Abstract
Type 2 diabetes mellitus (T2DM) is a condition marked by insulin resistance and hyperglycemia. Static training, as a form of exercise intervention, is increasingly demonstrating benefits for improving T2DM. However, the underlying mechanisms remain inadequately elucidated. Research suggests that insulin-like growth factor 2 (IGF-2) could be a significant contributor to this phenomenon. In this study, static training significantly reduced fasting and random blood glucose, total cholesterol, triglycerides, and LDL, while increasing HDL and improving insulin sensitivity. Transcriptomics suggested that static training might regulate the IGF-2 pathway and related genes. WB and PCR results showed that IGF-2 expression was upregulated in the static training group, and IGF-2 inhibitors attenuated these effects, whereas IGF-2 overexpression enhanced them. In conclusion, static training improves insulin resistance in T2DM mice via the IGF-2/IGF-1R pathway, suggesting its potential as a therapeutic intervention.
Similar content being viewed by others
Introduction
Diabetes is a chronic metabolic disorder characterized by insulin secretion defects, hyperinsulinemia, insulin resistance, and pancreatic β-cell dysfunction1. In recent years, the incidence of diabetes has gradually increased due to poor lifestyle and dietary habits, affecting approximately 537 million adults worldwide2,3.
Currently, clinical treatments for type 2 diabetes mellitus (T2DM) mainly include pharmacological interventions such as metformin, insulin injection therapies, and surgical options, which are associated with side effects including gastrointestinal issues, hypoglycemia, and weight gain. Although more personalized treatments are increasingly employed clinically, the escalating medical demands and significant economic burdens continue to make prevention and management of this disease a major challenge, underscoring its status as a persistent global public health issue4,5,6.
Physical exercise, as a lifestyle intervention for patients, is emphasized by diabetes associations both domestically and internationally for its critical role in the treatment of diabetes7,8. Static training9,10,11, a special exercise style characterized by endurance, high-intensity interval activities, and flexibility, significantly enhances physical activity with good patient tolerance and aligns with contemporary recommendations for exercise interventions in T2DM, gaining increasing application in clinical interventions12,13. In static training, patients maintain a relatively fixed body position and joint angle during training, facilitating skeletal muscle contraction at rest.
Extensive research indicates that static training can enhance the efficiency of glucose uptake, oxidation, and utilization in the muscles of patients with T2DM, thereby regulating glucose and lipid metabolism and reducing insulin resistance. This suggests the potential benefits of static training in the prevention and treatment of diabetes, though the specific biological regulatory mechanisms remain unclear10,14.
Insulin-like growth factor 2 (IGF-2), a protein with functions similar to insulin, potentially influences the onset and progression of diabetes15. IRS1 (Insulin Receptor Substrate 1), a key protein in the IGF-2-mediated signaling pathway, mainly expressed in skeletal muscle and adipose tissue, and mediates the effect of insulin on glucose uptake16,17. IRS2 plays a complementary role in insulin signaling, potentially influencing glucose transport indirectly. The two molecules work in concert within the insulin signaling pathway18,19. The IGF-2 pathway enhances insulin sensitivity and impacts tissue glucose uptake and utilization. This study aims to investigate whether static training influences the expression of IRS1 through the IGF-2/IGF-1R signaling pathway, thus affecting GLUT expression in skeletal muscles and alleviating insulin resistance, providing experimental and theoretical support for the application of static training in the prevention and treatment of T2DM.
Results
Static training enhanced metabolism and improved insulin resistance in T2DM mice
Firstly, a T2DM model was established and validated. Compared with the control group, mice in the model group exhibited significantly increased body weight (p < 0.01,), food consumption (p < 0.01), fasting blood glucose (FBG, p < 0.001), random blood glucose (RBG, p < 0.001), and water consumption (p < 0.01). (Fig. 1A).
Comprehensive Analysis of Physiological and Metabolic Changes in Mice After Intervention, (A) Comparison of weight, diet, FBG, RBG, and water intake between control and model groups. (B) Post-intervention changes in weight, diet, FBG, RBG, and water intake across the five groups. (C) ITT results and AUC for ITT and GTT, highlighting intervention effects on insulin sensitivity. (D) Post-intervention lipid metabolism markers (TC, TG, LDL, HDL) across all groups.(E) Post-intervention insulin levels, HOMA-IR, HbA1c, and TyG, showing group differences. For modeling, the control group has n = 9, and the model group has n = 41. After successful modeling, the sample size for each group is n = 6. Compared to the static training group: p < 0.05, ** p < 0.01, *** p < 0.001.
Next, static strength training was introduced. The results showed a slight decrease in the body weight of the mice (p < 0.01). Dietary intake also significantly declined, especially in the static training group (p < 0.01). In terms of glucose metabolism, static training led to notable decreases in both fasting blood glucose (FBG, p < 0.001) and random blood glucose (RBG, p < 0.001), with effects comparable to those of metformin. While water consumption significantly increased in the model group (p < 0.01), a non-significant decrease was observed in the static training group (Fig. 1B).
To further verify the benefit of static training on T2DM, insulin sensitivity and glucose tolerance tests were conducted. The results demonstrated that static training significantly improved insulin sensitivity and led to the most pronounced reduction in blood glucose levels (p < 0.01). (p < 0.01). In the glucose tolerance test (GTT), static training markedly enhanced glucose tolerance, outperforming the metformin group (p < 0.01, Fig. 1C). As for insulin sensitivity, static training decreased insulin levels (INS) (p < 0.001), HOMA-IR (p < 0.001), and glycosylated hemoglobin (HbA1c), and the TyG index (p < 0.01, Fig. 1E) indices, improving long-term glycemic control.
Additionally, abnormalities in lipid metabolism are typically closely associated with insulin resistance in type 2 diabetes (T2DM). Therefore, the effect of static exercise on lipid metabolism was investigated. As shown from the data, static training significantly improved lipid metabolism, evidenced by reductions in total cholesterol (TC) (p < 0.01), triglycerides (TG) (p < 0.01), and low-density lipoprotein (LDL) levels (p < 0.01), along with substantial increases in high-density lipoprotein (HDL) levels (p < 0.001) (Fig. 1D). In summary, static training markedly ameliorated metabolic disorders and insulin resistance in T2DM mice.
