Abstract
Hematological malignancies are a class of neoplasms that include a variety of diverse diseases that all develop from and change into lymphatic and bone marrow cells. Hematological malignancies significantly contribute to illness and mortality in African nations. The prevalence of these malignancies has not been evaluated in this continent. The purpose of this systematic review and meta-analysis was to assess the pooled prevalence of hematological malignancies in Africa. From October to November 2023, the electronic databases PubMed, Google Scholar, Web of Science, Research Gate, Embase, and Scopus were extensively searched to identify pertinent research. The Newcastle‒Ottawa Quality Scale for cross-sectional studies was used to assess the quality of the included studies. The analysis tool used was STATA-14. To calculate the pooled prevalence of hematological malignancies, a random effects model was used. Heterogeneity was measured by using the I2 value. Subgroup analysis was conducted for country, age of study subjects, population type, study design, and publication year. We evaluated publication bias through the implementation of a funnel plot and Egger’s test and conducted a sensitivity analysis. A total of 34 published articles including 43,099 study participants were included. The pooled prevalence of hematological malignancies was 27.30%. There was high heterogeneity, with an I2 value of 99.2%. Leukemia had the highest pooled prevalence (53.69%) among the hematological malignancy types, followed by lymphoma (38.36%). According to subgroup analysis conducted in African countries, Kenya had the highest pooled prevalence (44.69%). On the other hand, the lowest pooled prevalence reported in Nigeria (20.52%). Furthermore, the age-based subgroup analysis of the study participants revealed that children had a greater pooled prevalence of hematological malignancies than adults (60.92% vs. 17.02%), respectively. In African populations, the pooled prevalence of hematological malignancies was 27.30%. This suggests that there is a significant prevalence of hematological malignancy, necessitating regular monitoring and accurate diagnosis.
Trial registration PROSPERO CRD42023427152.
Similar content being viewed by others
Introduction
Hematological malignancies, along with other forms of cancer, are emerging as a significant public health concern and a global priority due to their increasing impact on illness and death rates. These types of cancer now account for approximately one-fifth of the most commonly identified cancer types and rank as the second leading cause of cancer-related deaths1,2,3.
A class of neoplasms known as hematological malignancies comprises a wide range of disorders that start with lymphatic and bone marrow cells and then transform into these cells. Malignancies, which are clonal diseases, induce independent cell division, lower normal hemopoietin levels, and invasion of organs and tissues4,5.
The World Health Organization (WHO) divided hematologic malignancies into myeloid neoplasms, lymphoid neoplasms, mast cell disorders, and histiocytic neoplasms based on the role that cell lines play in neoplastic transformation6,7. Different neoplasms are described and characterized using a combination of biological features, immunophenotype, genetic, and clinical features of the patients8. There are several different types of hematological malignancies that can be classified, including leukemia, lymphoma, multiple myeloma, myelodysplastic syndrome, polycythemia vera (PV), myeloproliferative neoplasms (MPNs), and primary myelofibrosis9,10. Acute and chronic forms of leukemia can be distinguished by a variety of factors, including the type of cells involved, their differentiation, morphology, cytochemical characteristics, and immunephenotyping11,12. Acute leukemia includes acute myeloblastic leukemia (AML) and acute lymphoblastic leukemia (ALL), whereas chronic leukemia includes chronic lymphocytic leukemia (CLL) and chronic myeloid leukemia (CML)5,13,14.
The most prevalent type of leukemia in children is ALL. However, only approximately 25% of adult ALL cases affect the T-cell phenotype, while in approximately 75% of cases, the remaining cases affect the B-cell phenotype15.
Chronic leukemia is characterized by the unregulated proliferation and growth of mature, differentiated hematopoietic system cells in the bone marrow and peripheral circulation9. Myeloid hematopoietic cell lines, which include granulocytes, monocytes, erythrocytes, and megakaryocytes, are affected by a collection of heterogeneously associated disorders, is chronic myeloid leukemia16. However, chronic lymphocytic leukemia affects mature lymphoid cells with B and T-cell phenotypes17.
Lymphoid malignancies, such as lymphoma can affect lymph nodes and/or other extramedullary locations. The two types of lymphomas are often categorized as Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL), and both are managed using quite different approaches. While the vast majority of NHLs have mature B, T, or natural killer cell phenotypes, up to 25% of childhood NHLs originate from precursor (immature) lymphoblasts. Extramedullary myeloid proliferation is uncommon and typically occurs in AML, in which case myeloid sarcomas (extramedullary accumulation of myeloid blasts) are present. With the exception of Langerhans cell histiocytosis, which is seen considerably more frequently, especially in children, and whose neoplastic character is debatable, histiocytic and dendritic cell neoplasms are typically rare11,12.
The etiology of hematological malignancies is not fully understood. Numerous risk factors, including benzene, formaldehyde, organic solvents, agricultural pesticides and herbicides, smoking, prior chemotherapy and radiation therapy for cancer, immunological and genetic disorders, and infection with oncogenic retroviruses, have been linked to the development and increased risk of hematological malignancies in epidemiological studies5,18.
