Abstract
Bevacizumab (BEV) combined with standard chemotherapy with carboplatin and paclitaxel (CP) as the front-line treatment for newly diagnosed advanced-stage epithelial ovarian cancer (EOC) is a promising treatment option. In Thailand, combined BEV and CP for patients with high-risk EOC is not yet covered. This study aimed to explore the cost-effectiveness of combined BEV and CP for subgroups with high-risk EOC. Cost-utility analysis was conducted to compare the effectiveness of CP alone versus combined BEV and CP according to the Gynecologic Oncology Group-218 (GOG-218) and the Gynecologic Cancer Intergroup International Collaboration on Ovarian Neoplasms 7 (ICON-7) regimens in patients with EOC. The decision tree model and Markov model were applied, and incremental cost-effectiveness ratios (ICERs) were analyzed. Data on direct medical costs were obtained from cost databases in Thailand. Details about all clinical parameters and direct non-medical costs were obtained from published studies. Utility information was collected by interviewing patient subgroups with high-risk EOC. One-way and probabilistic sensitivity analyses were performed to evaluate parameter uncertainties. Based on the provider and societal perspectives, the ICERs of CP and the GOG-218 regimen were $31,266 and $31,966 per quality adjusted life year (QALY) gained, respectively. Meanwhile, the ICERs of CP and the ICON-7 regimen were $14,331 and $15,003 per QALY gained, respectively. The probabilities of cost-effectiveness for using BEV as the GOG-218 and ICON-7 regimens were 0% and 3%, respectively, based on the willingness-to-pay threshold in Thailand ($4,571 per QALY gained). The median progression-free survival of patients who received combined BEV and CP was the most important parameter leading to more benefit from using BEV. BEV as the GOG-218 or ICON-7 regimen may not be cost-effective for patient subgroups with high-risk EOC in Thailand. However, BEV as the ICON-7 regimen is more likely to be effective.
Similar content being viewed by others
Introduction
In 2020, ovarian cancer was the eighth most common cancer in the female population and had the seventh highest mortality rates worldwide1. In Thailand, it was also the eighth most common type of cancer in women, accounting for approximately 4% of all female cancers2. Approximately two-thirds of patients present with advanced-stage ovarian cancer3 (i.e., stage III or IV) in accordance with the International Federation of Gynecology and Obstetrics4. In 2021, Liu et al. performed a systematic review5. Based on two landmark randomized controlled trials (i.e., the Gynecologic Oncology Group [GOG-218]6,7 and the Gynecologic Cancer Intergroup International Collaboration on Ovarian Neoplasms 7 [ICON-7])8, this study compared the benefit of bevacizumab (BEV) plus standard chemotherapy with carboplatin and paclitaxel (CP) versus CP alone on progression-free survival (PFS) in patients with newly diagnosed advanced-stage epithelial ovarian cancer (EOC). According to the ICON-7 trial, BEV in patients with stage III cancer with residual disease > 1 cm who underwent debulking surgery and those with stage IV cancer (i.e., high-risk subgroups) had a survival benefit9. Meanwhile, the GOG-218 trial revealed overall survival (OS) benefit for BEV only in patients with stage IV disease, whereas no OS difference was observed in the broader population5. Moreover, BEV had evident side effects such as hypertension, gastrointestinal events, and thromboembolic events6,7,8,9.
The value for money of BEV for EOC was interesting because of its high cost and long treatment duration in the GOG-218 (21 cycles)6 and ICON-7 trials (18 cycles)8. According to a systematic review of economic evaluations of systemic treatment for ovarian cancer in all cases10, BEV based on the GOG-218 and ICON-7 trials in the US was not cost-effective11,12,13,14,15. Meanwhile, for high-risk groups (i.e., patients with stage III EOC with residual disease > 1 cm after debulking surgery and those with stage IV EOC), BEV based on the ICON-7 trial was more likely to be beneficial in terms of value of money13,14 Consistently, an economic evaluation study in Canada reported that the probability of using BEV as the ICON-7 protocol for high-risk groups16 was 56% based on the $100,000 willingness-to-pay (WTP) threshold of Canadians per quality adjusted life year (QALY) gained. In contrast, unlike CP alone, combined BEV and CP was not a cost-effective treatment for high-risk patients with EOC in the United Kingdom17 and Belgium18. However, there is no cost-effectiveness study on the combined use of BEV and standard chemotherapy with CP in developing or middle-income countries such as Thailand. Although previous cost-effectiveness analyses have been performed in high-income countries, their results are not directly transferable to Thailand due to different healthcare contexts, resource constraints, and willingness-to-pay thresholds.
