Abstract
Hepatocellular carcinoma (HCC) is a prevalent and fatal tumor globally, characterized by a complex pathogenesis and poor prognosis. Despite significant advancements in the application of immune checkpoint inhibitors (ICIs) for cancer treatment, the efficacy of immunotherapy in HCC remains suboptimal. PSMD2, a crucial regulator of the ubiquitin-proteasome system, has attracted increasing attention for its involvement in various cancers; however, its functions and mechanisms in HCC are still poorly understood. This study aims to investigate the expression of PSMD2 in HCC, its association with prognosis, and its interaction with immune checkpoints, thus establishing a foundation for further exploration of its role in immune evasion in HCC. We analyzed the expression levels of PSMD2 in HCC and adjacent normal tissues utilizing the GEPIA and TIMER databases. Cox regression analysis was performed using R software to evaluate the relationship between PSMD2 expression and prognosis. Furthermore, we assessed the correlation between PSMD2 and immune cell infiltration, as well as immune checkpoints, including PD1, PD-L1, and CTLA-4, using R tools. Additionally, we examined the association between PSMD2 expression and immune therapy response through Tumor Immune Dysfunction and Exclusion (TIDE) analysis. Finally, we constructed a protein-protein interaction (PPI) network using the STRING database and Cytoscape software, followed by Gene Set Enrichment Analysis (GSEA). PSMD2 was significantly overexpressed in HCC and was closely correlated with poor prognosis (HR = 1.61, P = 2.0e-4). Immune infiltration analysis demonstrated that PSMD2 was positively correlated with several immune checkpoint genes, including PD1, PD-L1, and CTLA-4, as well as various immune cell types. TIDE analysis indicated that elevated PSMD2 expression was significantly associated with increased immune evasion potential and a poor response to immunotherapy. Furthermore, GSEA enrichment analysis revealed that PSMD2 is primarily enriched in the p53 signaling pathway, the ubiquitin-mediated proteolysis pathway, and other cancer-related pathways. The elevated expression of PSMD2 in HCC is not only correlated with poor prognosis but may also play a role in immune evasion by modulating tumor immunity, thereby affecting patient responses to immunotherapy. Consequently, PSMD2 presents a promising novel therapeutic target and potential biomarker for immunotherapy in HCC.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent malignancies globally1, accounting for approximately 90% of primary liver cancers2. Although early-stage HCC can be effectively treated with curative surgery or local therapies, achieving a 5-year survival rate of up to 70% in some patients3, the prognosis for the majority remains poor4. In China, approximately 70% of HCC cases are diagnosed at an advanced stage, where curative options are limited and the 5-year survival rate is less than 20%5,6. In recent years, systemic therapies, including targeted treatments and immune checkpoint inhibitors (ICIs), have opened new treatment avenues for various cancer types7, including advanced HCC8. However, the efficacy of ICIs remains suboptimal, with only 10–35% of patients experiencing sustained clinical benefit9.
Despite the remarkable success of immune checkpoint inhibitors (ICIs), such as PD-1/PD-L1 and CTLA-4 inhibitors, in cancer treatment, their efficacy is often compromised by the tumor microenvironment (TME) and mechanisms of immune evasion10. This situation can lead to both primary and adaptive resistance to therapy11. The TME in HCC is particularly complex, with immune cells playing a crucial role in the disease’s development and progression, as well as in modulating the response to immunotherapy12. However, the precise mechanisms regulating immune responses within the HCC microenvironment remain poorly understood13. During tumorigenesis, the protein degradation system, particularly the ubiquitin-proteasome pathway, plays a critical role in regulating cellular processes such as proliferation, apoptosis, and immune modulation14. PSMD2 (26 S proteasome non-ATPase regulatory subunit 2) is a key regulator within this system and is implicated in the development of various cancers, including breast cancer, liver cancer, and lung adenocarcinoma15. It significantly influences the proteasome degradation mechanism, regulates cell proliferation and apoptosis, and interacts with the immune system through various related signaling pathways, such as JAK-STAT, Toll-like receptors, P53, and MAPK, thereby participating in cancer occurrence and progression16,17,18. In HCC, PSMD2 can affect cell proliferation and apoptosis by regulating the p38-JNK and AKT signaling pathways, which in turn influence cellular lipid metabolism19. It is considered a potential prognostic biomarker and therapeutic target related to immunogenic cell death20. However, the specific role of PSMD2 in HCC and its impact on the immune microenvironment remain unexplored.
Based on this background, this study aims to investigate the expression of PSMD2 in HCC and its relationship with prognosis, immune checkpoint gene expression, and immune cell infiltration. By elucidating the role of PSMD2 in the HCC immune microenvironment, we hope to provide new insights into its potential as a biomarker and therapeutic target for immunotherapy in HCC.
Materials and methods
Data acquisition and processing
PSMD2 expression analysis in HCC
We obtained PSMD2 expression data from HCC and adjacent normal tissues using the TIMER (http://timer.cistrome.org/) and GEPIA databases (http://gepia2.cancer-pku.cn/). The expression of PSMD2 in tumor and normal tissues was analyzed with the TIMER database, and statistical significance was assessed using the Wilcoxon rank-sum test. Furthermore, immunohistochemistry (IHC) results from the Human Protein Atlas (HPA) database (https://www.proteinatlas.org/) were employed to validate the protein expression of PSMD2 in HCC tissues.
Immunofluorescence experiments
Immunofluorescence experiments were performed on a tissue microarray containing 13 HCC and 2 cholangiocarcinoma samples to validate the distribution of PSMD2 protein in tumor and adjacent normal tissues.
Experimental procedure
-
(1)
Paraffin section dewaxing to water: Sequentially immerse the sections in environmentally friendly dewaxing solution I for 10 min, followed by solution II for 10 min, and solution III for 10 min. Next, immerse in absolute ethanol I for 5 min, followed by ethanol II for 5 min, and ethanol III for 5 min. Finally, rinse the sections with distilled water.
-
(2)
Antigen retrieval: During the retrieval process under high pressure, it is essential to prevent excessive evaporation of the buffer solution and to avoid drying the sections. After retrieval, allow the slides to cool naturally. Subsequently, immerse the slides in PBS (pH 7.4) and perform a shaking wash three times on a decolorization shaker, with each wash lasting five minutes.
-
(3)
Circle and serum blocking: After slightly drying the sections, use a histochemical pen to delineate the tissue by drawing a circle around it. Add BSA for blocking. Allow the blocking to proceed for 30 min.
-
(4)
Add primary antibody (PSMD2 dilution ratio: 1:200): Drop the prepared primary antibody and incubate the sections flat in a humid box at 4 °C overnight.