According to a global cancer statistics report, it was estimated that there was a total of 437,000 new cases of leukemia and 309,000 deaths related to cancer from leukemia worldwide by 2018. There were nine cancer-related deaths19. Leukemia accounted for 3.4% of all new cancer cases and 3.8% of all cancer-related deaths worldwide in 202020. The burden of hematological malignancy was also high in Africa reported by studies done in Africa. Hematological malignancies can affect anyone at any age, but children and elderly people are the most susceptible. Men have slightly higher prevalence rates of leukemia than women do for all types of the disease, and these disparities may be impacted by factors such as geography and ethnic origin like Africa region18.
The impact of hematological malignancies in developing countries is high due to early death of children, loss of family, loss of productivity due to a disability, afflicted patients and their families psychologically, and high medical cost that affects the socioeconomic and health welfare of the population. The prevalence of these malignancies has been studied in different parts of the world, but little is known of their prevalence in the Africa. Also, the prevalence of hematological malignancies in Africa has not yet been reviewed by a formal national census or national health registry. In addition, there has been few meta-analysis reports globally21,22. However, these studies predominantly reported the pooled prevalence of hematological malignancy among specific study populations. Through our searching, this may be the first meta-analysis and systematic review of the pooled prevalence of hematological malignancies in Africa. Therefore, evaluating the pooled prevalence of hematological malignancies in the African population is the primary goal of this analysis.
Methods
Reporting and protocol registration
This systematic review and meta-analysis adhered to the guidelines set by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)23. The research protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42023427152.
Search strategy and study selection
All articles regarding hematological malignancies were retrieved through a systematic search of electronic databases such as PubMed/Central, Google Scholar, Web of Science, and Scopus from October to November 2023 and to ensure the presence of recently published additional articles, online data bases and grey literatures were rechecked again from February 3rd to 7th, 2025. Additionally, grey literatures were scrutinized from African Journal Online (AJOL), WHO IRIS, Hinary, university repositories and ResearchGate websites. To ensure comprehensiveness, a direct Google search was performed using the reference lists of the included studies to uncover additional pertinent studies that may have been overlooked during the electronic database searches. To identify other pertinent studies that were missed during the electronic database searches, a snowball search was also carried out utilizing the bibliographies of the identified studies. An exhaustive search strategy was employed using the condition, context, population, and outcome of interest (CoCoPop) framework to formulate questions, and all eligible studies were accessed by employing Medical Subject Headings (MeSH) terms and combination keywords including prevalence, distribution and magnitude, “hematological malignancy”, leukemia, lymphoma and cancer and Africa. Boolean operators (“OR” and “AND”) were used as necessary in the advanced search databases for finding all relevant papers. Detailed article search strategies and search engines were indicated in (Supplementary file 1). Studies that overlapped and were found in several databases were excluded. Duplicate studies were excluded, and four separate reviewers independently screened the titles and abstracts of all possibly eligible studies. Then, the full texts of potentially eligible studies that reported the prevalence of hematological malignancies were added to the collections for extraction. Disagreements among the authors during data extraction were resolved by discussion.
The eligibility criteria
Original articles that reported the prevalence of hematological malignancies among the African population were included. In addition, the study diagnosis of hematological disease by clinical and laboratory methods (complete blood count and peripheral morphology and/or bone marrow and/or cytochemistry and/or cytogenetic and/or molecular and/or immunophenotyping) were included. Also, the studies classified hematological malignancy by FAB (French-American-British) or WHO classification criteria were included. Only studies reported in English were included. However, case reports, editorial letters, and review articles were not included.
Outcome variables
The outcome variable for this study is the pooled prevalence of hematological malignancies among African populations.
Data extraction
Four reviewers worked independently to extract data from the eligible studies into Microsoft Excel sheets. The information extracted from each study included the name of the first author, publication year, region, study subjects, study characteristics, study design, sample size, prevalence of hematological malignancies, prevalence of leukemia, prevalence of lymphoma, prevalence of multiple myeloma, prevalence of myeloproliferative and prevalence of different types of leukemia and lymphoma.
Risk of bias assessment
Three evaluators conducted a thorough assessment of the methodological and substantive quality of eligible studies was assessed using the Newcastle‒Ottawa Quality Scale for cross-sectional studies. The critical appraisal checklist was applied during the assessment, considering studies with an average quality score of 50% or more (star 5–9) as being of high and moderate quality (low risk of bias) and consequently included in the analysis. However, the studies that had low quality scores were excluded from the analysis. The three reviewers (ZM, SA and HD) independently conducted quality assessments and meticulously verified their results. Any discrepancies were resolved through in-depth discussions and cross-verification of data. If consensus could not be reached, a senior reviewer (AG) provided the final decision.