In Thailand, the use of BEV in addition to standard chemotherapy with CP for high-risk subgroups with EOC has not been included in the National List of Essential Medicines (NLEM), a pharmaceutical reimbursement list in Thailand. Therefore, to confirm whether BEV should be included in the NLEM, the Subcommittee for the Development of NLEM requested cost-effectiveness information on combined BEV and standard chemotherapy with CP in high-risk patients. This study aimed to explore the value for money of combined BEV and standard chemotherapy with CP for high-risk patients with EOC based on the provider and societal perspectives in Thailand. Our findings provide novel, locally relevant evidence for policy makers within Thailand’s unique socioeconomic setting.
Materials and methods
Ethical approval and consent
This study was conducted in accordance with the relevant guidelines and regulations, including the Declaration of Helsinki for ethical principles in medical research involving human subjects and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH-GCP) guidelines for Good Clinical Practice. The study protocol was reviewed and approved by the Institutional Review Board (IRB) of the Faculty of Medicine Vajira Hospital (study code 314/64 E). Written informed consent was obtained from all participants or their legal guardians prior to their participation in the study.
Economic analysis
Cost-utility analysis was conducted to analyze and compare costs and health outcomes in terms of QALY using the hybrid decision tree and Markov model based on provider and societal perspectives during the lifetime period with a cycle length of 3 weeks. The target population in this study was a high-risk subgroup of patients with EOC, who were initially diagnosed with stage IIIB– IIIC EOC with residual tumor > 1 cm after debulking surgery and stage IV EOC. The mean age of the patients was 50 years19, with an ECOG performance status score of 0–2. Although the ICON-7 and GOG-218 trials had a higher mean age, we chose 50 years based on local registry data indicating Thai patients tend to be diagnosed at slightly younger ages than Western populations. The interventions evaluated were adjuvant treatments (i.e., BEV combined with standard chemotherapy with CP [6 cycles] as the front-line therapy according to the GOG-218 and ICON-7 regimens versus standard chemotherapy with CP alone). For the arm of standard chemotherapy, the same dose, schedule, and number of CP cycles were applied with six area under the receiving operating characteristic curve (AUC) of carboplatin and 175 mg/m2 of body surface area of paclitaxel every 3 weeks for 6 cycles. The BEV regimen in the GOG-218 study was 15 mg/kg every 3 weeks, with a total of 21 cycles. Meanwhile, the use of 7.5 mg/kg every 3 weeks for 18 cycles in the ICON-7 study was evaluated.
Model and analyses
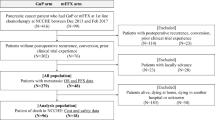
The decision tree and Markov models were generated using Microsoft Office Excel 2010 (Microsoft Corp., Redmond, WA, the USA). Figure 1 presents a decision tree indicating that two adjuvant alternative treatments with CP alone and adding BEV as the front-line therapy were compared. For each option, high-risk patients with EOC could be classified as platinum-sensitive or platinum-resistant. Then, they could enter the Markov model comprising three health statuses (i.e., progression-free, progression, and death) (Fig. 2). All high-risk patients with EOC could start at the progression-free state, and they might remain at the same health state or present with disease progression or death. Patients who remained at progression state might die or have the same health status. Two decision trees were separately created for the two trials with the same model structure as shown in Fig. 1.