-
(5)
Add secondary antibody (CY3-labeled goat anti-rabbit IgG dilution ratio: 1:300): Place the slides in PBS (pH 7.4) and wash them three times using a decolorization shaker, with each wash lasting five minutes. Subsequently, add the appropriate secondary antibody and incubate in the dark at room temperature for 50 min.
-
(6)
DAPI counterstaining of nuclei: Place the slides in PBS (pH 7.4) and wash them three times on a decolorization shaker, with each wash lasting five minutes. Subsequently, add the DAPI staining solution and incubate the slides at room temperature in the dark for ten minutes.
-
(7)
Mounting: Use an anti-fluorescence quenching mounting medium for mounting.
-
(8)
Image acquisition: The excitation and emission wavelengths for various fluorochromes are as follows: DAPI has an excitation wavelength range of 330 to 380 nm and an emission wavelength of 420 nm. The excitation wavelength for CY3 is between 510 and 560 nm, with an emission wavelength of 590 nm. For CY5, the excitation wavelength ranges from 608 to 648 nm, while the emission wavelength is between 672 and 712 nm. Additionally, the excitation wavelength for the fluorochrome with a 488 nm emission is between 465 and 495 nm, and it emits light at 515 to 555 nm.
Analysis method
Aipathwell is a digital pathological image analysis software developed by Servicebio, utilizing artificial intelligence learning. It employs the principles of AI deep learning to conduct algorithm training on extensive datasets, which are then integrated into an automated image analysis system. The specific process is as follows:
-
(1)
Tracking: The area to be measured along the tissue can be automatically located and delineated. Additionally, manual positioning can be performed based on specific requirements.
-
(2)
Color selection: Manually select the target fluorescence signal based on HSI (Hue, Saturation, Intensity) and utilize software for repeated identification to ensure that all positive signals are accurately selected. The established color selection standard must be consistently applied to the same index across all sections within the same batch.
-
(3)
Calculation: According to the requirements, the software automatically identifies and locates the nuclei of DAPI blue fluorescence while expanding the cytoplasmic range. It reverses the decolorization of the single-channel fluorescence image (where the positive signal appears black and the background is white), reads the black positive signals, and calculates various parameters, including the number of positive cells, area, integrated optical density (IOD), and tissue area.
-
(4)
Analysis: Calculate the area to be measured step by step at high magnification. After completion, automatically calculate and generate analysis results for each item based on the original basic data and algorithm formula, and generate a report.
Prognostic analysis
The Kaplan-Meier method (http://kmplot.com) and the Cox proportional hazards model21 were employed using GEPIA and the ‘survival’ package (https://cran.r-project.org/web/packages/survival/) in R software to evaluate the relationship between PSMD2 expression and HCC prognosis, specifically focusing on overall survival (OS) and progression-free survival (PFS). Patients were categorized into high and low expression groups based on PSMD2 expression levels, and the log-rank test was utilized to compare survival differences between these two groups. Additionally, receiver operating characteristic (ROC) curve analysis was conducted to assess the predictive accuracy of PSMD2 for prognosis.
Correlation analysis between PSMD2 and immune checkpoints
Immune checkpoint gene expression analysis
We utilized the R software package ‘survival’ to analyze the correlation between PSMD2 and various immune checkpoint genes, including PD-1, PD-L1, CTLA-4, TIGIT, LAG3, and HAVCR2, in HCC. The expression levels of PSMD2 were categorized into high and low expression groups. Subsequently, Spearman correlation analysis was conducted to evaluate the relationship between PSMD2 and the expression of these immune checkpoint genes.
TIDE score assessment
The Tumor Immune Dysfunction and Exclusion (TIDE) online tool (http://tide.dfci.harvard.edu/) was employed to evaluate the relationship between PSMD2 expression and the response to immunotherapy. The TIDE score serves as an indicator of the potential for tumor immune evasion. HCC patients were categorized into high and low PSMD2 expression groups based on their expression levels, and their TIDE scores were compared to infer the association between PSMD2 expression and immunotherapy response.
Immune cell infiltration analysis
CIBERSORT immune cell infiltration assessment
The CIBERSORT algorithm (https://cibersortx.stanford.edu/) was employed to analyze the proportions of immune cell infiltration in the tumor tissues of patients with HCC. We compared the levels of immune cell infiltration between the high and low PSMD2 expression groups to evaluate the correlation between PSMD2 and various types of immune cells, including CD8 + T cells, macrophages, and regulatory T cells (Tregs).
TIMER immune cell infiltration analysis
Further analysis of PSMD2 and immune cell infiltration—including regulatory T cells (Tregs), dendritic cells, and macrophages—was conducted using the TIMER tool. Cox proportional hazards models were constructed to evaluate the clinical relevance of PSMD2 expression, immune cell infiltration, tumor stage, age, and gender.
Protein-protein interaction (PPI) network analysis
The STRING database (https://string-db.org/) was utilized to construct a protein-protein interaction (PPI) network involving PSMD2 and other proteins, applying an interaction score threshold of 0.4. Cytoscape software (http://www.cytoscape.org/) was employed to visualize the PPI network and to identify proteins that interact directly with PSMD2, with particular emphasis on its interactions with immune checkpoint genes.
Gene set enrichment analysis (GSEA)
Gene set enrichment analysis (GSEA) was conducted using GSEA 4.0 (http://software.broadinstitute.org/gsea/msigdb) to investigate the functional enrichment of PSMD2 in HCC. HCC samples from TCGA(https://cancergenome.nih.gov/) were categorized into high and low expression groups based on PSMD2 expression levels. Gene sets from the MSigDB database (http://software.broadinstitute.org/gsea/msigdb) were selected for the enrichment analysis. A random permutation of 2000 iterations was performed, and an absolute normalized enrichment score (NES) greater than 1, along with a P-value of less than 0.05, was considered statistically significant.
Statistical analysis
All data analyses were conducted using the R software environment (version 3.2.7). Continuous variables were compared with the Student’s t-test or Mann-Whitney U test, while categorical variables were analyzed using the chi-square test or Fisher’s exact test. Spearman correlation analysis was employed to evaluate the relationship between PSMD2 expression, immune checkpoint genes, and immune cell infiltration. Survival data were analyzed using the Kaplan-Meier method, and differences between groups were compared using the log-rank test. A P-value of less than 0.05 was considered statistically significant.