Following the staining procedures, histological examination via hematoxylin and eosin (HE) staining revealed that the gastrocnemius muscle fibers in the control group were tightly packed, with regular shapes, uniform sizes, and distinct striations. In contrast, the model group showed irregularly shaped, partially atrophied fibers with widened interstitial spaces. The metformin group displayed muscle fibers with improved arrangement and increased regularity. Similarly, both the aerobic exercise and static training groups exhibited closely packed muscle fibers, with interstitial spaces approaching normalcy and minimal signs of damage or inflammation (Fig. 2A). Additionally, periodic acid-Schiff (PAS) staining indicated substantial glycogen reserves in the gastrocnemius tissue of the control group, with a pronounced purple hue. Conversely, the staining intensity in the T2DM model group was markedly diminished or absent, suggesting a significant reduction in glycogen storage. Notably, the static training group demonstrated enhanced staining intensity, indicating improved glucose utilization and glycogen synthesis in the muscle (Fig. 2B).
Effects of Static Strength Training on Histological, Ultrastructural, and Molecular Changes in Muscle Tissue: (A) H&E staining of muscle tissues. (B) PAS staining of muscle tissues. (C) Electron microscopy of muscle structure. Scale bar = 100 μm.Compared to the static training group: p < 0.05, ** p < 0.01, *** p < 0.001.
Ultrastructural analysis revealed intact gastrocnemius muscle cells in the control group, with orderly fibers, abundant mitochondria, well-defined cristae, and distinct Z-line and H-band structures. The model group showed severe degeneration, disorganized fibers, reduced, irregular mitochondria, disrupted cristae, vacuoles, and blurred Z line and H band. The metformin group displayed mild degeneration with irregular mitochondria and partial cristae fragmentation. The aerobic exercise group showed moderate degeneration, including mitochondrial swelling and cristae defects. In contrast, the static training group had intact muscle cells with well-arranged fibers, abundant, uniform mitochondria, clear cristae, and no autophagic structures (Fig. 2C).
Static training regulates gene expression and metabolic pathways in T2DM mice through the IGF-2 signaling pathway
To explore the underlying mechanisms by which static training alleviates insulin resistance in T2DM mice, transcriptomic analysis of mouse serum was performed. The results revealed alterations in several important genes and signaling pathways, particularly a notable regulation of IGF-2.
Pearson correlation analysis showed high consistency in gene expression within groups, especially in the static training (SE) and model (DM) groups, with coefficients close to 1 (0.95–0.99), confirming experimental reproducibility. In contrast, correlations between different treatment groups were lower (0.85–0.90), indicating a significant impact of static training on gene expression (Fig. 3A). FPKM distribution analysis further demonstrated improved gene expression in the SE group, partially restoring levels closer to the control (Ctrl) and surpassing the model group (DM) (Fig. 3B). SNP and InDel analysis revealed comparable variant counts across groups, with slightly more indels in SE, indicating minimal impact on experimental outcomes (Fig. 3C).
Transcriptomic Analysis of Gene Expression, Enrichment, and Pathway Alterations Across Control, Model, and Static Training Groups. (A) Heat map of Pearson correlation analysis between each sample, showing the correlation of gene expression levels among different groups (Ctrl, DM, SE). The darker the color, the higher the correlation. (B) FPKM (number of fragments per kilobase transcript per million alignment fragments) distribution of each sample, showing the distribution of gene expression levels in the control group (Ctrl), model group (DM), and static training group (SE). (C) number of SNPS and indels in different samples. (D) FPKM density distribution map of each group. (E) Gene enrichment analysis between the static training group (SE) and the model group (DM), blue bars represent GO items enriched for down-regulated genes, and red bars represent GO items enriched for up-regulated genes. (F) pathway enrichment analysis between static training (SE) and model group (DM), bubble size represents the number of enriched genes, and color represents the q value. (G) Gene enrichment analysis between model group (DM) and blank group (Ctrl). (H) pathway enrichment analysis between model group (DM) and control group (Ctrl). (I) statistical map of differentially expressed genes (DEG). (J-L) Volcano Plots show differentially expressed genes, red indicates significantly up-regulated genes, blue indicates significantly down-regulated genes, and green indicates no significantly changed genes.
FPKM density analysis indicated that gene expression in the static training group fell between control and model levels, suggesting partial restoration (Fig. 3D). Gene enrichment20 showed upregulation in metabolic and immune response genes (e.g., GO:0006955, GO:0008152) and downregulation in cell cycle and DNA repair genes (Fig. 3E). Pathway analysis21 confirmed enrichment in insulin signaling and inflammatory pathways in the static training group, while the model group showed downregulated metabolism pathways, aligning with T2DM pathology (Fig. 3F and H).
DEG analysis identified 200 upregulated and 300 downregulated genes in SE vs. DM (Fig. 3I), with volcano plot analysis showing a limited subset of significant changes (Fig. 3J and L).
Pathway enrichment analysis22 suggested that static training may modulate immune responses to improve T2DM pathology, including “IL-7 signaling” and “inflammatory response” (Fig. 4A and B). Notably, IGF-2 has garnered particular attention due to its close association with inflammation and metabolism, playing a critical role in the onset and progression of T2DM. Heat map analysis showed decreased IGF-2 levels in the DM group and increased levels in the SE group, suggesting that static training may exert its effects through IGF-2. (Fig. 4C).
Pathway and Gene Expression Analysis Between Static Training, Model, and Control Groups: (A) pathway enrichment analysis between SE group and DM group. (B) pathway enrichment analysis of DM group and Ctrl group. (C) heatmap of gene expression between SE group and DM group. (D) heatmap of gene expression between SE group and Ctrl group. (E) heatmap of gene expression between DM group and Ctrl group. (F) GO enrichment analysis between DM group and Ctrl group.