Statistical analysis
A Microsoft Excel worksheet was used for the data extraction, and STATA version 14 with metan commands was used for the meta-analysis. The point estimate and 95% confidence interval of the prevalence of hematological malignancies for the included studies were calculated. Due to the high heterogeneity reported, the national pooled prevalence of hematological malignancies was calculated using a random-effects model. The DerSimonian‒Laird method was used to estimate the between-study variance. Cochrane’s Q test and I2 statistics were used to estimate the percentage of variability in effect estimates due to heterogeneity rather than chance alone to assess heterogeneity21. Subgroup analysis was performed by country, publication year, age of the study subject, population type as general population (suspected hematological malignancy) and cancer case (diagnosed as cancer and suspected to cancer), and study design. Meta-regression was also performed for possible sources of heterogeneity for country, publication year, study population, age of the study subject as children (age < 18 years) and adult (age ≥ 18 years), and sample size. Furthermore, the presence of publication bias was evaluated by visually examining the symmetry of the funnel plot and analyzing the statistical outcomes of Egger’s test22,23. A sensitivity analysis was conducted to evaluate the influence of an individual study on the overall combined effect size.
Results
Selection and identification of studies
A total of 1293 records were found after a systematic search of studies on the prevalence of hematological malignancies. After conducting a thorough screening for duplication and eligibility, a total of 34 studies met the inclusion criteria for this systematic review and meta-analysis. All phases of the procedure were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA checklist 2020) indicated in (Supplementary file 2)24 (Fig. 1).
Study characteristics
A total of 34 articles were included in this systematic review and meta-analysis a from various countries in the Africa, including 13 studies in Nigeria24,25,26,27,28,29,30,31,32,33,34,35,36, 8 studies in Ethiopia37,38,39,40,41,42,43,44, 6 studies in South Africa45,46,47,48,49,50, 2 studies in Kenya51,52, 1 study in Tanzania53, 1 study in Togo54, 1 study in eastern Morocco55, 1 study in Eritrean56 and 1 study in Zimbabwe57 (Fig. 2). The studies involved 43,099 study participants. The sample sizes of the studies ranged from 7139 to 12,67140. All the included studies employed retrospective study designs, except for 3 studies. Out of the 34 studies that were included in this systematic review and meta-analysis, 20 studies included participants from all age groups, while 16 studies specifically focused on individuals diagnosed with cancer. Of the 34 studies included in this systematic review and meta-analysis, 25 reported the prevalence of hematological malignancies, while 31 articles reported the prevalence of leukemia with 5470 cases and 26 articles reported the prevalence of lymphoma that consists of 6740 cases. From the total, 10 studies diagnose hematological malignancy through bone marrow examination, 9 studies use basic lab tests (CBC and peripheral morphology) along with bone marrow examination, 3 studies additionally use immunohistochemistry, and 3 studies also incorporate imaging (X-ray and ultrasound) (Table 1). Every study that was included had a quality score higher than fifty percent or from star 5–8. The studies evaluated by Newcastle‒Ottawa Quality Scale for cross-sectional studies the result showed that 22 studies had high qualities (7–8 stars) and 12 studies had moderate qualities (5–6 stars) (Supplementary file 3).
Geographical distribution of hematological malignancy. Map showing Africa countries for which data were included in the systematic review and meta-analysis created using https://www.mapchart.net/world-subdivisions.html. The different color shading indicates the prevalence of hematoloogical malignancy in each country.
Prevalence of hematological malignancies
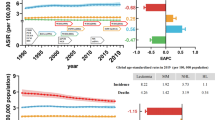
In this systematic review and meta-analysis, the pooled prevalence of hematological malignancies in Africa was 27.307% (95% CI 22.39–32.21%). Overall, the prevalence of hematological malignancies among the African population is variable, ranging from 7.0% reported in Nigeria (2022) to 71.88% reported in South Africa (2023). There was high heterogeneity, with an I2 of 99.2% (Fig. 3).
The pooled prevalence of the type of hematological malignancy
In this review, when considering different types of hematological malignancies, leukemia had the highest pooled prevalence of 53.69%, followed by lymphoma with a prevalence of 38.36%. Multiple myeloma accounted for only 12.27% of the patients. Among the various subtypes of leukemia, chronic myeloid leukemia (CML) had the highest pooled prevalence at 27.47%, followed by chronic lymphocytic leukemia (CLL) at 27.25%. Conversely, acute myeloid leukemia (AML) had the lowest pooled prevalence among the subtypes of leukemia (19.74%). Among the lymphoma types, non-Hodgkin lymphoma (NHL) had the highest pooled prevalence, representing 66.01% of the cases (Table 2).