Results
PSMD2 gene expression and its relationship with prognosis in HCC
PSMD2 expression in HCC
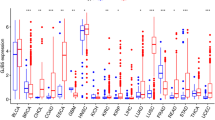
Analysis using the GEPIA database (Fig. 1-a) revealed that PSMD2 expression was significantly elevated in the majority of tumor tissues within the TCGA database compared to normal tissues. The highest expression levels were observed in squamous cell carcinoma of the lung, head and neck squamous carcinoma, cervical squamous carcinoma, glioblastoma, and melanoma. Although PSMD2 expression in HCC was relatively lower than in other tumor types, it remained significantly higher than in adjacent normal tissues.
Furthermore, we utilized the TIMER database to analyze the differential expression of PSMD2 between tumor and adjacent normal tissues, with the results illustrated in a boxplot (Fig. 1-b). The Wilcoxon test results indicated that PSMD2 expression was significantly higher in HCC tissues than in normal tissues (P < 0.001), denoted by ***.
To further validate tissue-level expression, immunohistochemical (IHC) analysis from the Human Protein Atlas (HPA) database confirmed the high expression of PSMD2 in HCC tissues, while low or negligible expression was observed in normal liver tissues (Fig. 1-c-h).
Analysis of Immunofluorescence experimental results
We detected the expression of the PSMD2 protein in liver cancer tissues and adjacent tissues using immunofluorescence experiments. The results demonstrated that the ratio of positive area and the positive fluorescence density of the PSMD2 protein in liver cancer tissues were significantly higher than those in adjacent tissues (P < 0.05) (Fig. 1-i, j). This finding indicates that the PSMD2 protein is widely distributed in hepatocellular carcinoma tissues, which is consistent with the expression results from the Human Protein Atlas (HPA) database. This aligns with its function as a ubiquitin receptor that recognizes substrates and delivers them to the 20 S core particle for degradation, thereby influencing the occurrence and progression of HCC.
These findings suggest a widespread distribution of the PSMD2 protein in HCC tissues, consistent with its high expression patterns observed in other cancers. Although PSMD2 expression in HCC is relatively lower compared to other tumors, it remains significantly higher than in normal tissues, indicating its potential role in the development and progression of HCC.
(a) PSMD2 expression in tumor and non-tumor tissues. (b) Distribution of PSMD2 expression in tumor and adjacent normal tissues. (c-h) Immunohistochemistry (IHC) analysis from the Human Protein Atlas (HPA) database showing high PSMD2 expression in HCC tissues (c-e) and low or no expression in normal liver tissues (f-h). (i) Immunofluorescence analysis of PSMD2 protein distribution in HCC tissues. (j) The positive area ratio and fluorescence intensity of PSMD2 protein in HCC tissues are significantly higher than in adjacent normal tissues (*P < 0.05, **P < 0.01).
Prognostic analysis of PSMD2 expression in HCC
We retrieved publicly available gene sequencing data from TCGA for 372 HCC samples and 50 normal tissues (Fig. 2-a). The expression of PSMD2 was significantly elevated in HCC tissues compared to normal tissues (P < 0.05). Based on PSMD2 expression levels, HCC patients were categorized into high and low expression groups. Kaplan-Meier survival curves were generated using the GEPIA platform and the ‘survival’ package in R software to evaluate the prognostic value of PSMD2. The log-rank test was employed to assess statistical differences between groups. Kaplan-Meier survival analysis demonstrated that patients with high PSMD2 expression exhibited significantly shorter overall survival (OS) and progression-free survival (PFS) (P < 0.05, Fig. 2-b, c). Additionally, the area under the curve (AUC) values for predicting one-year, three-year, and five-year survival rates in the ROC curve were all above 0.6, with the AUC at one year exceeding 0.7. These results indicate that PSMD2 exhibits excellent predictive efficacy and is strongly time-dependent, thereby demonstrating its predictive accuracy in forecasting long-term outcomes for HCC patients (Fig. 2-d). Moreover, Cox regression analysis was conducted to identify factors affecting the prognosis of liver cancer patients (Table 1). As shown in Table 1, univariate analysis revealed that pathologic stage (HR = 2.986, P < 0.001), T stage (HR = 3.203, P < 0.001), and PSMD2 expression (HR = 0.559, P = 0.027) were significantly associated with the overall survival (OS) of liver cancer patients. Further multivariate analysis suggested that PSMD2 expression (HR = 0.585, P = 0.047) serves as an independent prognostic factor. These findings further support PSMD2 as a potential prognostic biomarker in HCC.
(a) PSMD2 expression in HCC samples from the TCGA database. (b, c) Kaplan-Meier survival curves showing that high PSMD2 expression predicts shorter overall survival (OS) and progression-free survival (PFS) (P < 0.05). (d) Time-dependent ROC curve for PSMD2 expression (AUC1, 3, 5 > 0.6, 95% CI [0.63–0.77]).
PSMD2 expression in different tumor stages
We further analyzed the differential expression of PSMD2 in HCC samples across various tumor stages using data from TCGA database. The expression levels of PSMD2 at different clinical stages were compared using an unpaired Student’s t-test, while analysis of variance (ANOVA) was employed to assess differences across multiple groups. The results indicated significant variations in PSMD2 expression among four tumor types: breast cancer (BRCA), clear cell renal carcinoma (KIRC), HCC (LIHC), and bladder cancer (BLCA) (Fig. 3-a, b). Furthermore, significant differences in PSMD2 expression were observed between distinct pathological T stages (T1, T2, T3) in HCC, with statistical significance denoted by *P < 0.05 and **P < 0.01 (Fig. 3-c). These findings suggest that PSMD2 expression may fluctuate across tumor stages, underscoring its potential role in tumor progression.
Prognostic analysis of PSMD2 in pancarcinoma
Furthermore, we utilized the R software package to analyze the relationship between gene expression and prognosis across various tumor types, evaluating the statistical significance of prognosis using the Log-rank test. Our results indicated that in 13 tumor types, high PSMD2 expression was significantly associated with poor prognosis. For instance, in hepatocellular carcinoma (TCGA-LIHC, N = 341, P = 2.0e-4, HR = 1.61 [1.25, 2.07]), high PSMD2 expression predicted reduced survival rates. Additionally, low PSMD2 expression correlated with poorer prognosis in both colorectal adenocarcinoma (TCGA-READ, N = 90, P = 0.03, HR = 0.48 [0.24, 0.94]) and uveal melanoma (TCGA-UVM, N = 74, P = 0.05, HR = 0.46 [0.21, 1.00]), as illustrated in Fig. 3-d.
These findings suggest that the expression level of PSMD2 across different cancer types is significantly correlated with prognosis, particularly in HCC, where elevated PSMD2 expression predicts a poor prognosis.