Other immune-related genes, such as PEG3 and GST family members, also differed significantly between groups, with notable increases in genes like MB, C7, and IGF-2 in the SE group compared to Ctrl, suggesting links to static training effects (Fig. 4D). Conversely, the DM group displayed upregulated immune genes (IRF7, IFIT1) and downregulated metabolic genes (FBP2, PAK1), indicating compromised metabolism in T2DM (Fig. 4D-E). GO enrichment analysis further emphasized immune system and stress response gene enrichment in the DM group (Fig. 4F), aligning with observed inflammatory responses. Overall, transcriptome data suggested that static training alleviated metabolic dysfunction and insulin resistance in T2DM mice by modulating the IGF-2 pathway and related genes.
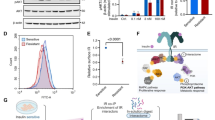
To confirm the critical role of IGF-2 in static training treatment, further validation was conducted. Firstly, Real-time PCR analysis of IRS-1, IRS-2, IGF-IR, and IGF-2 mRNA levels in gastrocnemius tissue showed significantly lower expression in the T2DM group compared to controls (p < 0.001), indicating suppression by the disease. Following intervention, gene expression improved across all groups, with static training producing the most pronounced recovery, significantly elevated compared to the model group (p < 0.01) (Fig. 5A).
Subsequently, protein expression levels of IRS-1, IRS-2, IGF-IR, and IGF-2 were assessed by Western blot, confirming transcriptome findings. The model group showed significantly reduced levels compared to controls (p < 0.001). Following intervention, each treatment group displayed recovery, with the static training group showing the most significant restoration, nearly matching or surpassing control levels and differing significantly from the model group (p < 0.01) (Fig. 5B and C). This effect was particularly pronounced for IGF-1R and IRS-2, indicating that static training may improve glucose and lipid metabolism by modulating the IGF2 signaling pathway. These results suggest that static training may therapeutically enhance key molecules in the IGF signaling pathway.
IGF-2 inhibitors aggravate insulin resistance in T2DM mice
To further investigate the potential role of IGF-2 in enhancing skeletal muscle insulin resistance through static training, an experiment was conducted in which IGF2 inhibitor was used to intervene in T2DM mice (Fig. 6A). In the static training group, body weight was significantly reduced. However, after the IGF-2 inhibitor introduction, weight initially decreased but rebounded in later stages, potentially indicating an adaptive response (Fig. 6B). In contrast, body weight remained relatively stable in both the model and IGF-2 inhibitor plus model groups, with a slight increase toward the intervention’s end.
Effects of Static Strength Training Combined with IGF-2 Inhibition on Metabolic and Physiological Parameters in Mouse Models: (A) Comparison of FBG, RBG, diet, water intake, and AUC of ITT between control and model groups based on modeling standards. (B-C) Post-intervention changes in body weight, fasting blood glucose (FBG), random blood glucose (RBG), and insulin tolerance test (ITT) results across the model group, static strength training group, inhibitor-combined model group, and inhibitor-combined static strength training group, Modeling standards of AUC for glucose tolerance test (GTT) and insulin tolerance test (ITT) comparison between control and model groups, and glucose tolerance test (GTT) area under the curve (AUC) following interventions in each group. (D) Lipid metabolism indicators after intervention, including total cholesterol (TC), triacylglycerol (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) levels in each group. (E) Changes in insulin (INS) levels, glycosylated hemoglobin (Hb), homeostasis model assessment of insulin resistance (HOMA-IR). For modeling, the control group has n = 9, and the model group has n = 41. After successful modeling, the sample size for each group is n = 6. Compared to the static training group: p < 0.05, ** p < 0.01, *** p < 0.001.
For fasting blood glucose (FBG) and random blood glucose (RBG) levels, the static training group showed a significant reduction (p < 0.01). However, this effect was less pronounced in the group combining IGF-2 inhibition with static training (Fig. 5A). Blood glucose levels in the model and IGF-2 inhibitor plus model groups remained elevated, indicating persistent insulin resistance.In the insulin tolerance test (ITT), the static training group showed a statistically significant increase in insulin sensitivity (p < 0.01). In contrast, the IGF-2 inhibitor plus model group displayed a weaker improvement, though some sensitivity enhancement was noted (Fig. 6B).
An analysis of the area under the curve (AUC) for the ITT (Fig. 6B-C) indicated that the static training group had the lowest AUC, reflecting the greatest enhancement in insulin sensitivity (p < 0.01). In contrast, the AUC for the IGF-2 inhibitor plus static training group was higher, suggesting that IGF-2 inhibition slightly attenuated the effect of static exercise. In the glucose tolerance test (GTT) and its AUC analysis, the static training group demonstrated superior glucose regulation (p < 0.01). Conversely, the IGF-2 inhibitor plus model group showed the highest AUC, indicating impaired glucose tolerance.
Lipid profile analysis further supported these findings. The static training group achieved the most substantial reductions in total cholesterol (TC), triglycerides (TG), and low-density lipoprotein (LDL) levels, along with an increase in high-density lipoprotein (HDL) (p < 0.01). However, these improvements were less pronounced in the IGF-2 inhibitor plus static training group compared to static training alone ((Figs. 6D)).
Additionally, reductions in insulin levels (INS), glycosylated hemoglobin (HbA1c), and the HOMA-IR index were significant in the static training group (p < 0.01), whereas these improvements were less pronounced in the IGF-2 inhibitor plus static training group (Fig. 6E).
Histological examination using hematoxylin and eosin (HE) staining revealed disorganized muscle fibers, enlarged inter-fiber gaps, and muscle rupture and necrosis in the model group, indicating substantial muscle damage. The static training group, however, exhibited orderly fiber arrangement, intact tissue structure, reduced gaps, and less muscle damage. In the IGF-2 inhibitor plus model group, muscle damage was exacerbated, with further enlarged gaps. Nevertheless, static training markedly improved fiber alignment and reduced damage, even under IGF-2 inhibition (Fig. 7A).