The model assumptions in this study were as follows: (1) all patients could receive CP alone or CP plus BEV (the GOG-218 or ICON-7 regimens) as planned without disease progression during these upfront treatments; (2) If the disease progressed, all patients could completely receive palliative chemotherapy, which were platinum-sensitive at 70% (receiving 5 AUC of carboplatin plus liposomal doxorubicin 30 mg/m2 for 6 cycles)20 and platinum-resistant at 30% (receiving liposomal doxorubicin 40 mg/m2 for 6 cycles); and (3) the utility values during obtaining BEV either GOG-218 or ICON-7 regimens were equal. The results were presented in terms of the incremental cost-effectiveness ratio (ICER), which was calculated as the difference in costs divided by the difference in QALYs between adding BEV to CP from either the GOG-218 or ICON-7 trial and CP alone.
Model parameters
Cost parameters
The value for money of using BEV was reflected based in the provider and societal perspectives. Thus, direct medical costs (DMCs) and direct non-medical costs (DnMCs) were incorporated. For DMCs, all costs from the initial diagnosis of high-risk EOC, treatment, follow-up, and palliative treatment were collected. All investigation and surgical procedure costs for diagnosis were identified as pre-treatment costs and obtained from the Standard Cost List for Health Technology Assessment21. The costs of all drugs including CP, BEV, medications for the treatment of important side effects, second-line chemotherapy, and supportive and symptomatic drugs were collected from the Drug and Medical Supply Information Center of Thailand22.
Moreover, DnMCs incurred by patients and their families throughout the cancer treatment times including costs of transportation, food, informal care, and income loss of caregivers were obtained from our published study23. A discount rate of 3% was used to convert all future costs to year 202224. Table 1 shows the all cost parameters in both Thai Baht (THB) and United States Dollar, applying 35 THB per US dollar for the exchange rate.
Clinical parameters
The clinical outcomes were extracted from the GOG-2186,7 and ICON-7 (only the high-risk subgroup) studies8,9. The PFS rate, OS rate, median PFS, and OS were converted to the transition probabilities of progression-free status to disease progression and progression to death with a cycle length of 3 weeks. The incidence rates of important side effects including neutropenia, febrile neutropenia, bowel perforation, thromboembolic, and hypertension between adjuvant treatment with CP and adding BEV to each regimen were also reviewed from the two studies6,7,8. An age-specific death rate from the World Health Organization database was used to calculate the transition probabilities of progression-free status to death25. Table 2 presents all clinical parameters.
Utility parameters
The utility data were collected by interviewing 117 patients with high-risk EOC who received front-line therapy with either CP alone or adding BEV or those who with progression-free or disease-progression status after treatment completion at the Faculty of Medicine Vajira Hospital from February to May 2022. The Euro-Quality of Life Five-Dimension-Thai version (EQ-5D-TH) questionnaire was applied to collect the utility values for each health status26. Table 1 summarizes these utility values. Readers are referred to Reference 26 for additional details regarding data collection and calculation methods of utility values for Thailand.
Uncertainty analysis
To handle the uncertainty of each parameter, one-way sensitivity of various ICER values was performed if each parameter was changed. All costs, except those of BEV, varied, ranging from 90 to 110%. Meanwhile, the cost of BEV ranged from 75 to 125%. All probabilities and utility parameters used their 95% confidence interval (CI) for uncertainty analysis. The discount rate ranged from 0–6%.27 The Tornado diagram was used to display the most significant parameters, which had an impact on ICER. In addition, probabilistic sensitivity analyses (PSA) were conducted using the Monte Carlo simulation with 1,000 replications and presented as cost-effectiveness acceptability curves. In Thailand, the societal WTP threshold of 160,000 THB or $4,571 was applied to interpret which option is cost-effective28. Moreover, threshold sensitivity analysis was performed to evaluate the cost-effective price.