(a) PSMD2 expression in different tumor types and their correlation with tumor stages. (b) Correlation between PSMD2 expression and HCC tumor stages (Pr(> F) = 0.0263). (c) PSMD2 expression across different pathological T stages in HCC (*P < 0.05, **P < 0.01). (d) Prognostic analysis of PSMD2 expression across various tumor types. High PSMD2 expression is significantly associated with poor prognosis in certain tumors, such as hepatocellular carcinoma (TCGA-LIHC N = 341, P = 2.0e-4, HR = 1.61 [1.25–2.07]).
Correlation between PSMD2 and immune checkpoints
Immune checkpoint gene expression and PSMD2 correlation
We analyzed the correlation between PSMD2 expression and various immune checkpoint genes utilizing the TCGA database. The results indicated that high PSMD2 expression was positively correlated with the expression of immune checkpoint genes, including PD-1 (CD274), PDCD1, CTLA4, TIGIT, LAG3, HAVCR2, and TNF (P < 0.05). This suggests that PSMD2 may facilitate tumor immune evasion by regulating these critical immune checkpoint molecules. However, no significant correlation was observed between PSMD2 and the immune checkpoint genes CD40 and CD40LG (P > 0.05) (Fig. 4-a, b).
TIDE score and PSMD2 expression
Using the TIDE website, we assessed the relationship between PSMD2 expression and the response to immune therapy. A higher TIDE score indicates a greater likelihood of tumor immune evasion and a lower chance of benefiting from immune therapy. Our study revealed that the TIDE score was significantly lower in the PSMD2 low-expression group compared to the high-expression group (****P < 0.0001, Fig. 4-c). This suggests that patients with high PSMD2 expression may have a greater likelihood of immune evasion and a poorer response to immunotherapy. These findings provide compelling evidence that PSMD2 may play a critical role in regulating immune responses within the HCC tumor microenvironment, thereby supporting its potential as a target for immunotherapy.
(a) Visualization of PSMD2 high and low expression levels and their correlation with immune checkpoint infiltration. (b) Correlation between PSMD2 expression and immune evasion markers (CD274, CTLA4, PDCD1, TIGIT, CD40LG, LAG3, HAVCR2, TNF, CD40). (c) PSMD2 immune therapy prediction through TIDE scoring.
Correlation between PSMD2 and immune cell infiltration
CIBERSORT analysis of immune cell infiltration
Using the CIBERSORT algorithm, we analyzed the correlation between PSMD2 expression and immune cell infiltration in HCC tissues. Our results indicated that PSMD2 expression was negatively correlated with the infiltration levels of CD8 + T cells, B cells, dendritic cells, and cytotoxic cells, while showing a positive correlation with macrophages, helper T cells, and Th2 cells (Fig. 5-a). These findings demonstrate a significant association between PSMD2 expression and immune cell infiltration.
TIMER analysis of immune cell infiltration and clinical relevance
We further analyzed the correlation between PSMD2 expression and the infiltration of regulatory T cells (Tregs) (Fig. 5-b). No significant differences were observed in Tregs infiltration levels between the high and low PSMD2 expression groups in the low-expression cohort; however, a significant difference was noted in the high-expression group. Additionally, we employed TIMER software to construct a Cox proportional hazards model to evaluate the clinical relevance of Tregs infiltration, PSMD2 expression, age, gender, clinical stage, and overall survival (OS) events (Table 2). The results indicated that no significant correlation was found between PSMD2 expression and age or gender, while a significant correlation was observed with tumor stage (Stage 3: P < 0.001, Stage 4: P < 0.05), OS events (P < 0.001), and Tregs infiltration levels (P < 0.05) (Fig. 6-a, b, c, d, e, f).
Protein-protein interaction (PPI) network of PSMD2
Using the STRING database, we constructed a Protein-Protein Interaction (PPI) network of PSMD2, which includes 13 mRNA molecules: TP53, TNF, CD274, CD4, GAPDH, CD8A, PDCD1LG2, CTLA4, LAG3, HAVCR2, TIGIT, EIF4G1, and TUBA1C (Fig. 7-a). Cytoscape was employed for visualization purposes (Fig. 7-b). The analysis revealed that PSMD2 directly interacts with TP53, EIF4G1, TNF, and GAPDH, all of which play significant roles in tumorigenesis and development.
Gene set enrichment analysis (GSEA) of PSMD2
We conducted Gene Set Enrichment Analysis (GSEA) to explore the potential biological processes and signaling pathways associated with PSMD2 in HCC. The results revealed that samples exhibiting high PSMD2 expression were significantly enriched in several pathways pertinent to tumor development, including the p53 signaling pathway, ubiquitin-mediated proteolysis, insulin signaling pathway, neurotrophin signaling pathway, and other cancer-related pathways (Fig. 8-a, b, c, d) (www.kegg.jp/kegg/kegg1.html)22,23. These findings imply that PSMD2 may play a critical role in influencing essential biological processes related to tumor cell growth, proliferation, apoptosis, and metabolism.
Summary table
The result summary table of this article is presented in Table 3.
Discussion
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide, with both its incidence and mortality rates continuing to rise globally24. Although significant advancements have been made in HCC treatment strategies in recent years25, particularly with the application of immune checkpoint inhibitors (such as anti-PD-1/PD-L1 drugs), providing new therapeutic opportunities for patients with advanced HCC26, the prognosis for most patients remains poor27. It is particularly noted that repurposing existing drugs and administering various vitamins such as vitamin D as preventive measures have regulatory effects and a positive impact on HCC28.Therefore, identifying new immunotherapy targets to improve clinical outcomes in HCC patients is critically important. In this study, we used bioinformatics analyses to investigate the expression characteristics and biological functions of PSMD2, a non-ATPase regulatory subunit of the 26 S proteasome29, in HCC, with a particular focus on its potential role in regulating the tumor immune microenvironment. We conducted multiple analyses to explore the oncogenic role of PSMD2 and its correlation with immune infiltration.