Effects of Static Strength Training and IGF-2 Inhibition on Muscle Tissue Structure and IGF-2 Signaling Pathway (n = 3): (A) H&E staining of muscle tissues from the model group, static strength training group, IGF-2 inhibitor combined with model group, and IGF-2 inhibitor combined with static strength training group, showing differences in muscle fiber structure.(B)PAS staining of muscle tissues.(C) Western blot analysis of IRS-1, IRS-2, IGF-1R, and IGF-2 protein expression in muscle tissues from the different groups. β-actin serves as the loading control. (D) Quantification of IRS-1, IRS-2, IGF-1R, and IGF-2 protein levels, showing the relative expression in each group. (E) RT-qPCR analysis of IRS-1, IRS-2, IGF-1R, and IGF-2 mRNA expression levels, comparing the four experimental groups. Scale bar = 100 μm.Compared to the static training group: * p < 0.05, ** p < 0.01, *** p < 0.001.
PAS staining results supported the HE staining findings, showing a significant reduction in glycogen storage in the model group’s muscle tissue, indicating impaired glucose metabolism. In contrast, the static training group exhibited increased glycogen storage, evidenced by heightened PAS staining intensity, suggesting improved muscle glucose metabolism. In the IGF-2 inhibitor group, glycogen levels were further reduced, implying that IGF-2 inhibition worsened glycogen metabolism disturbances. However, static training combined with IGF-2 inhibition significantly enhanced glycogen storage compared to the IGF-2 inhibitor model group (Fig. 7B).
Western blot analysis of IRS-1, IRS-2, IGF-1R, and IGF-2 protein levels aligned with mRNA level changes, indicating that static training significantly boosted these protein levels. IGF-2 inhibition markedly reduced their expression, underscoring IGF-2’s regulatory role. In the IGF-2 inhibitor plus static training group, protein levels recovered compared to the IGF-2 inhibitor group but remained lower than in the static training group alone, highlighting IGF-2’s importance in maintaining IGF pathway integrity and the influence of static training on IGF-2 (Fig. 7C and D).
Real-time PCR showed that static training significantly upregulated IRS-1, IRS-2, IGF-1R, and IGF-2 mRNA levels. In contrast, these gene expressions were notably reduced in the IGF-2 inhibitor group, reinforcing IGF-2’s crucial regulatory role. Although gene expression partially recovered in the combined IGF-2 inhibition and static training group, levels were still lower than in the static training group alone (Fig. 7E).
Overexpression of IGF-2 improves insulin resistance in T2DM mice
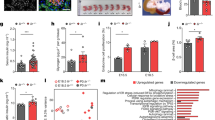
To further explore whether static training enhances skeletal muscle insulin sensitivity via the IGF-2 pathway, we conducted an additional experimental phase using IGF-2 overexpression adenovirus in the gastrocnemius muscle. The group receiving IGF-2 overexpression combined with static training showed significant weight loss in the first six weeks (p < 0.05), with a minor rebound later, though body weight remained below baseline. Blood glucose levels, including fasting (FBG) and random (RBG), were significantly reduced in this group (p < 0.05), outperforming other groups. Insulin tolerance test (ITT) results indicated enhanced insulin sensitivity, with the IGF-2 overexpression plus static training group achieving the best outcomes (p < 0.05). Lipid parameters improved notably, with reductions in total cholesterol (TC), triglycerides (TG), and LDL, and increased HDL (p < 0.05). Insulin levels, HbA1c, and HOMA-IR index were also significantly improved (p < 0.05), with the lowest AUC in the glucose tolerance test (GTT) (Fig. 8).
Metabolic Effects of IGF-2 Overexpression and Static Strength Training on Mouse Models (n = 6): (A-B) Post-intervention changes in body weight, fasting blood glucose (FBG), random blood glucose (RBG), and insulin tolerance test (ITT) results across the control virus + model group, IGF-2 overexpression + model group, control virus + static strength training group, and IGF-2 overexpression + static strength training group. The area under the curve (AUC) of ITT is also shown, Post-intervention area under the curve (AUC) of glucose tolerance test (GTT) and the glucose tolerance test (GTT) results over time for the different groups. (C) Post-intervention lipid metabolism markers, including total cholesterol (TC), triacylglycerol (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) levels in the four groups. (D) Post-intervention levels of glycosylated hemoglobin (HbA1c), insulin (INS), homeostasis model assessment of insulin resistance (HOMA-IR), and triglyceride-glucose index (TyG). Compared to the static training group: p < 0.05, ** p < 0.01, *** p < 0.001.
Histological analysis via HE staining showed relatively preserved muscle structure in the IGF-2 overexpression group despite diabetes, indicating protection against diabetes-induced muscle damage. Conversely, muscle fibers in the blank virus plus static training group were loosely organized, suggesting mild degeneration, while the blank virus plus model group showed severe degeneration and necrosis (Fig. 9A). PAS staining confirmed relatively intact glycogen storage in the IGF-2 overexpression group, with localized reduction, while the blank virus plus static training group displayed reduced glycogen, and the model group showed severe glycogen depletion (Fig. 9B).
Effects of Static Strength Training and IGF-2 Overexpression on Muscle Tissue Structure, Protein, and mRNA Expression (n = 3): (A) H&E staining of muscle tissues from the AAV + static strength training, AAV + model, blank AAV + static strength training, and blank AAV + model groups, highlighting muscle fiber morphology. (B) PAS staining of muscle tissues. (C) Immunofluorescence images showing IGF-2 expression in muscle tissues for blank AAV and IGF-2 overexpression groups. (D) Western blot analysis showing protein expression levels of IRS-1, IRS-2, IGF-1R, and IGF-2 in different groups. β-actin is used as the loading control. (E) Quantification of IRS-1, IRS-2, IGF-1R, and IGF-2 protein levels, displaying relative protein expression across the groups. (F) RT-qPCR analysis of IRS-1, IRS-2, IGF-1R, and IGF-2 mRNA expression levels, comparing the four experimental groups. Scale bar = 100 μm. Compared to the static training group: p < 0.05, ** p < 0.01, *** p < 0.001.