Subgroup analysis
Subgroup analysis of various African countries revealed that the highest pooled prevalence of hematological malignancies, 44.69% (95% CI 10.82, 78.55), was observed in Kenya, followed by 42.76% (95% CI 14.31–71.21) in South Africa. On the other hand, Nigeria reported the lowest pooled prevalence of 20.52% (95% CI 13.56–27.48). Furthermore, when considering the publication year of the studies, the highest pooled prevalence of 39.05% (95% CI 20.80, 57.29) was reported in the period of 2011–2015, followed by 25.04% (95% CI 15.65–34.44) in 2016–2020. The lowest prevalence of 18.81% (95% CI 15.37–22.25) was reported in studies published prior to 2011. The pooled prevalence of hematological malignancies was 60.92% (95% CI 48.26–73.57) among children and 17.02% (95% CI 10.55, 23.49) among adults. Similarly, the pooled prevalence of hematological malignancies was 28.34% (95% CI 11.83–44.85) among the general population and 23.97% (95% CI 21.79–32.13) among specific groups. Based on diagnostic criteria, the highest pooled prevalence of hematological malignancy detected at diagnosis by bone marrow examination alone was 36.59% (95% CI 21.81–51.37), while the lowest prevalence, 12.81% (95% CI 9.02–16.59), was observed when immunohistochemistry was included. The presence of low heterogeneity (34.80%) was noted in studies diagnosing hematological malignancy through the incorporation of immunohistochemistry (Table 3).
Meta-regression
The meta-regression showed that the most significant source of heterogeneity was the age of the study subject (p < 0.001) (Table 4).
Publication bias
In this review, both the symmetry of the funnel plot and the results of Egger’s test (P = 0.003) indicated the existence of publication bias (Fig. 4). So, nonparametric trim and fill analysis was performed.
Trim-and-fill analysis of the pooled prevalence of hematological malignancies in Africa
Due to the detection of publication bias, a non-parametric trim and fill analysis was conducted. When trimming and filling analysis was conducted using a random model, fourteen studies were imputed, and the total number of studies was thirty nine. Following the inclusion of those additional studies, the revised pooled prevalence of hematological malignancies in Africa was 10.90% (95% CI 1.42–20.39%) (Table 5) and (Fig. 5).
Heterogeneity exploration and sensitivity analysis
To assess the possible sources of heterogeneity, a Galbraith plot was computed and indicated that one study was found outside of the 95% CI or not found between 2 and − 2 (Fig. 6). But, according to the sensitivity analysis, the exclusion of individual studies did not significantly affect the overall pooled prevalence of hematological malignancies. The pooled effect size, when any single study was omitted, remained within the 95% confidence interval of the overall pooled effect size. This suggests that the absence of any particular study did not have a substantial impact on the overall pooled prevalence of hematological malignancies (Fig. 7).
Discussion
This meta-analysis and systematic review aimed to determine the total frequency of hematological malignancies in Africa. This thorough study revealed that the pooled prevalence of hematological malignancies in Africa was 27.30%. The results of this systematic review and meta-analysis were consistent with those of a study conducted in the UK, which reported a prevalence of 29% for hematological malignancies among COVID-19 patients58. In contrast, our analysis revealed a greater prevalence of hematological malignancies among patients in Iran59. Another systematic review reported a prevalence of 14% for hematological malignancies among patients with membranous nephropathy21, and a review reported a prevalence of 16.5% for hematological malignancies among patients with SARS-CoV-2 infection22. On the other hand, the prevalence of hematological malignancies among COVID-19 patients was lower than the findings of a study that reported a pooled prevalence of 34.3%60 and a study conducted in Pakistan where the prevalence was found to be 45.36%61. These variations in prevalence could be attributed to differences in sample size, study population, geographical location, sociodemographic factors, type of sample, and diagnostic methods.
In this systematic review and meta-analysis, we found that among the different types of hematological malignancies, leukemia had the highest pooled prevalence (53.69%), followed by lymphoma (38.36%). While this provides valuable epidemiological insights, further investigation of potential risk variables such as ethnicity, genetic susceptibility, history of viral infections (e.g., EBV and HTLV-1), and environmental exposures may improve the interpretation of our findings. Understanding these characteristics may aid in the development of more specific prevention efforts, early detection programs, and focused public health interventions to lower the burden of hematological malignancies. This finding aligns with a study conducted in Pakistan (2019) among adults61 as well as studies conducted in Bangladesh (2014) among patients of all ages62 and in India (2008)63. However, in contrast to our findings, a study conducted in Bangladesh (2020)64 and Latin America (2019)65 among adult patients, as well as a worldwide study66, reported that lymphoma neoplasm was the most common hematological malignancy. The variation in these findings could be attributed to differences in sample size, study population, geographical location, sociodemographic factors, type of sample, age groups included in the study, and methods of diagnosis.
In this systematic review and meta-analysis, among the leukemia types, the most common was CML (27.47%), followed by CLL (27.25%). This review is similar to a study conducted in India (2016)67. However, the most prevalent type of leukemia was acute myeloid leukemia in studies conducted in Pakistan (2019) among adults61, Bangladesh (2014)62, Bangladesh (2010)68 and India (2008)63. The possible justification for this variation might be the variations in sample size, study population, sociodemographic pattern, and age of the study subject.
According to the current systematic review and meta-analysis, among the lymphoma types, the most common was NHL (66.01%). These findings are similar to those of studies conducted in Bangladesh (2014)62, worldwide66, Korea (2012) and India (2012)69. Therefore, NHL was the most common type of lymphoma in this review and other studies.