PSMD2, a member of the PSMD gene family, plays a critical role in the degradation of ubiquitinated proteins30. By influencing proteasomal degradation mechanisms, it regulates cell proliferation and apoptosis processes31, and interacts with the immune system, thereby contributing to cancer development and progression32. High expression levels of PSMD2 have been linked to the malignancy and poor prognosis of various tumors33. Research indicates that PSMD2 can affect breast cancer cell proliferation and cell cycle progression by promoting the proteasomal degradation of p21 and p2734. In lung adenocarcinoma, PSMD2 expression is significantly upregulated in tumor tissues and correlates with immune cell infiltration levels35. In colorectal cancer, PSMD2 inhibits cell proliferation by blocking the NF-kappaB signaling pathway36, while in esophageal squamous cell carcinoma, it promotes tumor progression by inhibiting autophagy37. Furthermore, the role of PSMD2 in the tumor immune microenvironment (TME) has garnered increasing attention. Studies have demonstrated that PSMD2 is directly associated with immune cell infiltration and immune evasion mechanisms in the tumor microenvironment of bladder cancer38. In lung adenocarcinoma, PSMD2 expression correlates with the abundance of tumor-infiltrating lymphocytes (TILs)39. Additionally, the role of PSMD2 in cancer prognosis and immunotherapy has received significant attention; for instance, in thyroid cancer, PSMD2 is part of a four-gene prognostic model that predicts prognosis and immune therapy response40.Additionally, high expression levels of PSMD2 are positively correlated with Th2 cell infiltration and the expression of immune evasion markers in cancers, while showing a negative correlation with the infiltration of natural killer T (NKT) cells and CD8 + T cells41. These findings suggest that PSMD2 may play a significant role in reshaping the tumor microenvironment and enhancing cancer immunotherapy. Therefore, PSMD2 serves not only as an important biomarker but also as a potential therapeutic target for future cancer treatments. However, the role and mechanisms of PSMD2 within the immune microenvironment of HCC remain to be fully elucidated.
Our data indicate that PSMD2 expression is significantly elevated in HCC tissues compared to normal liver tissues. Both our immunofluorescence results and the analysis from the Human Protein Atlas (HPA) database reveal a widespread distribution of PSMD2 protein in liver cancer cells. This observation aligns with its expression pattern in other malignancies, such as lung cancer, breast cancer, and colorectal cancer, where high levels of PSMD2 expression are frequently associated with adverse clinical outcomes. This suggests that PSMD2 may act as a promoter of HCC progression. Furthermore, we observed that elevated PSMD2 expression in HCC correlates with poor prognosis, indicating that increased PSMD2 levels may serve as a potential marker for HCC development and could be utilized as a biomarker for liver cancer detection and immunotherapy.
Notably, through the analysis of the correlation between PSMD2 and immune checkpoint genes, we found that high PSMD2 expression is significantly positively correlated with immune checkpoint molecules such as PD-1, PD-L1, and CTLA4. This suggests that PSMD2 may facilitate tumor immune evasion by regulating these molecules, aligning with existing studies on the mechanisms of immune checkpoints in HCC42. One reason for the limited efficacy of immune checkpoint inhibitors is that tumors weaken the killing effect of immune cells through immune evasion mechanisms43, and high PSMD2 expression may further exacerbate this process. Targeted interventions against PSMD2 could enhance the efficacy of immune checkpoint inhibitors and improve the prognosis of HCC patients. Our study underscores the importance of PSMD2 as both a biomarker and a potential therapeutic target in HCC. High PSMD2 expression is not only closely associated with poor prognosis in HCC patients, but it may also influence the response to tumor immune therapy by regulating immune cell infiltration and immune checkpoint activity within the tumor immune microenvironment. This finding provides a theoretical foundation for developing PSMD2-targeted therapeutic strategies, particularly in immunotherapy. Future research should further explore the specific molecular mechanisms of PSMD2 in HCC and its potential functions in other tumor types to strengthen the foundation for clinical applications.
The immune microenvironment plays a crucial role in the pathogenesis of HCC. The immune microenvironment of HCC is not only complex44 but also constantly changing, comprising various immune cells such as T cells, B cells, and Kupffer cells45. These cells regulate immune tolerance in the liver, maintaining physiological homeostasis while also being exploited by tumor cells to evade immune surveillance46,47. In this study, by analyzing the relationship between PSMD2 expression and immune evasion-related genes, as well as immune cell infiltration, we found that high PSMD2 expression is associated with the expression of several immune evasion-related genes and is negatively correlated with the infiltration levels of CD8 + T cells, B cells, dendritic cells, and cytotoxic cells, while positively correlated with macrophages, helper T cells, and Th2 cells. This is consistent with previous studies, suggesting that PSMD2 may promote HCC progression by activating and mobilizing relevant genes and immune cells with immunosuppressive characteristics, in which the PSMD2 gene and its immune-associated gene network may play a key role48. Given that T cells play a vital role in the development and treatment of HCC49, regulatory T cells (Tregs) in the tumor microenvironment suppress the function of cytotoxic T cells (CD8 + T cells), leading to their exhaustion. Exhausted T cells exhibit impaired function, reduced cytokine production, diminished proliferation capacity, and weakened cytotoxicity, which hinder the effectiveness of immunotherapy50. Therefore, we further analyzed the correlation between PSMD2 and Treg cells. The results indicated that when PSMD2 expression is high, Tregs infiltration levels significantly increase, while the infiltration of CD8 + T cells, B cells, and dendritic cells decreases. Tregs in the tumor microenvironment suppress the function of effector T cells, helping tumor cells evade immune surveillance51,52. PSMD2 may facilitate the establishment of an immunosuppressive microenvironment in HCC by modulating the activity of Tregs, thereby influencing the efficacy of immunotherapy. Our findings indicate that PSMD2 may indirectly affect the mechanisms of tumor immune evasion through the regulation of immune checkpoint molecules, subsequently impacting the success of immunotherapy. Thus, PSMD2 not only contributes to the progression of HCC by altering the intrinsic characteristics of tumor cells but also exacerbates tumor advancement by manipulating immune responses within the tumor microenvironment.
To analyze the interaction between PSMD2 proteins, we constructed a protein-protein interaction (PPI) network involving PSMD2 and 13 mRNAs (TP53, TNF, CD274, CD4, GAPDH, CD8A, PDCD1LG2, CTLA4, LAG3, HAVCR2, TIGIT, EIF4G1, TUBA1C) using the STRING database. The results indicated that PSMD2 directly interacts with TP53, EIF4G1, TNF, and GAPDH. Notably, EIF4G1 and TUBA1C have been reported to be associated with immune infiltration in lung cancer, and EIF4G1 shows a significant correlation with PD-L1 expression53,54. PD-L1 is recognized as one of the most critical markers of immune evasion55 and is linked to T cell exhaustion across various cancers, including HCC56, which aligns closely with our previous findings. Furthermore, we identified TP53 as a hub protein within the PSMD2-related PPI network. TP53 is frequently referred to as the “guardian of the genome,” as it plays a pivotal role in preventing cancer development by regulating the cell cycle, apoptosis, and DNA repair57. The TP53 gene encodes the p53 protein, acknowledged as a tumor suppressor58. Previous studies have established a regulatory feedback loop between p53 and MDM2, wherein MDM2 interacts with the p53 protein, leading to its polyubiquitination and subsequent degradation by the 26 S proteasome, thereby reducing p53 levels. Activated p53 can induce the transcription of MDM2, resulting in increased MDM2 protein levels, which subsequently inhibits p53 activity by promoting its degradation59. This intricate regulation of the p53-MDM2 axis is essential for preventing unnecessary or excessive cell cycle arrest or apoptosis. Dysregulation of the p53-MDM2 interaction may lead to uncontrolled cell proliferation, contributing to tumorigenesis and tumor progression60,61. Therefore, we hypothesize that PSMD2 may influence the development and progression of HCC by participating in the degradation of p53, thereby modulating the MDM2-p53 axis.