Western blot analysis revealed that static training significantly enhanced IRS-1, IRS-2, IGF-1R, and IGF-2 protein expression. Notably, the IGF-2 overexpression plus static training group showed the highest expression of IRS-1 and IRS-2 (p < 0.001), suggesting effective upregulation of upstream insulin signaling components. IGF-1R expression was significantly higher in the IGF-2 overexpression group, especially when combined with static training (p < 0.001), reinforcing the role of IGF-2 in enhancing insulin receptor expression. IGF-2 protein levels were significantly elevated in the overexpression group, particularly with static training, surpassing all other groups (p < 0.001) (Fig. 9C–E).
Real-time PCR corroborated that static training upregulated IRS-1, IRS-2, IGF-1R, and IGF-2 mRNA levels, with the IGF-2 overexpression plus static training group showing the highest expression of IRS-1 and IRS-2 (p < 0.001), indicating improved insulin signaling and reduced insulin resistance. Elevated IGF-1R and IGF-2 expression in this group further supports enhanced insulin sensitivity in skeletal muscle under diabetic conditions (Fig. 9F).
Discussion
IGF-2 is a multifunctional growth factor interacting with IGF-1 receptors, influencing cellular growth, differentiation, and metabolism via PI3K/Akt and MAPK pathways23, and modulating T2DM progression by affecting insulin signaling and inflammatory responses. Aberrant IGF-2 expression disrupts insulin receptor substrates (IRS1 and IRS2)24,25,26, leading to impaired insulin signaling, reduced glucose transporter (GLUT) activity in the liver and muscle, and inadequate insulin secretion due to effects on pancreatic beta cells27,28. Additionally, IGF-2 regulates inflammation, further exacerbating T2DM pathology29,30,31.
This study explored the effects of static training on insulin resistance in the skeletal muscle of T2DM mice. While static training showed some improvements in insulin sensitivity, several limitations should be addressed.
Firstly, the effectiveness of static training is influenced by factors such as intensity, duration, and frequency. The protocol used here may not fully replicate common human exercise patterns. Static training’s impact on insulin resistance might be less significant than aerobic exercise, which is well-known for improving insulin sensitivity and metabolism. Whether static training can produce lasting improvements in skeletal muscle insulin resistance remains uncertain, and the specific conditions used in this study may limit the generalizability of the results.Secondly, static training does not fully simulate more complex exercise types, such as aerobic or resistance training, which are known to improve insulin sensitivity more comprehensively. Future studies should compare different exercise forms to assess static training’s relative effectiveness.Lastly, this study focused mainly on phenotypic changes, with limited exploration of molecular mechanisms. Further research is needed to determine whether static training modulates key signaling pathways, such as IGF2, IRS1, and IRS2, in improving insulin resistance. Understanding how static training affects other metabolic factors, like fatty acid metabolism and protein synthesis, could provide more insight into its mechanisms.
In conclusion, while static training offers some benefits in improving insulin resistance in T2DM mice, it may not be as effective as other exercise forms. Future research should further explore its mechanisms and compare its effects with other types of exercise.The findings underscore IGF-2 as a critical molecule in metabolic health regulation, suggesting potential therapeutic applications for T2DM.
Materials and methods
Animals
A total of 130 male SPF C57BL/6J mice, aged 8 weeks, were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Certificate No.: SCXK (Beijing) 2016-0011). The animals were acclimated for one week under standard environmental conditions (23 ± 2 °C, 50–70% humidity, 12-hour light/dark cycle) with adlibitum access to food and water. The experimental protocol was approved by the Animal Ethics Committee of Nanjing University of Chinese Medicine (Ethics No.: 202310A049). The mice were euthanized by isoflurane inhalation at a concentration of 2% and then subjected to orbital blood collection, followed by cervical dislocation.
Materials
Metformin hydrochloride tablets (Glucophage, batch number: H20023370, Shanghai Squibb Pharmaceutical Co., Ltd.), streptozotocin (STZ, product number: S8050, Beijing Solarbio Science & Technology Co., Ltd.), sodium citrate buffer solution (product number: C1013, Beijing Solarbio Science & Technology Co., Ltd.), isoflurane (RWD Life Science Co., Ltd), insulin injection (batch number: H10890001, Jiangsu Wanbang Biopharmaceuticals Co., Ltd.), total RNA rapid extraction reagent (product number: YFXM0011P, Nanjing Yifeixue Biotechnology Co., Ltd.), glycated hemoglobin (HbA1c) assay kit (product number: JEB-12766, Nanjing Jin Yibo Biotechnology Co., Ltd.), serum insulin (INS) ELISA kit (product number: JEB-12607, Nanjing Jin Yibo Biotechnology Co., Ltd.), total cholesterol (TC) assay kit (product number: A111-1-1, Nanjing Jiancheng Bioengineering Institute), triglyceride (TG) assay kit (product number: A110-1-1, Nanjing Jiancheng Bioengineering Institute), high-density lipoprotein (HDL) assay kit (product number: A112-1-1, Nanjing Jiancheng Bioengineering Institute), low-density lipoprotein (LDL) assay kit (product number: A113-1-1, Nanjing Jiancheng Bioengineering Institute), reverse transcription kit (product number: 15662ES03, Shanghai Yisheng Biotechnology Co., Ltd.), RT-qPCR SYBR Green Mix (product number: 11201ES03, Shanghai Yisheng Biotechnology Co., Ltd.), IGF-2 antibody (product number: A2086, ABclone, China), IGF-1R antibody (product number: A0243, ABclone, China), IRS-1 antibody (product number: A0245, ABclone, China), IRS-2 antibody (product number: A7945, ABclone, China), PCR primers were synthesized by Shanghai Sangon Biotech Co., Ltd. Glucometer (model: Xiangxie Injection Zhun No. 20172400337, Sinocare Inc.), microplate reader (model: MODEL680, BioTeK Instruments, USA), real-time PCR system (model: 7900, ABI, USA), biological tissue freezing embedding machine (model: EG1150(C), Leica, Germany), microtome (model: CM1900, Leica, Germany), microscope (model: E100, Nikon, Japan), transmission electron microscope (model: HT7700, Hitachi, Japan).
