Abstract
This study aims to investigate the causal relationship between Body Mass Index (BMI) and the severity of Psoriasis (PsO) using bidirectional Mendelian Randomization (MR) and regression analyses. We conducted a multicenter study which combined bidirectional MR analyses with regression analyses. The MR analyses included 366,776 individuals from the largest up-to-date published BMI Genome-Wide Association Study (GWAS) data. Regression analyses were performed on 1,979 patients with psoriasis from 12 participating centers (from October 31, 2019, to May 31, 2022). We assessed the impact of BMI on PsO severity using odds ratios (ORs) and regression coefficients for three key measures: the Psoriasis Area and Severity Index (PASI), Body Surface Area (BSA), and Dermatology Life Quality Index (DLQI). Two independent MR analyses revealed a significant causal association between BMI and PsO development. The first MR1 analysis showed that an increased BMI is significantly associated with a higher risk of psoriasis, with odds ratios of 2.28 (95% CI 1.33–3.92; p = 0.003). A subsequent MR2 analysis yielded consistent results, presenting an odds ratio of 2.37 (95% CI 1.16–4.85; p = 0.018) using the inverse-variance weighted method. Logistic regression showed that for every 1-unit increase in BMI (unadjusted covariates), the risk of severe psoriasis (PASI ≥ 10, BSA ≥ 10%, DLQI ≥ 10) increased by 6%, 6%, and 3%, respectively. Linear regression analysis revealed that each unit increase in BMI (not standardised) was associated with an increase of 0.25 units in the mean PASI score (p < 0.001), 0.34 units in the BSA score (p = 0.001), and 0.14 units in the DLQI score (95% CI 0.05–0.23; p = 0.001). From both the genetic and clinical severity assessment perspectives, it has been verified that abnormal weight gain is correlated with the severity of the condition in psoriasis patients. Clinicians should prioritize weight management and nutritional balance in the management of psoriatic disease. Clinicaltrials.gov: ChiCTR1900024852, date of registration: 2019-07-31.
Similar content being viewed by others
Introduction
Psoriasis (PsO) represents a spectrum of chronic inflammatory conditions, with plaque psoriasis (psoriasis vulgaris) being the most common cutaneous manifestation1,2. This condition affects approximately 2–4% of the global population2, impacting not only physical manifestations but also significantly impairing quality of life and psychological well-being3.
The prevalence of obesity has been increasing at an alarming rate, with more than 5% of children has been increasing at an alarming rate, representing a major public health challenge in the twenty-first century4,5,6,7. Studies have linked obesity to various comorbidities, including diabetes, hypertension, and rheumatic diseases8. PsO ofen leads to reduced physical activity among affected individuals, which may contribute to weight gain2,3,9. Furthermore, although research has indicated a potential link between obesity and psoriasis10, the precise nature of this association, particularly its directionality and magnitude, remains unclear.
Furthermore, although research has indicated a potential link between obesity and psoriasis, the precise nature of this association, particularly its directionality and magnitude, remains unclear11,12. Recent meta-analyses and Mendelian Randomization (MR) studies have demonstrated a causal effect of elevated body mass index (BMI) on the development of psoriatic disease manifestations (cutaneous and musculoskeletal domains)8,13,14. However, these studies have only established that BMI can causally contribute to the onset of psoriasis, without addressing the relationship between BMI and psoriasis severity or stratifying BMI categories. A comprehensive investigation of the obesity-psoriasis relationship is crucial for developing effective prevention and treatment strategies to address this public health issue. MR analysis enables the investigation of causal relationships by utilizing genetic variations to randomly assign individuals to genotype groups, analogous to randomized controlled trials. Logistic regression is employed to predict the probability of an event occurring, particularly when the dependent variable is categorical15. Linear regression is used to establish a linear relationship between one or more independent variables and a continuous dependent variable16. These methods provide a robust theoretical and methodological foundation for analyzing causality and trend correlations.
In this study, we employed a combination of bidirectional Mendelian randomization, linear regression, and logistic regression, leveraging extensive genetic data and clinical patient samples to investigate the potential causal relationship between BMI and psoriasis susceptibility. The findings offer valuable insights into clinical psoriasis management strategies and are expected to inform personalized treatment approaches, improve patient outcomes, and ultimately enhance the quality of life for individuals with psoriasis.
Methods
Study design
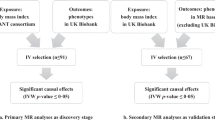
Our study aimed to investigate the relationship between BMI and PsO through a genetic epidemiologic approach complemented by observational analysis. Initially, a bidirectional MR analysis was undertaken to estimate the direct causal effect of BMI on PsO (Fig. 1A). Three key hypotheses were tested in our analysis: (1) Instrumental variables (IVs) were highly correlated with BMI subtypes, ensuring a robust genetic instrument for the analysis; (2) IVs were independent of confounding factors between BMI and PsO, confirming the absence of external influences on the observed relationship; and (3) IVs did not influence PsO through pathways other than BMI, validating the direct causal effect of BMI on PsO. The first analysis explored the causal relationship with BMI as the exposure and PsO as the outcome, while the second analysis examined the reverse causality, using PsO as the exposure and BMI as the outcome. Subsequently, we performed an extensive, multicenter retrospective analysis of PsO patients (Fig. 1B).