PSMD2 is intricately involved in the recognition and binding of ubiquitinated proteins. Any aberration in the function of PSMD2 has the potential to disrupt the proteasome-mediated degradation process of p53. Moreover, PSMD2 may also participate in the ubiquitination and degradation of MDM2 through the proteasome pathway. Consequently, PSMD2 may directly impact the equilibrium of the MDM2-p53 axis by influencing both p53 and MDM2. Additionally, through its involvement in the tumor necrosis factor (TNF) signaling pathway, PSMD2 can activate the NF-κB pathway62, thereby regulating the expression of MDM2. This mechanism suggests that PSMD2 may indirectly affect the MDM2-p53 axis by modulating the NF-κB pathway. Furthermore, PSMD2 plays a role in regulating the classical Wnt signaling pathway, which is integrally involved in processes such as cell proliferation, differentiation, and apoptosis, and it also interacts with the p53 pathway63. Variations in the activity of PSMD2 can potentially influence the Wnt pathway, which may, in turn, have an indirect impact on the MDM2-p53 axis. Moreover, PSMD2 is implicated in the regulation of post-translational protein modifications, including methylation and acetylation64. These modifications can influence the transcriptional activity of the p53 and MDM2 promoters and may also affect chromatin remodeling. Alterations in chromatin structure can modulate the accessibility of transcription factors to DNA65, thereby regulating the expression of the p53 and MDM2 genes and ultimately influencing the MDM2-p53 axis. However, to date, no study has clearly delineated the direct interactions among the proteins within the PSMD2-MDM2-p53 axis. It is hoped that future research will involve experimental validation to further explore and confirm our proposed hypothesis.
Finally, we conducted pathway enrichment analysis of PSMD2 using gene set enrichment analysis (GSEA) to systematically explore the biological processes and signaling pathways in which PSMD2 may be involved. The results indicated that PSMD2 is primarily enriched in several tumor development-related pathways, including the p53 signaling pathway, the ubiquitin-mediated proteolysis pathway, the insulin signaling pathway, and the neurotrophin signaling pathway. Notably, the p53 signaling pathway plays a crucial role in regulating the cell cycle, apoptosis, and DNA repair, while the ubiquitin-mediated protein degradation system is vital for maintaining cellular homeostasis and signal transduction. This finding supports our previous hypothesis that PSMD2 may participate in the MDM2-P53 axis in HCC, where PSMD2, as a non-ATPase subunit of the 26 S proteasome, could contribute to HCC development by modulating this axis. Furthermore, PSMD2 may enhance tumor cell survival and proliferation while inhibiting tumor cell apoptosis by activating key tumor-promoting signaling pathways, such as JAK-STAT, MAPK, and PI3K-Akt66. Therefore, the elevated expression of PSMD2 may facilitate HCC progression and immune evasion by influencing these biological processes.
In conclusion, this study is the first to systematically reveal the elevated expression of PSMD2 in HCC and its critical role in regulating the immune microenvironment. PSMD2 not only serves as a potential marker of poor prognosis in HCC but may also represent a novel target for future immunotherapy. Targeting PSMD2 holds significant promise for enhancing the response to immunotherapy and improving clinical outcomes in HCC patients. These findings provide a robust scientific foundation for the development of new treatment strategies for HCC and establish a theoretical framework for future basic research and clinical translation.
This study highlights the significant role of PSMD2 in HCC. However, our findings predominantly rely on data sourced from public databases, which may introduce issues related to data heterogeneity. Therefore, in future research, we aim to further validate the correlation between PSMD2 and the clinical characteristics and prognosis of HCC patients by collecting and analyzing clinical samples, including peripheral blood and liver tissue. Additionally, our exploration of the role of PSMD2 in regulating immune checkpoint genes and immune cell infiltration is still preliminary; thus, the detailed molecular mechanisms warrant further investigation. This will provide more substantial evidence and support for a deeper understanding of the crucial role of PSMD2 in the onset and progression of hepatocellular carcinoma. Furthermore, our findings offer new insights and targets for the early diagnosis and treatment of HCC.
Conclusion
-
1.
PSMD2 is highly expressed in HCC and is closely associated with the prognosis of HCC.
-
2.
PSMD2 is significantly correlated with the infiltration of several key immune checkpoints and is related to immune evasion, making it a potential target for immunotherapy in liver cancer.
-
3.
PSMD2 expression levels are significantly associated with important immune cells in the HCC immune microenvironment, suggesting that PSMD2 may play a critical role in regulating the immune microenvironment in HCC.
-
4.
PSMD2 may influence liver cancer development by participating in the MDM2-P53 axis.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- TME:
-
Tumor microenvironment
- TILs:
-
Tumor infiltrating lymphocytes
- DEGs:
-
Differentially expressed genes
- IRGs:
-
Immune related genes
- DEIRGs:
-
Differential expressed immune-related genes
- ICI:
-
Immune checkpoint inhibitors
- PD-1:
-
Programmed cell death protein 1
- CTLA4:
-
Cytotoxic T-lymphocyte-associated protein 4
- TCGA:
-
The cancer genome atlas
- HPA:
-
Human protein atlas
- PBS:
-
Phosphate buffered saline
- BSA:
-
Bovine serum albumin
- DAPI:
-
4’,6-diamidino-2’-phenylindole
- IHC:
-
Immunohistochemical
- IOD:
-
Integrated optical density
- Tregs:
-
Regulatory T cells
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
References
Ali, N. A., Hamdy, N. M., Gibriel, A. A. & El Mesallamy, H. O. Investigation of the relationship between CTLA4 and the tumor suppressor RASSF1A and the possible mediating role of STAT4 in a cohort of Egyptian patients infected with hepatitis C virus with and without hepatocellular carcinoma. Arch. Virol. 166 (6), 1643–1651 (2021).