Model establishment
The control group received a standard diet (Cat#: XTCON50J, Jiangsu Xietong, Nanjing, China), while the experimental group was fed a high-fat diet (Cat#: XTHF60, Jiangsu Xietong, Nanjing, China). After six weeks, a low dose of streptozotocin (STZ) was administered. Animals were weighed, and fasting glucose levels were measured following intraperitoneal STZ injections at 40 mg/kg daily for five days. The STZ solution was prepared under light-protected conditions and used within 30 min. Fasting blood glucose levels were measured on days 3 and 7 post-STZ, with successful T2DM modeling defined by two consecutive fasting glucose readings ≥ 16.7 mmol/L32.
Manual therapy intervention
After establishing the T2DM insulin resistance mouse model, mice were randomly assigned to groups: model, positive drug, aerobic exercise, and static training. The control group received standard feeding without exercise and 0.2 ml of saline lavage daily for eight weeks. The model group continued a high-fat diet with no exercise and received the same saline lavage regimen for eight weeks. The positive drug group was also on a high-fat diet without exercise and received 0.2 ml of metformin hydrochloride solution (200 mg/kg, China check, batch H20023370, Shanghai Squibb Co., LTD.), dissolved in saline, daily for eight weeks33.
The aerobic exercise group was on a high-fat diet and underwent treadmill exercise at moderate intensity (approx. 60% VO2max) with a 0° incline34, 17 m/min speed, for 30 min, once daily, five days a week for eight weeks, following Bedford animal exercise standards. The static training group, also on a high-fat diet, performed static training where mice were suspended by their hind feet on a horizontal bar with the abdomen curled, head elevated, and upper limbs held by a derrick, inducing isometric biceps brachii contractions. Static sessions lasted 30 min, once daily, five days a week, for eight weeks35.
To investigate the mechanisms of skeletal muscle insulin resistance in T2DM mice following massage training, three experimental phases were conducted. In the second experiment, an IGF-2 inhibitor was added to the original interventions, creating four groups: control, inhibitor + T2DM model, inhibitor + aerobic exercise, and inhibitor + static training. In the third experiment, an IGF-2 overexpression adenovirus was introduced into the gastrocnemius muscle to explore the impact of Tuina static training on T2DM insulin resistance. Mice were grouped as IGF-2 overexpression adenovirus + T2DM model, IGF-2 overexpression adenovirus + aerobic exercise, IGF-2 overexpression adenovirus + static training, control adenovirus + T2DM model, control adenovirus + aerobic exercise, and control adenovirus + static training.
Daily observation indicators
Routine observations were made to record parameters including mental state, activity level, color changes, water intake, and urine output in mice. Weekly interventions were performed at fixed times to collect tail blood samples for random blood glucose measurements using a glucose meter. Fasting blood glucose levels were measured similarly after a 12-hour fast. Additionally, oral glucose tolerance tests (OGTT) and insulin tolerance tests (ITT) were conducted pre- and post-intervention to track blood glucose changes.
For OGTT, mice were fasted for 12 h before receiving a 1.5 g/kg oral dose of 50% glucose solution. Blood glucose was measured at 0, 30, 60, 90, and 120 min to plot a curve and calculate the area under the curve (AUC). In the ITT, mice were fasted for 6 h, followed by an intraperitoneal injection of insulin at 1 U/kg body weight. Blood glucose was measured at the same time points (0, 30, 60, 90, and 120 min) to plot a graph and calculate AUC.
Methods of tissue retention
Forty-eight hours later after the final exercise session, mice from each group were fasted for 12 h and then euthanized for tissue collection. Body weights were recorded, and blood samples were obtained via orbital collection and centrifuged at 1925 G for 10 min to obtain serum. Liver and skeletal muscle tissues were dissected, rinsed with saline, blotted dry, weighed, and divided. Samples were rapidly frozen in liquid nitrogen, with a portion fixed in 4% paraformaldehyde. Tissues stored in liquid nitrogen were subsequently transferred to a -80 °C freezer for further analysis.
Enzyme-linked immunosorbent assay
Blood samples were collected, and serum was isolated by centrifugation. Samples and standards were added to antibody-coated microplates and incubated for antigen binding. After washing to remove unbound material, enzyme-labeled secondary antibodies were applied, incubated, and washed again. A substrate solution was then added, triggering an enzyme reaction that produced a color change. The reaction was stopped, and absorbance was measured at a specific wavelength using a microplate reader. Glycosylated hemoglobin (HbA1c) and serum insulin concentrations were determined by comparing absorbance values with a standard curve, following ELISA kit instructions. The insulin resistance index (HOMA-IR) was calculated as HOMA-IR36 = (fasting blood glucose (FPG, mmol/L) x fasting insulin (FINS, µU/mL)) / 22.5.
Biochemical indicator detection
Biochemical markers such as triglyceride (TG), total cholesterol (TC), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) were assessed according to the methodology established by Nanjing Jiancheng BioEngineering Institute located in Nanjing, Jiangsu Province.
Hematoxylin and Eosin (H&E) staining
After fixing the gastrocnemius muscles in 4% paraformaldehyde, they were embedded in paraffin and sectioned into 4-micron slices. Sections were dewaxed in xylene twice for 5–10 min each, followed by sequential soaking in 100%, 95%, 85%, and 70% ethanol for 2–5 min each, then hydrated in tap water. The sections were stained with hematoxylin for 5–10 min, rinsed, briefly differentiated in acid alcohol, rinsed again, and counterstained in ammonia water. They were subsequently stained with eosin for 1–3 min, quickly rinsed, dehydrated in graded ethanol, and cleared in xylene twice for 5–10 min each. Finally, sections were mounted with neutral gum and examined under a microscope.