Our result revealed significant heterogeneity in pooled prevalence, which might be attributed to various factors. As a result, we performed post hoc subgroup analysis according to many factors, including country, publication year, study design, age of the study subject and population type. Variations in the prevalence of hematological malignancies were observed in different African countries; the highest pooled prevalence of 44.69% was observed in Kenya, and the lowest pooled prevalence was reported in Nigeria (20.52%). This discrepancy might be ethnicity, target population, sample size, type of sample, participants’ immunological status and method of diagnosis. Subgroup analysis by publication year revealed that the highest pooled prevalence was reported in 2011–2015 (30.67%), while the lowest prevalence was recorded in years after 2011 (18.81%). This variation it might be target population, sample size, and diagnostic criteria. Finally, the subgroup analysis by the age of the study subjects revealed that the highest pooled prevalence of hematological malignancies was among children (60.92%), and the lowest pooled prevalence was among adults (17.02%). This difference might be type of sample, participants’ immunological status and method of diagnosis.
The subgroup analysis based on diagnostic criteria revealed that low heterogeneity (34.80%) was observed in studies diagnosing hematological malignancy through the incorporation of immunohistochemistry. Therefore, the variability in diagnostic criteria may be the cause of heterogeneity. The subgroup analyses based on publication year, country, study design, age of the study subject and population type may not fully elucidate all underlying factors contributing to heterogeneity in the pooled prevalence. Variations in study populations, including differences in demographic characteristics, ages, sex, participants’ immunological status, and diagnostic criteria for hematological malignancy across different regions, could introduce heterogeneity. Furthermore, changes in assay methodologies, laboratory protocols, and equipment calibration may lead to heterogeneity in hematological malignancy diagnosis among studies, potentially altering the pooled prevalence. Unmeasured confounding variables, such as comorbidities, and lifestyle factors, could also influence study outcomes and contribute to heterogeneity in the pooled prevalence. The meta-regression also showed that the most significant source of heterogeneity was the age of the study subject.
The publication bias was identified in this systematic review and meta-analysis by the symmetry of the funnel plot and the Egger’s test results. Hence, trim and fill analysis was computed. The pooled prevalence of hematological malignancy was 10.90% following a trim and fill analysis that included fourteen additional studies. The trim-and-fill analysis of the final model (Observed and imputed model) revealed a significant decline in pooled prevalence of hematological malignancies in Africa. This further suggests that evidences reporting lower estimates may be underrepresented in the literature. Thus, the current finding might overestimate the true prevalence of hematological malignancies in Africa. However, the findings of the sensitivity analysis showed that no single study had an impact on the pooled effect size. By eliminating each study in turn, the pooled prevalence of hematological malignancies was determined, and the resulting computed pooled prevalence was within 95% confidence intervals of the total pooled prevalence. Generally, the findings of the present study suggested that further large-scale studies are required and that there is a need for special concerns for high-risk groups to minimize the risk of hematological malignancies.
There are various limitations to this research. First, all the listed studies were conducted in Africa. To begin with, the publications included in this meta-analysis were primarily from a small number of countries, which may have introduced geographical bias. There was high heterogeneity amongst the studies, which may have influenced the results’ interpretability. Despite doing subgroup analyses, the heterogeneity persisted except diagnostic criteria, implying that potential confounding factors impacting the pooled prevalence were not adequately examined in this meta-analysis. In addition, only research published in English were included, which may have resulted in language bias. These limitations may have an impact on the conclusions in this research for the overall prevalence of hematological malignancies in Africa. Another limitation is that most studies included in the meta-analysis were conducted using a retrospective study design. I recommend that researchers conduct prospective studies.
Conclusion
The pooled prevalence of hematological malignancies in Africa was 27.30%, according to this systematic study and meta-analysis. This encourages clinicians to consider hematological malignancies to request appropriate standard testing to confirm suspected hematological malignancies and treat those patients early. This study highlights the necessity of regular monitoring and accurate diagnosis of hematological malignancies in Africa. Given the limited healthcare resources and diagnostic facilities in many regions, routine surveillance can help track disease prevalence, identify high-risk populations, and support early detection efforts. Accurate diagnosis is crucial for ensuring appropriate treatment, as misdiagnosis or delays can lead to poor clinical outcomes. Implementing advanced diagnostic techniques, such as immunophenotyping and molecular methods, alongside strengthening healthcare infrastructure, is essential for improving patient survival and informing effective public health strategies. Furthermore, it serves as a wake-up call to international, continental, and national health bureaus, as well as other stakeholders, to develop targeted prevention and control strategies for hematological malignancy disorders. This review also provides useful information for policymakers and other stakeholders. Furthermore, the data could be used for future complementary research and evidence-based decision-making. I recommend that researchers conduct prospective studies and diagnose hematological malignancies using advanced techniques such as cytogenetics, immunophenotyping, and molecular methods.