Results
Cost-utility analysis
For the patients’ life span, the amount of money for adding BEV as the GOG-218 and ICON-7 regimens increased by $38,614 and $16,650 from the provider perspective, and $39,479 and $17,430 from the societal perspective. Meanwhile, the QALY values gained from CP alone were 1.24 and 1.16, respectively. Compared with CP alone from the provider and societal perspectives, the ICER values of using BEV in the GOG-218 and ICON-7 regimens were $31,266 and $31,966 and $14,331 and $15,003 per QALY gained, respectively. Table 3 depicts all the results.
Uncertainty analysis
According to our cost-utility analysis, the ICER values of BEV in the ICON-7 arm were notably lower than those in the GOG-218 arm. Therefore, the Tornado diagram represents the one-way sensitivity analysis results of adding BEV based on the ICON-7 regimen (Fig. 3). The three most influential parameters were (1) the transition probability from progression-free to progression under BEV, (2) the transition probability from progression-free to progression under CP alone, and (3) the transition probability from progression to death under BEV. Lower transition probabilities for BEV translate into higher PFS or OS benefits, thus lowering the ICER. Conversely, higher transition probabilities for CP alone reduce outcomes with standard therapy, making BEV relatively more cost-effective. If the probability of changing from progression-free to progression and the probability of changing from progression to death when using BEV decreased to the lower value of their 95% CI (increased PFS rate after the diagnosis and OS rates after progression), the benefit of BEV increased. The lower cost was used to raise one QALY from $15,003 to $6,472 and $9,524 based on increasing PFS and OS rates, respectively. Meanwhile, an increased probability of changing from progression-free to progression to the upper end of the 95% CI of the standard chemotherapy with CP (decreased PFS rate after diagnosis) also supported the value of using BEV with reduced ICER to $9,461 per QALY gained. A 25% discounted cost for BEV had influential consequence at the fourth effect with decreasing ICER to $11,246 per QALY gained. However, if the cost of BEV was discounted by 70%, the ICER of the additional BEV was below the WTP threshold at $4,486/QALY, and it became the most powerful parameter. The rest parameters including utility values, discount rate, DMCs, and DnMCs had less effect on the changing of ICER itself.
If all uncertainties of parameters were adjusted simultaneously with consideration of the WTP threshold, the probabilities of cost-effectiveness for using BEV as the GOG-218 and ICON-7 regimen were evaluated. Considering the WTP threshold of Thailand ($4,571 or 160,000 THB), the cost-effectiveness of BEV administered according to the GOG-218 regimen could not be demonstrated when compared to standard CP. Specifically, the ICER of BEV exceeded the threshold by a significant margin, indicating that the additional cost of treatment with BEV was not justified by the associated gains in QALYs. As illustrated in Fig. 4, our findings suggest that BEV in this regimen does not represent a cost-effective option under Thailand’s current healthcare funding limitations. The first value of ICER, which showed the effectiveness of BEV at 1% was $14,286. Meanwhile, using BEV as the ICON-7 regimens, the cost-effectiveness was at 3% of the WTP threshold (Fig. 5).
Figure 6 shows the ICER value of using both BEV regimens in terms of the cost-effectiveness plane and the WTP threshold of Thailand in each level. All ICER values were placed in the right upper quadrant of the cost-effectiveness plane. If the WTP threshold ($4,571) was considered, almost the ICER values of BEV added as the GOG-218 protocol was begun above the WTP line at three times that Thailand’s threshold ($13,713). However, the ICER values of adding BEV as the ICON-7 regimen was below this line in approximately 50%.
Discussion
BEV is one type of targeted cancer therapy, which is an expensive drug. It should be noted that current guidelines recommend genomic testing for newly diagnosed advanced ovarian cancer to identify patients who may benefit from PARP inhibitors (e.g., olaparib, niraparib, rucaparib), alone or in combination with BEV, in the maintenance setting. While these novel strategies may alter the therapeutic landscape and cost-effectiveness calculations, evaluating their relative cost-effectiveness was beyond the scope of our study.
