (2024).
Wrobel, L. J., Gayet-Ageron, A. & Le Gal, F. A. Effects of Beta-Blockers on Melanoma Microenvironment and Disease Survival in Human. Cancers 12 (2020). https://doi.org/10.3390/cancers12051094
Tang, J., Li, Z., Lu, L. & Cho, C. H. β-Adrenergic system, a backstage manipulator regulating tumour progression and drug target in cancer therapy. Sem. Cancer Biol. 23, 533–542. https://doi.org/10.1016/j.semcancer.2013.08.009 (2013).
Cole, S. W. & Sood, A. K. Molecular pathways: Beta-Adrenergic signaling in Cancer. Clin. Cancer Res. 18, 1201–1206. https://doi.org/10.1158/1078-0432.Ccr-11-0641 (2012).
Chaudhary, K. R. et al. Effects of β-Adrenergic antagonists on chemoradiation therapy for locally advanced Non-Small cell lung Cancer. J. Clin. Med. 8 (5), 575. https://doi.org/10.3390/jcm8050575 (2019).
Wang, H. M. et al. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann. Oncol. 24, 1312–1319. https://doi.org/10.1093/annonc/mds616 (2013).
Shah, S. M. et al. Does β-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br. J. Clin. Pharmacol. 72, 157–161. https://doi.org/10.1111/j.1365-2125.2011.03980.x (2011).
von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370, 1453–1457. https://doi.org/10.1016/S0140-6736(07)61602-X (2007).
Curtin, L. R. et al. CL. National health and nutrition examination survey: sample design, 2007–2010. Vital Health Stat. 2 (160):1–23 (2013 Aug).
Udumyan, R. et al. Beta-blocker use and urothelial bladder cancer survival: a Swedish register-based cohort study. Acta Oncol. 61, 922–930. https://doi.org/10.1080/0284186X.2022.2101902 (2022).
Strell, C. et al. Use of beta-blockers in patients with ductal carcinoma in situ and risk of invasive breast cancer recurrence: a Swedish retrospective cohort study. Breast Cancer Res. Treat. 207, 293–299. https://doi.org/10.1007/s10549-024-07358-y (2024).
Lofling, L. L. et al. beta-blockers and breast cancer survival by molecular subtypes: a population-based cohort study and meta-analysis. Br. J. Cancer. 127, 1086–1096. https://doi.org/10.1038/s41416-022-01891-7 (2022).
Daly, C. J. & McGrath, J. C. Previously unsuspected widespread cellular and tissue distribution of β-adrenoceptors and its relevance to drug action. Trends Pharmacol. Sci. 32, 219–226. https://doi.org/10.1016/j.tips.2011.02.008 (2011).
Zhang, X. et al. Chronic stress promotes gastric cancer progression and metastasis: an essential role for ADRB2. Cell. Death Dis. 10, 788. https://doi.org/10.1038/s41419-019-2030-2 (2019).
Jin, M., Wang, Y., Zhou, T., Li, W. & Wen, Q. Norepinephrine/beta(2)-Adrenergic receptor pathway promotes the cell proliferation and nerve growth factor production in Triple-Negative breast Cancer. J. Breast Cancer. 26, 268–285. https://doi.org/10.4048/jbc.2023.26.e25 (2023).
Dong, Z. K., Wang, Y. F., Li, W. P. & Jin, W. L. Neurobiology of cancer: adrenergic signaling and drug repurposing. Pharmacol. Ther. 264, 108750. https://doi.org/10.1016/j.pharmthera.2024.108750 (2024).
Jin, M. Z. J. W. The updated landscape of tumor microenvironment and drug repurposing. Signal. Transduct. Target. Ther. 25;5(1):166 (2020 Aug). https://doi.org/10.1038/s41392-020-00280-x
Le Pechoux, C. et al. Postoperative radiotherapy versus no postoperative radiotherapy in patients with completely resected non-small-cell lung cancer and proven mediastinal N2 involvement (Lung ART): an open-label, randomised, phase 3 trial. Lancet Oncol. 23, 104–114. https://doi.org/10.1016/S1470-2045(21)00606-9 (2022).
Haseltine, J. M. et al. Association of cardiac calcium burden with overall survival after radiotherapy for non-small cell lung cancer. Phys. Imaging Radiat. Oncol. 25, 100410. https://doi.org/10.1016/j.phro.2023.01.001 (2023 Jan 5).
Jiang, W., Li, X. Y., Yang, X. & Cardiac Fibrosis, Y. Cellular effectors, molecular pathways, and Exosomal roles. Front. Cardiovasc. Med. 8, 715258. https://doi.org/10.3389/fcvm.2021.715258 (2021 Aug 16).
Globig, A. M. et al. The beta(1)-adrenergic receptor links sympathetic nerves to T cell exhaustion. Nature 622, 383–392. https://doi.org/10.1038/s41586-023-06568-6 (2023).
Mancia, G. et al. M, 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J Hypertens 41(12), 1874–2071 (2023). Dec 1 https://doi.org/10.1097/HJH.0000000000003480
Pei, Y. et al. The influence of baseline platelet on mortality risk in stroke and cancer patients: a cross-sectional analysis of the NHANES database. BMC Neurol. 25, 30. https://doi.org/10.1186/s12883-025-04043-5 (2025).
Felip, E. et al. Adjuvant Atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 398, 1344–1357. https://doi.org/10.1016/S0140-6736(21)02098-5 (2021).
Antonia, S. J. et al. Durvalumab after chemoradiotherapy in stage III Non-Small-Cell lung Cancer. N Engl. J. Med. 377, 1919–1929. https://doi.org/10.1056/NEJMoa1709937 (2017).
Spigel, D. R. et al. Five-Year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III Non-Small-Cell lung Cancer. J. Clin. Oncol. 40, 1301–1311. https://doi.org/10.1200/JCO.21.01308 (2022).
Bradley, J. D. et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus Paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 16, 187–199. https://doi.org/10.1016/S1470-2045(14)71207-0 (2015).
Acknowledgements
We would like to express our gratitude to the Affiliated Cancer Hospital of Shandong First Medical University for providing us with their learning platform. And thank you to the patients who contributed information to medical research.
Funding
This work was funded by the National Natural Science Foundation of China (82172676, 82373217), the Distinguished Young Scholars of Shandong Provincial Science Fund (ZR2024JQ032), the Shandong Natural Science Foundation Major Basic Research Project (ZR2023ZD26), the State Key Program of National Natural Science of China (82030082).
Author information
Authors and Affiliations
Contributions
D.C, and Y.M designed the study and drafted the manuscript. X.L, P.L and Y.Q were responsible for data collection and organization. X.L and Y.M performed data analysis and interpreted the results. All authors made significant contributions to the work and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liang, X., Li, P., Qin, Y. et al. Beta-adrenergic receptor blockers improve survival in patients with advanced non-small cell lung cancer combined with hypertension undergoing radiotherapy. Sci Rep 15, 10702 (2025). https://doi.org/10.1038/s41598-025-93205-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-93205-z