Data availability
Data is provided within the manuscript or supplementary information.
Abbreviations
- ALL:
-
Acute lymphocytic leukemia
- AML:
-
Acute myeloid leukemia
- CLL:
-
Chronic lymphocytic leukemia
- CML:
-
Chronic myeloid leukemia
- HL:
-
Hodgkin lymphoma
- NHL:
-
Non-Hodgkin lymphoma
References
Kocarnik, J. M. et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: A systematic analysis for the global burden of disease study 2019. JAMA Oncol. 8(3), 420–444 (2022).
Fitzmaurice, C. et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 3(4), 524–548 (2017).
Burns, R., Leal, J., Sullivan, R. & Luengo-Fernandez, R. Economic burden of malignant blood disorders across Europe: A population-based cost analysis. Lancet Haematol. 3(8), e362–e370 (2016).
Olaniyi, J. A. Flow cytometric immunophenotyping of hematological malignancies: the way forward in Nigeria. Pathol. Lab. Med. Int. 3, 17–24 (2011).
Rodriguez-Abreu, D., Bordoni, A. & Zucca, E. Epidemiology of hematological malignancies. Ann. Oncol. 18, i3–i8 (2007).
Swerdlow, S. H. et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127(20), 2375–2390 (2016).
Arber, D. A. et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute Leukemia. Blood 127(20), 2391–2405 (2016).
Harris, N. L. et al. The world health organization classification of hematological malignancies report of the clinical advisory committee Meeting, Airlie House, Virginia, November 1997. Mod. Pathol. 13(2), 193–207 (2000).
Taylor, J., Xiao, W. & Abdel-Wahab, O. Diagnosis and classification of hematologic malignancies on the basis of genetics. Blood 130(4), 410–423 (2017).
Hossain, M. S. et al. Diagnosed hematological malignancies in Bangladesh - a retrospective analysis of over 5000 cases from 10 specialized hospitals. BMC Cancer 14, 438 (2014).
Swerdlow, S. H., Campo, E., Harris, N. L., Jaffe, E. S., Pileri, S. A. & Stein, H., et al. WHO classification of tumours of haematopoietic and lymphoid tissues: International agency for research on cancer Lyon, (2008).
Choi, J. H. & Ro, J. Y. The 2020 WHO classification of tumors of soft tissue: selected changes and new entities. Adv. Anatomic Pathol. 28(1), 44–58 (2021).
Hamayun, M., Khan, S. A. & Muhammad, W. Investigation on the prevalence of leukemia in North West Frontier Province of Pakistan. Turkish journal of cancer. 35(3), (2005).
Tefferi, A., Thiele, J. & Vardiman, J. W. The 2008 World Health Organization classification system for myeloproliferative neoplasms: Order out of chaos. Cancer: Interdiscip. Int. J. Am. Cancer Soc. 115(17), 3842–3847 (2009).
Onciu, M. Acute lymphoblastic leukemia. Hematol. Oncol. Clin. North Am. 23(4), 655–674 (2009).
Shet, A. S., Jahagirdar, B. N. & Verfaillie, C. M. Chronic myelogenous leukemia: Mechanisms underlying disease progression. Leukemia 16(8), 1402–1411 (2002).
Oertel, J., Kastner, M., Bai, A. R., Kleiner, S. & Huhn, D. Analysis of chronic lymphoid leukaemias according to FAB. Leuk Res. 16(9), 919–927 (1992).
Bochtler, T. et al. Hematological malignancies in adults with a family predisposition. Dtsch. Arztebl. Int. 115(50), 848–854 (2018).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68(6), 394–424 (2018).
Lin, X. et al. Global, regional, and national burdens of leukemia from 1990 to 2017: A systematic analysis of the global burden of disease 2017 study. Aging (Albany NY) 13(7), 10468–10489 (2021).
Leeaphorn, N. et al. Prevalence of cancer in membranous nephropathy: A systematic review and meta-analysis of observational studies. Am. J. Nephrol. 40(1), 29–35 (2014).
Jafari, M. et al. Proportion of hematological cancer patients with SARS-CoV-2 infection during the COVID-19 pandemic: A systematic review and meta-analysis. Hematol. Transfus. Cell Ther. 44(2), 225–234 (2022).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 88, 105906 (2021).
Babatunde, A., Amiwero, C., Olatunji, P. & Durotoye, I. Pattern of haematological malignancies in Ilorin, Nigeria: A ten year review. Internet J. Hematol. 5(2), 1–8 (2008).
Akinbami, A., Dada, M., Dosunmu, A. & Balogun, M. Adult haematooncology cases: A six year review at Lagos State University Teaching Hospital Ikeja. Internet J. Hematol. 6(1), (2009).
Nwannadi, I., Alao, O., Bazuaye, G., Halim, N. & Omoti, C. The epidemiology of haematological malignancies at the university of Benin teaching hospital: A ten-year retrospective study. Internet J. Epidemiol. 9(2), 1–6 (2010).