PAS staining
Following fixation of the gastrocnemius muscles with 4% paraformaldehyde, they were embedded in paraffin and sliced into 4-micron sections. After standard deparaffinization, the sections were treated with a 0.5% periodic acid solution for 5–8 min, followed by rinsing in tap water and distilled water. Subsequently, the sections were subjected to Schiff reagent staining for 10–20 min at room temperature in the absence of light, followed by a 10-minute rinse in tap water. Hematoxylin staining was then applied for 1 min, followed by rinsing in tap water, differentiation with hydrochloric acid, additional rinsing in tap water, blue restoration with ammonia, and a final rinse in tap water. The sections were then dehydrated using a gradient of ethanol, clarified with xylene, sealed with neutral gum, and ultimately examined under a microscope.
Ultrastructural examination of gastrocnemius muscle
The calf specimen was treated with glutaraldehyde and subsequently fixed with osmic acid. Following dehydration using a gradient of ethanol, the samples underwent displacement in acetone. Subsequently, the samples were embedded in resin and sectioned into ultrathin slices (~ 70 nm). These sections were then mounted on a copper mesh and subjected to double staining with uranyl acetate and lead citrate. Subsequently, the ultrastructure of gastrocnemius muscle cells was examined under transmission electron microscopy (TEM), allowing for a detailed analysis of muscle fiber characteristics, as well as the morphology and distribution of organelles such as mitochondria and endoplasmic reticulum.
Western blot
After lysing, homogenizing, and performing low-temperature centrifugation, total protein was extracted from the samples. The protein concentration was measured using a BCA protein assay kit. Equal amounts of protein were then loaded onto gels for electrophoresis and transferred onto membranes. The membranes were blocked with 5% skim milk for 2 h, followed by overnight incubation with the primary antibody at 4 °C. After washing, the membranes were incubated with a secondary antibody for 2 h at room temperature. Finally, after additional washes, chemiluminescent detection was performed, and band intensities were analyzed using Quantity One software v4.6.6 ( Bio-Rad, USA) (https://www.bio-rad.com/en-cn/product/quantity-one-software).
RT-PCR
RNA was isolated from gastrocnemius muscle tissue using TRIzol lysate and chloroform. The purity of the extracted RNA was evaluated by measuring the OD260/OD280 ratio, followed by reverse transcription into cRNA. The reference gene β-actin was selected based on Ct values, and the relative expression of mRNA in each group was calculated using the ΔΔCt method. Primers were synthesized by Sangon Bioengineering (Shanghai) Co., LTD. The specific primers utilized in this process are detailed in the accompanying Table 1.
Serum mRNA sequencing and analysis
Total RNA was extracted from mouse serum samples, and quality was assessed. A transcriptome library was constructed and sequenced using the NovaSeq 6000 platform by a designated provider. Differential gene expression analysis was performed on a cloud platform with selection criteria of |log2FC| ≥ 1.3 and FDR < 0.05. Differentially expressed genes (DEGs) were identified between the blank vs. model and model vs. static training groups. KEGG pathway enrichment analysis for DEGs in the static training group was conducted via the Orvison Biology platform (Orvison Biotechnology, Shenzhen, China) (https://www.omicsmart.com). A Venn diagram identified common DEGs between the static training vs. model and model vs. blank groups. Core genes were selected based on statistical significance (p < 0.05) and expression levels (TPM > 10), and validated by RT-PCR, with cDNA synthesized from total RNA and PCR amplification conducted per manufacturer’s instructions.
Statistical analysis
The results are presented as mean and standard deviation (SD). Statistical significance between different groups was determined by one-way-ANOVA using GraphPad Prism v9.0 (GraphPad Software, USA) (https://www.graphpad.com/), and P < 0.05 was considered statistically significant.
Ethics approval
The experimental protocol was approved by the Animal Ethics Committee of Nanjing University of Chinese Medicine (Ethics No.: 202310A049). All procedures were performed in accordance with the ethical standards of the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health, and ARRIVE guidelines.
Data availability
Data is provided within the manuscript or supplementary information files and available from the corresponding author on reasonable request.
References
Collaborators, G. D. Global, regional, and National burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the global burden of disease study 2021. Lancet 402, 203–234. https://doi.org/10.1016/s0140-6736(23)01301-6 (2023).
Sun, H. et al. IDF diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119. https://doi.org/10.1016/j.diabres.2021.109119 (2022).
ElSayed, N. A. et al. Classification and diagnosis of diabetes: Standards of care in Diabetes-2023. Diabetes Care. 46, S19–S40. https://doi.org/10.2337/dc23-S002 (2023).
Committee, A. D. A. P. P. Pharmacologic approaches to glycemic treatment: standards of medical care in Diabetes-2022. Diabetes Care. 45, 125–143. https://doi.org/10.2337/dc22-S009 (2022).
Sarafidis, P. A., Tsapas, A. & Empagliflozin Cardiovascular outcomes, and mortality in type 2 diabetes. N Engl. J. Med. 374, 1092. https://doi.org/10.1056/NEJMc1600827 (2016).
Gerstein, H. C. et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 394, 121–130. https://doi.org/10.1016/s0140-6736(19)31149-3 (2019).
Davies, M. J. et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care. 45, 2753–2786. https://doi.org/10.2337/dci22-0034 (2022).
Shuyu, Y. Guidelines for the prevention and management of diabetes in primary care with traditional Chinese medicine (2022). J. Tradit. Chin. Med. 63, 2397–2414 (2022).
Jia, Y., Huang, H., Yu, Y., Jia, H. & Zhang, Y. Four kinds of traditional Chinese exercise therapy in the treatment of type 2 diabetes: A systematic review and network meta-analysis. Syst. Rev. 12, 231. https://doi.org/10.1186/s13643-023-02384-1 (2023).
Wu, Y., Wei, Q., Fang, Z. & Lin, F. Influence of traditional Chinese medicine health preservation Gongfa on fasting blood glucose and fasting insulin in pre-diabetes patients. Acta Med. Mediterranea. 35, 745–749 (2019).