Several cost-effectiveness studies have shown that the use of BEV in ovarian cancer as the upfront therapy was not effective. However, these studies were produced in four western countries including the United States11,12,13,14,15, Canada16, the United Kingdom17, and Belgium18. Therefore, this is the first study conducted in Asian countries, which have different contexts from western countries particularly in terms of income and health preference. Therefore, it is important to explore the efficiency of BEV against ovarian cancer in each country before its application in respect of health policy maker. Previous studies have revealed the probability of increasing the value for adding BEV to standard chemotherapy with CP in this subgroup condition. Therefore, this study evaluated a high-risk subgroup, which comprises patients with stage III EOC with residual disease > 1 cm after debulking surgery and stage IV EOC12,13,14,16. Our results revealed that based on the level of WTP threshold in Thailand, adding BEV based on the GOG-218 or ICON-7 regimens was not cost-effective in provider and societal perspectives. The study results were similar to those of studies previously published11,12,13,14,15,16,17,18.
Using BEV based on the GOG-218 protocol had a higher cost than that based on the ICON-7 protocol. In particular, the cost is approximately 1.5 times higher due to a greater dose and longer treatment duration ($61,815 vs. $39,032). Nevertheless, the survival benefit did not respond to a difference in the BEV protocol. Thus, the ICER for one QALY gained of BEV for the GOG-218 study was approximately two times more than that of BEV for the ICON-7 study. If the ICER from adding more BEV as both the GOG-218 and ICON-7 studies were deliberated based on the WTP level of Thailand ($4,571), these ICER values were 7 times ($31,966) and 3 times ($15,003) greater than the threshold for GOG-218 and ICON-7, respectively, from a societal perspective. Based on two previous health economic studies, which used the data from both GOG-218 and ICON-7 studies14,18, BEV as the GOG-218 regimen was not effective. This finding was similar to that of the study of Metha et al.14 By contrast, a study performed in Belgium showed that BEV as the GOG-218 regimen had a better value than the ICON-7 regimen from a governmental perspective18. This remarkable difference was caused by the estimated value of the hazard ratios of patients with stage IV EOC in the ICON-7 study. The authors declared that this value was not appropriate based on their valuation method. There was a minimal difference in terms life year between CP alone and adding BEV. This was reflected on a small number of incremental QALY and a high ICER per one QALY gained18.
In accordance with the Tornado diagram from the ICON-7 study, the three most common consequences for changing the efficiency of BEV were the performance of CP and BEV in a subgroup of patients with high-risk ovarian cancer. If BEV can increase survival benefit compared with CP, particularly PFS, based on the results of these two landmark studies, this targeted therapy can be increasing efficient. This influential parameter from our study was similar with that in the study of Metha et al.14 which reported the importance of the health status such as from progression-free to progression to ICER. In terms of increasing OS, Chan et al.13 and Lesnock et al.15 revealed the significance of this factor via one-way sensitivity analysis. The effect of BEV cost was based on the percentage of cost discounting. In our study, reducing the BEV cost by 70% from the baseline cost remarkably enhanced the value of adding BEV, and the ICER was below the WTP threshold. However, it is challenging to compare the efficiency of BEV from each study and particularly from different countries because of the dissimilarity of the WTP threshold and policy of each country. Based on the PSA, using BEV as the GOG-218 regimen was totally cost-ineffective. Meanwhile, using BEV as the ICON-7 regimen showed a 3% probability of cost-effectiveness at a WTP threshold of $4,571.
All parameters of the clinical outcomes including PFS, OS, and important side effects were derived from the two landmark studies6,7,8,9. The standard treatment was CP alone, which was the same regimen between these two studies. The difference in terms of effectiveness between CP and BEV from both the GOG-218 and ICON-7 studies was similar in terms of the median PFS. However, after disease progression, the dissimilarity of the performance of CP and BEV from these two studies was evident. A longer median OS time was just 3 months from adding more BEV as the GOG-218 protocol, and the difference between CP alone and combined BEV and CP was not significant7. When adding BEV in the ICON-7 study, the difference in terms of median OS was approximately 10 months9. However, the actual effectiveness of BEV in both the GOG-218 and ICON-7 regimens was not completely elucidated. Moreover, the treatments after disease progression should be considered. All treatments after the identification of novel diseases or the increase in CA-125 levels were independent, which inevitably affected the OS time in both studies. Therefore, all clinical parameters, which were extracted from randomized controlled trials, might not reflect treatment outcomes in real-world settings.