Babatunde, A., Olawumi, H., Durotoye, L., Shittu, A. & Akinwumi, O. Changing pattern of haematological malignancies in Ilorin, Nigeria: A 10 year retrospective review of 181 cases. Ann. Med. Res. 5, 1–6 (2016).
Egesie, O. J. et al. Prevalence and type of haematological malignancies among adults in a tertiary hospital in Jos-Nigeria: A sixteen-year retrospective analysis. Highland Med. Res. J. 17(2), 92–96 (2017).
Oe, B. O. B. Epidemiological pattern of adult haematological malignancies in a tertiary hospital in cross river state. Int. Res. J. Oncol. 2(1), 1–9 (2019).
Nwagu, M., Ikusemoro, A., Akinola, O. & Egbo, O. An eight year retrospective study of the prevalence and socio-demographic characteristics of adult blood cancers at a sub-urban teaching hospital in Nigeria. J. Res. Basic Clin. Sci. 1(3), 260–265 (2019).
Babatunde, A. et al. Evaluation of the Indications and diagnostic value of bone marrow examination in haematological disorders in Ilorin Nigeria: A review of 496 cases. Tropical J. Health Sci. 27(1), 27–33 (2020).
Dachi, R., Suleiman, D., Mustapha, F., Mohammed, A. & Adogu, I. Pattern of haematological malignancies in a tertiary health facility in Bauchi state, northeastern Nigeria. Jewel J. Med. Sci. 2(1), 80–87 (2021).
Out, T. & Ejikeme, U. The Frequency and pattern of haematological malignancies at a tertiary hospital in Abuja. Federal capital territory, North-Central Nigeria: 2005. (2021).
Ugwu, N. I. et al. Distribution pattern and prevalence of haematological cancers among adults in Abakaliki, South-Eastern Nigeria. Nigerian Postgraduate Med. J. 28(4), 266–272 (2021).
Onoja, A. et al. Prevalence and nature of adult hematological malignancies using bone marrow aspiration cytology in a tertiary health facility: A seven year retrospective review. Western J. Med. Biomed. Sci. 2(1), 43–49 (2021).
James, J. et al. Pattern of haematological malignancies in a tertiary hospital in yola, Nigeria: A three year retrospective review. Orient J. Med. 34(3–4), 84–90 (2022).
Getachev, A. Malignant lymphoma in western Ethiopia. East Afr. Med. J. 78(8), 402–404 (2001).
Weldetsadik, A. T. Clinical characteristics of patients with hematological malignancies at Gondar university hospital, North West Ethiopia. Ethiopian Med. J. 51(1), 25–31 (2013).
Yifru, S. & Muluye, D. Childhood cancer in Gondar university hospital, Northwest Ethiopia. BMC Res. Notes 8, 1–5 (2015).
Tigeneh, W., Molla, A., Abreha, A. & Assefa, M. Pattern of cancer in Tikur Anbessa specialized hospital oncology center in Ethiopia from 1998 to 2010. Int. J. Cancer Res. Mol. Mech. 1(1), 1 (2015).
Memirie, S. T. et al. Estimates of cancer incidence in Ethiopia in 2015 using population-based registry data. J. Global Oncol. 4, 1–11 (2018).
Kassahun, W. et al. Prevalence of leukemia and associated factors among patients with abnormal hematological parameters in Jimma Medical Center, Southwest Ethiopia: A cross-sectional study. Adv. Hematol. 2020, 1–7 (2020).
Enawgaw, B. et al. Hematological malignancies in the Northwest Ethiopia. PLoS One 16(12), e0260639 (2021).
Ebrahim, H., Fisha, T., Debash, H. & Bisetegn, H. Patterns of bone marrow confirmed malignant and non-malignant hematological disorders in patients with abnormal hematological parameters in Northeast Ethiopia. J. Blood Med. 13, 51–60 (2022).
Stefan, D. C., Baadjes, B. & Kruger, M. Incidence of childhood cancer in Namibia: the need for registries in Africa. Pan Afr. Med. J. 17, (2014).
Inamasu, T. et al. Retrospective case-series analysis of haematological malignancies in goldmining areas of South Africa. South Afr. Med. J. 108(10), 858–864 (2018).
Oelofse, D. & Truter, I. Incidence of haematological malignancies, Eastern Cape Province; South Africa, 2004–2013. Cancer Epidemiol. 53, 166–171 (2018).
Wilson, D. P., Tshabalala, W. S. & Pillay, S. Diagnostic outcomes of bone marrow aspirate and trephine biopsies performed at a hospital in KwaZulu-Natal, South Africa. Afr. J. Lab. Med. 9(1), 1–6 (2020).
Abdullah, I. et al. Indications and diagnostic value of bone marrow examination in HIV-positive individuals: A 3-year review at Tygerberg Hospital. Southern Afr. J. Infect. Dis. 36(1), 273 (2021).