Wang, H. et al. Effect of Elastic-band Baduanjin exercise on older adults with type 2 diabetes mellitus in China: Protocol for a randomised controlled trial. BMJ Open. 13, e073200. https://doi.org/10.1136/bmjopen-2023-073200 (2023).
Kanaley, J. A. et al. Exercise/Physical activity in individuals with type 2 diabetes: A consensus statement from the American college of sports medicine. Med. Sci. Sports Exerc. 54, 353–368. https://doi.org/10.1249/mss.0000000000002800 (2022).
Liu, Y. et al. Eight weeks of High-Intensity interval static strength training improves skeletal muscle atrophy and motor function in aged rats via the PGC-1α/FNDC5/UCP1 pathway. Clin. Interv Aging. 16, 811–821. https://doi.org/10.2147/cia.S308893 (2021).
Wu, Y. et al. in BASIC & CLINICAL PHARMACOLOGY & TOXICOLOGY. 33–33 (WILEY 111 RIVER ST, HOBOKEN 07030 – 5774, NJ USA).
Sélénou, C., Brioude, F., Giabicani, E., Sobrier, M. L. & Netchine, I. IGF2: development, genetic and epigenetic abnormalities. Cells https://doi.org/10.3390/cells11121886 (2022).
Tian, B. et al. Curcumin inhibits urothelial tumor development by suppressing IGF2 and IGF2-mediated PI3K/AKT/mTOR signaling pathway. J. Drug Target. 25, 626–636 (2017).
Buck, E. et al. Compensatory insulin receptor (IR) activation on Inhibition of insulin-like growth factor-1 receptor (IGF-1R): rationale for cotargeting IGF-1R and IR in cancer. Mol. Cancer Ther. 9, 2652–2664 (2010).
Long, Y. C., Cheng, Z., Copps, K. D. & White, M. F. Insulin receptor substrates Irs1 and Irs2 coordinate skeletal muscle growth and metabolism via the Akt and AMPK pathways. Mol. Cell. Biol. (2011).
Petersen, K. F. & Shulman, G. I. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am. J. Cardiol. 90, 11–18 (2002).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Kanehisa, M., Furumichi, M., Sato, Y. & Kawashima, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592 (2023).
Kanehisa, M. Toward Understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951 (2019).
Mu, Q. et al. Imp2 regulates GBM progression by activating IGF2/PI3K/Akt pathway. Cancer Biol. Ther. 16, 623–633. https://doi.org/10.1080/15384047.2015.1019185 (2015).
Devanathan, N. & Kimble-Hill, A. C. Systematic survey of the role of IGF in the link between diabetes and cancer. Indiana Univ. J. Undergrad. Res. 4, 17 (2018).
Luo, L. et al. Disruption of FOXO3a-miRNA feedback Inhibition of IGF2/IGF-1R/IRS1 signaling confers Herceptin resistance in HER2-positive breast cancer. Nat. Commun. 12, 2699 (2021).
Ge, Q. et al. Mulberry leaf regulates differentially expressed genes in diabetic mice liver based on RNA-seq analysis. Front. Physiol. 9, 1051 (2018).
Zhang, B. et al. Insulin-like growth factor 2 (IGF2)-fused lysosomal targeting chimeras for degradation of extracellular and membrane proteins. J. Am. Chem. Soc. 145, 24272–24283 (2023).
BUCHANAN, C. M., COOPER, G. J. & PHILLIPS, A. R. & Preptin derived from proinsulin-like growth factor II (proIGF-II) is secreted from pancreatic islet β-cells and enhances insulin secretion. Biochem. J. 360, 431–439 (2001).
Hui, Y. et al. Nonenzymatic function of DPP4 promotes diabetes-associated cognitive dysfunction through IGF-2R/PKA/SP1/ERp29/IP3R2 pathway-mediated impairment of Treg function and M1 microglia polarization. Metabolism 138, 155340 (2023).
Wang, T. et al. IGF2 promotes alveolar bone regeneration in murine periodontitis via inhibiting cGAS/STING-mediated M1 macrophage polarization. Int. Immunopharmacol. 132, 111984 (2024).
Setshedi, M. et al. Limited therapeutic effect of N-acetylcysteine on hepatic insulin resistance in an experimental model of alcohol‐induced steatohepatitis. Alcoholism: Clin. Experimental Res. 35, 2139–2151 (2011).
You, S. et al. Down-regulation of WWP2 aggravates type 2 diabetes mellitus-induced vascular endothelial injury through modulating ubiquitination and degradation of DDX3X. Cardiovasc. Diabetol. 22, 107. https://doi.org/10.1186/s12933-023-01818-3 (2023).
Shen, Q. et al. Platycodin D ameliorates hyperglycaemia and liver metabolic disturbance in HFD/STZ-induced type 2 diabetic mice. Food Funct. 14, 74–86. https://doi.org/10.1039/d2fo03308a (2023).
Furrer, R. et al. Molecular control of endurance training adaptation in male mouse skeletal muscle. Nat. Metab. 5, 2020–2035. https://doi.org/10.1038/s42255-023-00891-y (2023).
Huan, M. et al. Static strength training method for type 2 diabetic mice. J. Vis. Exp. https://doi.org/10.3791/65556 (2024).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. diabetologia 28, 412–419 (1985).
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Lizhen Gan and Pei Chen: Conceived and designed the study, performed key experiments, collected and analyzed data, and drafted the initial manuscript, Zhi Zhang, Xinyi He, Xia Wu, and Zhewei Chen: Conducted experimental procedures, participated in data acquisition, contributed to data analysis, and assisted in figure preparation, Qingbo Wei and Yunchuan: Provided overall supervision, contributed to the study conception and design, guided the experimental process, interpreted the results, and critically revised the manuscript for intellectual content.
Corresponding authors
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gan, L., Chen, P., Zhang, Z. et al. Static training improves insulin resistance in skeletal muscle of type 2 diabetic mice via the IGF-2/IGF-1R pathway. Sci Rep 15, 10662 (2025). https://doi.org/10.1038/s41598-025-94360-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-94360-z