The strength of this study is the that it used a method for collecting utility data. EQ-5D-TH, a standard questionnaire, was used to interview patients with high-risk EOC for each treatment and all health statuses. These were then transformed to standard utility value. Therefore, this parameter is more suitable and reliable than extraction from other diseases and other studies or cancer-specific questionnaire that had doubts about utility values. Moreover, the societal perspective was also considered in this study. All costs with a patient’s lifetime in this study covered all important stakeholders for disease treatment. However, the current study also had some limitations, which are a cause of concern. The utility values of each complication were not included in the model. There were some differences in terms of the percentage of these severe complications between CP and BEV from both RCTs. These could be affected by the health outcomes of each treatment. The next limitation was our consumption. The first consumption, which might not occur in real life in all patients, was the complete treatment as planned, particularly in the BEV arm. In addition, if the disease progressed, our assumption forced all patients to be treated with further palliative chemotherapy. Some patients may just have received treatment as symptomatic care or die during treatment. Hence, the lifetime of patients from the calculation, which was based on our consumption, had been overestimated. Therefore, the QALY values from the GOG-218 and ICON-7 studies were also overvalued, and the ICER values should be more than our estimation. Notably, BEV biosimilars have recently emerged, potentially lowering drug costs. According to the Drug and Medical Supply Information Center of Thailand, provisional cost data for some biosimilars have been available since 2022, but official pricing and reimbursement details remain limited. Should these biosimilars be introduced at substantially reduced prices, the incremental cost-effectiveness ratio of BEV in high-risk EOC may become more favorable. Moreover, our study did not evaluate the value of BEV between patients undergoing stage III suboptimal surgery and those undergoing stage IV suboptimal surgery in a subgroup of patients with high-risk cancer. Some important data such as PFS and survival time after disease progression at each stage (suboptimal surgery stage III vs. stage IV) were not complete. Therefore, they should be cautiously considered.
In addition to front-line therapy, BEV is also indicated for recurrent ovarian cancer in both platinum-sensitive and platinum-resistant settings. Nevertheless, we focused on the first-line high-risk setting. Future analyses may need to integrate the use of BEV in subsequent lines of therapy and explore its cost-effectiveness in these distinct clinical contexts. High-risk ovarian cancer subgroup is still associated with a poor prognosis. Maintenance BEV had a higher median PFS (approximately 5–6 months). However, the consistency of benefit for median OS was not able to show from GOG-218 and ICON-7 study. Due to the high cost of this targeted therapy, in Thailand, using BEV as the ICON-7 regimen is currently permitted only for the Civil Servant Medical Benefit Scheme. As a result of this health policy, most patients cannot assess and afford this drug. Our study result may support the information for policy makers to negotiate the appropriate drug price. There are several knowledge gaps with respect to the appropriate treatment in terms of effectiveness and efficiency for the high-risk EOC subgroup. There are two novel studies about this area, which investigated the effectiveness of combined CP and BEV29,30. The first study by the Arbeitsgmeinschaft Gynäkologische Onkologie Study Group used the control arm as the BEV maintenance from the GOG-218 protocol (21 cycles). Moreover, such a treatment was compared with 42 cycles of BEV (two times than the control arm) used as the intervention arm29. The early results showed that the prolonged use of BEV was not effective in improving both PFS and OS. The second study is the ICON-8B study, which used CP plus BEV from the ICON-7 study as the control arm30. This study was evaluated the difference in applying paclitaxel between standard regimen (175 mg/m2 every 3 weeks) and the regimen comprising 80 mg/m² per week for 3 weeks at 1 cycle. The dose, frequency, and number of cycle of BEV in the ICON-8B study were similar to those in the ICON-7 study30. Health economic evaluation was a secondary objective, and their outcomes are in the phase of exploration. Therefore, future studies must be conducted to evaluate predictive biomarkers. This approach may be useful in establishing individualized treatment for each patient. This concept is similar to screening all patients at high risk of disease recurrence. Hence, an appropriate treatment can be selected for each patient. However, each drug has its own cost. Therefore, cost-effectiveness studies must be performed evaluate the best value of each novel option before its use in real-life settings.