Youssef, S. S. & Hamdy, N. M. SOCS1 and pattern recognition receptors: TLR9 and RIG-I; novel haplotype associations in Egyptian fibrotic/cirrhotic patients with HCV genotype 4. Arch. Virol. 162 (11), 3347–3354 (2017).
Sung, H. et al. Global Cancer Statistics. : GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Cancer J. Clin. 71(3),209–249 (2021).
Eldosoky, M. A. et al. Diagnostic significance of hsa-miR-21-5p, hsa-miR-192-5p, hsa-miR-155-5p, hsa-miR-199a-5p panel and ratios in hepatocellular carcinoma on top of liver cirrhosis in HCV-Infected patients. Int. J. Mol. Sci. 24 (4), 3157 (2023).
Vogel, A., Meyer, T., Sapisochin, G., Salem, R. & Saborowski, A. Hepatocellular carcinoma. Lancet 400 (10360), 1345–1362 (2022).
RUFF, S. A. M. A. N. T. H. A. M. Highlighting novel targets in immunotherapy for liver cancer. Expert Rev. Gastroenterol. Hepatol. 16 (7–12), 1029–1041 (2022).
Li, B., Jin, J., Guo, D., Tao, Z. & Hu, X. Immune checkpoint inhibitors combined with targeted therapy: the recent advances and future potentials. Cancers (Basel). 15 (10), 2858 (2023).
Stein, S., Pishvaian, M. & Lee, M. S. et al. Safety and clinical activity of 1L atezolizumab plus bevaeizumab in a phase 1 h study in hepa-tocellula carcinoma. J Clin Oncol. (2020).
Schoenfeld, A. J. & Hellmann, M. D. Acquired resistance to immune checkpoint inhibitors. Cancer Cell. 37 (4), 443–455 (2020).
Elanany, M. M., Mostafa, D. & Hamdy, N. M. Remodeled tumor immune microenvironment (TIME) parade via natural killer cells reprogramming in breast cancer. Life Sci. 330, 121997 (2023).
Bagchi, S., Yuan, R. & Engleman, E. G. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu. Rev. Pathol. 16, 223–249 (2021).
Matsuyama, Y. et al. Proteasomal non-catalytic subunit PSMD2 as a potential therapeutic target in association with various clinicopathologic features in lung adenocarcinomas. Molcarcinogenesis 50 (4), 301–309 (2011).
Atta, H., Kassem, D. H., Kamal, M. M. & Hamdy, N. M. Targeting the ubiquitin proteasome system in cancer stem cells. Trends Cell. Biol. 35 (2), 97–101 (2025).
Yerlikaya, A., Kanbur, E., Stanley, B. A. & Tümer, E. The Ubiquitin-Proteasome pathway and epigenetic modifications in cancer. Anticancer Agents Med. Chem. 21 (1), 20–32 (2021).
Wang, S., Wang, H., Zhu, S. & Wang, Z. PSMD2 promotes the progression of bladder cancer and is correlated with immune infiltration. Front. Oncol. 12,1058506 (2022).
Tian, R. et al. RACK1 facilitates breast cancer progression by competitively inhibiting the binding of β-catenin to PSMD2 and enhancing the stability of β-catenin. Cell Death Dis. 14(10),685, Interaction between CD244 and SHP2 regulates inflammation in chronic obstructive pulmonary disease via targeting the MAPK/NF-κB signaling pathway.PLoS One. 19(10),e0312228(2024). (2023).
Li, Y. et al. Comprehensive analysis of PSMD family members and validation of PSMD9 as a potential therapeutic target in human glioblastoma. CNS Neurosci. Ther. 30 (2), e14366 (2024).
Tan, Y., Jin, Y., Wu, X. & Ren, Z. PSMD1 and PSMD2 regulate HepG2 cell proliferation and apoptosis via modulating cellular lipid droplet metabolism. BMC Mol. Biol. 20 (1), 24 (2019).
Bai, Y. et al. Dual network analysis of transcriptome data for discovery of new therapeutic targets in non-small cell lung cancer. Oncogene 42 (49), 3605–3618 (2023).
Beecroft, S. et al. Major variation in hepatocellular carcinoma treatment and outcomes in England: a retrospective cohort study. Frontline Gastroenterol. 14 (1), 19–24 (2022).
Therneau, T. M. & Grambsch, P. M. Modeling Survival Data: Extending the Cox Model (Springer, 2000).
Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M. & Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44, D457–D462 (2016).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Brown, Z. J. et al. Manage Hepatocellular Carcinoma. Rev. JAMA Surg. 158(4),410–420 (2023).
Singal, A. G., Kanwal, F. & Llovet, J. M. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat. Rev. Clin. Oncol. 20 (12), 864–884 (2023).
Xiong, Q. et al. ATG16 mediates the autophagic degradation of the 19S proteasomal subunits PSMD1 and PSMD2. Eur. J. Cell. Biol. 97 (8), 523–532 (2018).
Tian, S. et al. A viral movement protein targets host catalases for 26S proteasome-mediated degradation to facilitate viral infection and aphid transmission in wheat. Mol. Plant. 17 (4), 614–630 (2024).
Chen, Y. C. et al. Effect of vitamin D supplementation on primary dysmenorrhea: A systematic review and Meta-Analysis of randomized clinical trials. Nutrients 15 (13), 2830 (2023).
Bashore, C. et al. Targeted degradation via direct 26S proteasome recruitment. Nat. Chem. Biol. 19 (1), 55–63 (2023).
Zaky, W. et al. The ubiquitin-proteasome pathway in adult and pediatric brain tumors: biological insights and therapeutic opportunities. Cancer Metastasis Rev. 36 (4), 617–633 (2017).
Inui, N., Sakai, S. & Kitagawa, M. Molecular pathogenesis of pulmonary fibrosis, with focus on pathways related to TGF-β and the Ubiquitin-Proteasome pathway. Int. J. Mol. Sci. 22 (11), 6107 (2021).
Xu, X. et al. PSMD2 overexpression as a biomarker for resistance and prognosis in renal cell carcinoma treated with immune checkpoint and tyrosine kinase inhibitors. Cell. Oncol. (Dordr). 47 (5), 1943–1956 (2024).
Li, Y. etal. PSMD2 regulates breast cancer cell proliferation and cell cycle progression by modulating p21 and p27 proteasomal degradation. Cancer Lett. 430, 109–122 (2018).
Zhang, Z. et al. Asporin promotes cell proliferation via interacting with PSMD2 in gastric cancer.front biosci. (Landmark Ed). 24 (6), 1178–1189 (2019).