Ndlovu, S., Esterhuizen, T., Uys, R., van Zyl, A. & Kruger, M. Trends in childhood cancers at Tygerberg Hospital from 1994 to 2014. South African Journal of Child Health. 17(2), 93–98 (2023).
Okinda, N. & Riyat, M. Bone marrow examination findings at aga Khan University Hospital, Nairobi. East Afr. Med. J. 87(1), 4–8 (2010).
Mostert, S. et al. Epidemiology of diagnosed childhood cancer in Western Kenya. Arch. Dis. Childhood 97(6), 508–512 (2012).
Leak, S. A., Mmbaga, L. G., Mkwizu, E. W., Mapendo, P. J. & Henke, O. Hematological malignancies in East Africa-Which cancers to expect and how to provide services. PLoS One 15(5), e0232848 (2020).
Kueviakoe, I. M. et al. Hematological malignancies: Analysis of myelogram results over 21 years in lome teaching hospitals. Clin. Med. Res. 4(4), 111–115 (2015).
Elidrissi Errahhali, M., Elidrissi Errahhali, M., Boulouiz, R., Ouarzane, M. & Bellaoui, M. Distribution and features of hematological malignancies in Eastern Morocco: A retrospective multicenter study over 5 years. BMC Cancer 16(1), 1–10 (2016).
Belai, N., Ghebrenegus, A. S., Alamin, A. A., Embaye, G. & Andegiorgish, A. K. Patterns of bone marrow aspiration confirmed hematological malignancies in Eritrean National Health Laboratory. BMC Hematol. 19, 1–4 (2019).
Mandisodza, A. The epidemiology of leukaemia in Zimbabwe: A ten year retrospective study. Haematol. Int. J. 1, 1–7 (2017).
Bhogal, T. et al. Haematological malignancy and nosocomial transmission are associated with an increased risk of death from COVID-19: results of a multi-center UK cohort. Leuk Lymphoma 62(7), 1682–1691 (2021).
Keykhosravi, A., Neamatshahi, M., Navipour, E., Barabadi, Z. & Neamatshahi, M. Incidence prevalence and associated risk factors for leukemia and lyphoma occurrence in Iran: A systematic review and meta analysis. Int. J. Med. Rev. 8(2), 57–64 (2021).
Venkatesulu, B. P. et al. A systematic review and meta-analysis of cancer patients affected by a novel coronavirus. JNCI Cancer Spectr. 5(2), pkaa102 (2021).
Khan, S. et al. Pattern of adulthood hematological malignancies in Khyber Pakhtunkhwa. J. Blood Disord. Transfus. 10(2), 5 (2019).
Hossain, M. S. et al. Diagnosed hematological malignancies in Bangladesh-a retrospective analysis of over 5000 cases from 10 specialized hospitals. BMC Cancer 14, 1–7 (2014).
Kusum, A. et al. Hematological malignancies diagnosed by bone marrow examination in a tertiary hospital at Uttarakhand, India. Indian J. Hematol. Blood Transfus. 24, 7–11 (2008).
Lt, C. S., Rahid Sarwar, M., Lona, H., Halder, D. & Selim, S. Hematological malignancies pattern among Bangladeshi adults. JMSCR. 08(5), 11–19 (2020).
de Moraes, T. et al. Epidemiology of hematologic malignancies in real-world settings: Findings from the Hemato-oncology Latin America observational registry study. J. Global Oncol. 5, 1–19 (2019).
Ferlay, J. et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136(5), E359–E386 (2015).
Baviskar, J. B. Incidence of acute and chronic leukemias in rural area at tertiary care teaching hospital: a five years of study. Indian J. Pathol. Oncol. 3(4), 710–713 (2016).
Kibria, S., Islam, M., Chowdhury, A., Ali, M., Haque, M. & Mustanzid, S., et al. Prevalence of hematological disorder: a bone marrow study of 177 cases in a private hospital at faridpur. (2010).
Arora, N., Manipadam, M. T. & Nair, S. Frequency and distribution of lymphoma types in a tertiary care hospital in South India: Analysis of 5115 cases using the World Health Organization 2008 classification and comparison with world literature. Leuk Lymphoma 54(5), 1004–1011 (2013).
Acknowledgements
The authors would like to express their gratitude to the authors of the included research as well as the participants in their investigations.
Funding
There is no specific funding for this review.
Author information
Authors and Affiliations
Contributions
Z.M. conceptualized , wrote the manuscript and assess quality. D.M.B., B.E., T.G., and M.T. search and extraction were carried out. S.A . and H.D. conducted the quality assessment of the included studies. E.A., D.M.B. and Y.K. performed statistical analysis and interpretation of the data. H.E., E.A., and A.G. prepared Fig. 1–6. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Ethical approval and consent to participate were not required because this was a systematic review and meta-analysis that did not involve human or animal experiments.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mulatie, Z., Berta, D.M., Gedefie, A. et al. Prevalence of hematological malignancies in Africa: A systematic review and meta-analysis. Sci Rep 15, 9471 (2025). https://doi.org/10.1038/s41598-025-94428-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-94428-w