Conclusion
Using maintenance BEV as the GOG-218 or ICON-7 regimen is not cost-effective for a subgroup of patients with high-risk EOC in Thailand. However, adding BEV as the ICON-7 regimen can be effective. The performance of BEV in terms of median PFS is important in improving its value. Therefore, the price of BEV should be decreased by 70% before the inclusion of BEV for treating patients with high-risk EOC.
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
References
International Agency for Research on Cancer. (2018). http://globocan.iarc.fr. Accessed 2 Feb 2022.
Cancer in Thailand. Volume X 2016–2018. In: https://www.nci.go.th/th/File_download/Nci%20Cancer%20Registry/Cancer%20in%20Thailand%20IX(Unpublished%20Edition)03.pdf. Accessed 2 Feb 2022.
Li, H., Li, J., Gao, W., Zhen, C. & Feng, L. Systematic analysis of ovarian cancer platinum-resistance mechanisms via text mining. J. Ovarian Res. 13(1), 27 (2020).
Mutch, D. G. & Prat, J. 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol. Oncol. 133, 401–404 (2014).
Liu, S. et al. The use of bevacizumab in the modern era of targeted therapy for ovarian cancer: a systematic review and meta-analysis. Gynecol. Oncol. 161(2), 601–612 (2021).
Burger, R. A. et al. Gynecologic oncology group. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl. J. Med. 365(26), 2473–2483 (2011).
Tewari, K. S. et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J. Clin. Oncol. 37(26), 2317–2328 (2019).
Perren, T. J. et al. ICON7 investigators. A phase 3 trial of bevacizumab in ovarian cancer. N Engl. J. Med. 365(26), 2484–2496 (2011).
Oza, A. M. et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 16(8), 928–936 (2015).
Poonawalla, I. B., Parikh, R. C., Du, X. L., VonVille, H. M. & Lairson, D. R. Cost effectiveness of chemotherapeutic agents and targeted biologics in ovarian cancer: a systematic review. Pharmacoeconomics 33(11), 1155–1185 (2015).
Cohn, D. E., Kim, K. H., Resnick, K. E., O’Malley, D. M. & Straughn, J. M. Jr At what cost does a potential survival advantage of bevacizumab make sense for the primary treatment of ovarian cancer? A cost-effectiveness analysis. J. Clin. Oncol. 29(10), 1247–1251 (2011).
Barnett, J. C. et al. Cost effectiveness of alternative strategies for incorporating bevacizumab into the primary treatment of ovarian cancer. Cancer 119(20), 3653–3661 (2013).
Chan, J. K. et al. Bevacizumab in treatment of high-risk ovarian cancer–a cost-effectiveness analysis. Oncologist 19(5), 523–527 (2014).
Mehta, D. A. & Hay, J. W. Cost-effectiveness of adding bevacizumab to first line therapy for patients with advanced ovarian cancer. Gynecol. Oncol. 132(3), 677–683 (2014).
Lesnock, J. L., Farris, C., Krivak, T. C., Smith, K. J. & Markman, M. Consolidation Paclitaxel is more cost-effective than bevacizumab following upfront treatment of advanced epithelial ovarian cancer. Gynecol. Oncol. 122(3), 473–478 (2011).
Duong, M. et al. The cost-effectiveness of bevacizumab for the treatment of advanced ovarian cancer in Canada. Curr. Oncol. 23(5), e461–e467 (2016).
Hinde, S. et al. The Cost-Effectiveness of bevacizumab in advanced ovarian cancer using evidence from the ICON7 trial. Value Health. 19(4), 431–439 (2016).