Ying, K. Diverse Ras-related GTPase DIRAS2, downregulated by PSMD2 in a proteasome-mediated way,inhibits colorectal cancer proliferation by blocking NF-κB signaling. Int. J. Biol. Sci. 18 (3), 1039–1050 (2022).
Liu, Y. et al. PSMD2 contributes to the progression of esophageal squamous cell carcinoma by repressing autophagy. Cell. Biosci. 13, 67 (2023).
Salah Fararjeh, A., Al-Khader, A., Al-Saleem, M. & Abu Qauod, R. The prognostic significance of proteasome 26S subunit, Non-ATPase (PSMD) genes for bladder urothelial carcinoma patients. Cancer Inf. 20,11769351211067692 (2021).
Zhao, H. & Lu, G. Prognostic implication and immunological role of PSMD2 in lung adenocarcinoma. Front. Genet. 13, 905581 (2022).
Li, C. W. etal.A 4 Gene-based immune signature predicts dedifferentiation and immune exhaustion in thyroid cancer. J. Clin. Endocrinol. Metab. 106 (8), e3208–e3220 (2022).
Xuan, D. T. M. et al. Prognostic and immune infiltration signatures of proteasome 26S subunit, non-ATPase (PSMD) family genes in breast cancer patients. Aging (Albany NY). 13 (22), 24882–24913 (2021).
Fu, Y., Liu, S., Zeng, S. & Shen, H. From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 38 (1), 396 (2019).
Sheng, Q. J. et al. Programmed death 1, ligand 1 and 2 correlated genes and their association with mutation, immune infiltration and clinical outcomes of hepatocellular carcinoma. World J. Gastrointest. Oncol. 12 (11), 1255–1271 (2020).
Lin, J., Rao, D., Zhang, M. & Gao, Q. Metabolic reprogramming in the tumor microenvironment of liver cancer. J. Hematol. Oncol. 17 (1), 6 (2024).
Agirre-Lizaso, A. et al. Targeting the heterogeneous Tumour-Associated macrophages in hepatocellular carcinoma. Cancers (Basel). 15 (20), 4977 (2023).
Xue, R. et al. Liver Tumour Immune Microenvironment Subtypes Neutrophil Heterogeneity Nat. 612(7938),141–147 (2022).
Kurebayashi, Y. & etal. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology 68 (3), 1025–1041 (2018).
Zhang, Q. et al. DNAJA4 suppresses epithelial-mesenchymal transition and metastasis in nasopharyngeal carcinoma via PSMD2-mediated MYH9 degradation. Cell. Death Dis. 14 (10), 697 (2013).
Hofmann, M., Tauber, C., Hensel, N. & Thimme, R. CD8 + T cell responses during HCV infection and HCC. J. Clin. Med. 10 (5), 991 (2021).
Dituri, F. et al. Direct and indirect effect of TGFβ on Treg transendothelial recruitment in HCC tissue microenvironment. Int. J. Mol. Sci. 22 (21), 11765 (2021).
Sangro, B., Sarobe, P., Hervás-Stubbs, S. & Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 18 (8), 525–543 (2021).
Llovet, J. M. et al. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 19 (3), 151–172 (2022).
Thorsson, V. et al. The immune landscape of cancer [published correction appears in immunity. 51(2),411–412 (2019).
Tomida, S. et al. Identification of a metastasis signature and the DLX4 homeobox protein as a regulator of metastasis by combined transcriptome approach. Oncogene 26, 4600–4608 (2007).
Doroshow, D. et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 18 (6), 345–362 (2021).
Necchi, A. etal.Comprehensive assessment of immuno-oncology biomarkers in adenocarcinoma, urothelial carcinoma, and squamous-cell carcinoma ofthe bladder. Eur. Urol. 77 (4), 548–556 (2020).
Toledo, F. & Wahl, G. M. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat. Rev. Cancer. 6 (12), 909–923 (2006).
Vazquez, A., Bond, E. E., Levine, A. J. & Bond, G. L. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat. Rev. Drug Discov. 7 (12), 979–987 (2008).
Lin, W., Yan, Y., Huang, Q. & Zheng, D. MDMX in cancer: A Partner of P53 and a P. Indep. Effector Biol. 18, 53 (2024).
Aguilar, A. & Wang, S. Therapeutic strategies to activate p53. Pharmaceuticals (Basel). 16 (1), 24 (2022).
Koo, N., Sharma, A. K. & Narayan, S. Therapeutics targeting p53-MDM2 interaction to induce cancer cell death. Int. J. Mol. Sci. 23 (9), 5005 (2022).
Kung, C. P. & Weber, J. D. It’s Getting Complicated-A Fresh Look at p53-MDM2-ARF Triangle in Tumorigenesis and Cancer Therapy. Front. Cell. Dev. Biol. 10,818744 (2022).
Dhingra, R. et al. Proteasomal degradation of TRAF2 mediates mitochondrial dysfunction in Doxorubicin-Cardiomyopathy [published correction appears in circulation. 146(12)934–954 (2022).
Simon, M., Grandage, V. L., Linch, D. C. & Khwaja, A. Constitutive activation of the Wnt/beta-catenin signalling pathway in acute myeloid leukaemia. Oncogene 24 (14), 2410–2420 (2005).
Collins, G. A. & Goldberg, A. L. The logic of the 26S proteasome. Cell 169 (5), 792–806 (2017).
Audia, J. E. & Campbell, R. M. Histone modifications and cancer. Cold Spring Harb Perspect. Biol. 8 (4), a019521 (2016).
Shorning, B. Y., Dass, M. S., Smalley, M. J. & Pearson, H. B. The PI3K-AKT-mTOR pathway and prostate cancer: at the crossroads of AR, MAPK, and WNT signaling. Int. J. Mol. Sci. 21 (12), 4507 (2020).
Acknowledgements
Not applicable.
Funding
This study was supported by the Fund for the Key R&D Programmes of Ningxia (2022CMG02039) and the Natural Science Foundation of Ningxia (2022AAC02065). These funding bodies played no role in study design, data collection and analysis, and decision-making regarding publishing or preparing the manuscript.
Author information
Authors and Affiliations
Contributions
Li-Na Ma and Xiang-Chun Ding conceived the study. Mei-ying Gu, Wan-long Ma,Zinming Ma and Xiang-Chun Ding drafted the manuscript and critically revised it for important intellectual content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gu, MY., Ma, WL., Ma, Zm. et al. Expression of PSMD2 gene in hepatocellular carcinoma and its correlation with immune checkpoints and prognosis. Sci Rep 15, 10111 (2025). https://doi.org/10.1038/s41598-025-94504-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-94504-1