Neyt, M., Vlayen, J., Devriese, S. & Camberlin, C. First- and second-line bevacizumab in ovarian cancer: a Belgian cost-utility analysis. PLoS One. 13 (4), e0195134 (2018).
Kittisiam, T. et al. Prevalence of other cancers in ovarian cancer patient. Vajira Med. J. 62(1), 1–8 (2018).
Nguyen, J., Solimando, D. A. Jr & Waddell, J. A. Carboplatin and liposomal doxorubicin for ovarian cancer. Hosp. Pharm. 51(6), 442–449 (2016).
Standard Cost Lists for Health Technology Assessment. In: https://costingmenu.hitap.net/. Accessed 2 Feb2022.
Drug and Medical Supply Information Center of Thailand. In: http://dmsic.moph.go.th/index/dataservice/90/0. Accessed 2 Feb2022.
Katanyoo, K., Riewpaiboon, A., Chaikledkaew, U. & Thavorncharoensap, M. The cost of locally advanced cervical cancer in Thailand: an empirical study for economic analysis. Asian Pac. J. Cancer Prev. 22 (10), 3171–3179 (2021).
Permsuwan, U., Guntawongwan, K. & Buddhawongsa, P. Handling time in economic evaluation studies. J. Med. Assoc. Thai. 97 (Suppl 5), S50–S58 (2014).
Life table by country-Thailand. (2020). https://apps.who.int/gho/data/view.main.61640?lang=en. Accessed 2 Feb 2022.
Pattanaphesaj, J. et al. The EQ-5D-5L valuation study in Thailand. Expert Rev. Pharmacoecon Outcomes Res. 8, 551–558 (2018).
Chaikledkaew, U., Teerawattananon, Y. & Suksomboon, N. Health Intervention and Technology Assessment Program (Graphico Systems Co., 2009).
Riewpaiboon, A. Cost analysis. In: (eds Chaikledkaew, U. & Teerawattananon, Y.) Thai National Health Technology Assessment Guidelines. 2nd ed. 23–42 (Nonthaburi: Watcharin, 2013).
Pfisterer, J. et al. Optimal treatment duration of bevacizumab (BEV) combined with carboplatin and paclitaxel in patients (pts) with primary epithelial ovarian (EOC), fallopian tube (FTC) or peritoneal cancer (PPC): a multicenter open-label randomized 2-arm phase 3 ENGOT/GCIG trial of the AGO Study Group, GINECO, and NSGO (AGO-OVAR 17/BOOST, GINECO OV118, ENGOT Ov-15, NCT01462890). J Clin Oncol. no. 15_suppl (May 20, 2021) 5501–5501. Meeting Abstract | 2021 ASCO Annual Meeting I (2021) 39.
ICON8 Trials Programme. In: http://www.icon8trial.org/media/1738/icon8-trials-programme-protocol-v80_16apr20_clean.pdf Accessed 27 Feb 2023.
Acknowledgements
Special thanks to the patients and staff at the Faculty of Medicine Vajira Hospital for their cooperation and participation in this study.
Funding
We would like to thank the funding support from Food and Drug Administration, Thailand. This paper represents the views of the authors. The Health Economics Working Group (HEWG) under the Subcommittee for the Development of the National List of Essential Medicines (NLEM) in Thailand, appointed from 2019 to 2021, reviewed the technical component of the work but is not responsible for the study findings and the dissemination of the findings.
Author information
Authors and Affiliations
Contributions
K.K., U.C., and N.C. contributed to the conception and design of the study, performed the data analysis, and drafted the manuscript. U.C. provided expertise in health technology assessment, assisted with the methodological framework, and participated in the interpretation of results. N.C. contributed to the clinical input, facilitated data collection, and critically reviewed and revised the manuscript for important intellectual content. All authors approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Katanyoo, K., Chaikledkaew, U. & Chandeying, N. Cost effectiveness of bevacizumab plus carboplatin paclitaxel versus carboplatin paclitaxel as front line for advanced ovarian cancer in Thailand. Sci Rep 15, 9949 (2025). https://doi.org/10.1038/s41598-025-94455-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-94455-7