(A) Schematic diagrams of mendelian randomization studies. (a) Panel a shows forward Mendelian studies where Body Mass Index (BMI) is used as an instrumental variable to assess the causal effect of BMI on psoriasis, considering potential confounders. (b) Panel b depicts reverse Mendelian studies with psoriasis as the instrumental variable to determine its effect on BMI, also accounting for confounders. In Mendelian analysis 2, a Mendelian randomization study was conducted again with BMI as an exposure variable to assess its effect on psoriasis and to infer the causal relationship between genetic markers (BMI) and psoriasis outcomes, reducing sampling bias factors that often occur in observational studies. (B) Flowchart of analytical methods in cohort observational analysis. The flowchart shows the analytic approach used to assess the severity of psoriasis in a cohort observational study. On the left, severity of psoriasis was assessed using three criteria: PASI ≥ 10, BSA ≥ 10% and DLQI ≥ 10, followed by logistic regression analysis in which BMI was used as a continuous and categorical variable, respectively. On the right side, the severity of psoriasis was assessed directly by PASI, BSA, and DLQI metrics, followed by linear regression analyses, also using BMI as a continuous and categorical variable, respectively. BMI was grouped by using the BMI classification of the Chinese reference standard.
The "rule of 10s" classification is utilized to determine the severity of PsO. Under this standard, patients are categorized as having severe or moderate-to-severe psoriasis if they fulfill one or more of the following criteria: a Psoriasis Area and Severity Index (PASI) of 10 or above, over 10% Body Surface Area (BSA) involvement, or a Dermatology Life Quality Index (DLQI) exceeding 101,17,18. This standardized system offers a more objective evaluation of the disease’s impact on patients. Obesity is diagnosed through the calculation of Body Mass Index (BMI), defined as weight (in kilograms) divided by the square of height (in meters)8,12,19. BMI is the most widely used and recognized measure for assessing obesity8,19.
Patients were divided into groups based on the PASI: PASI < 10 and PASI ≥ 10. Similarly, categorization was also performed based on BSA (BSA < 10%, BSA ≥ 10%) and the DLQI (DLQI < 10, DLQI ≥ 10). According to the World Health Organization’s classification standards for BMI20,21, we categorized BMI into 5 levels (categorized as < 18.5 kg/m2 [BMI_group1], 18.5–24 kg/m2 [BMI_group2], 24–28 kg/m2 [BMI_group3], 28–30 kg/m2 [BMI_group4], and ≥ 30 kg/m2 [BMI_group5]). We used logistic regression to analyze the correlation between BMI (as both a continuous variable and in categorized groups) and PASI, BSA, and DLQI. Linear regression was applied to further analyze the correlations between BMI and these PsO severity indices.
Data source
For this study, we obtained our genome-wide association study (GWAS) data from two reputable sources: the European Bioinformatics Institute (EBI) OpenGWAS database and the FinnGen online database. The EBI GWAS Catalog offered extensive information that was especially valuable for genetic research related to body mass index (BMI), aiding in the understanding of genetic factors that influence body weight. We accessed a broad spectrum of BMI-related genetic data from the EBI open database, including data1, which had a sample size of 330,793 individuals (accession number: ebi-a-GCST90095039 for BMI1), and data2, which consisted of 359,983 individuals of European ancestry (accession number: ebi-a-GCST90018947 for BMI2). The first dataset included 2,401,077 SNPs (PMID: 35,399,580), while the second dataset included 19,066,885 SNPs (PMID: 34,594,039). Additionally, for PsO, we employed data (finn-b-PSORI_STRICT) from a meta-analysis, specifically using a stringent definition based on the International Classification of Diseases (ICD-10) criteria (L40-L400). This dataset included 334 cases with PsO and 212,242 controls of European descent.
SNPs selection
In this study, we identified single-nucleotide polymorphisms (SNPs) robustly associated with BMI and PV, achieving genome-wide significance at a P-value threshold of 5 × 10–8. To ensure the independence of the selected SNPs, those exhibiting linkage disequilibrium (LD) with an r^2 value exceeding 0.001 within a 10,000 kb clumping window were excluded. The strength of our instrumental variables (IVs) was evaluated using an F statistic greater than 10, a criterion established to minimize the influence of weak IVs on causal inference. The F statistic was calculated using the formula: F = (R2/(1 − R2)) × ((n − k − 1)/k)22, where 'n' denotes the sample size,‘k’ represents the number of IVs, and 'R^2' signifies the proportion of variance explained by the IVs. The ‘R2’ was derived from the minor allele frequency (MAF) and the β-value, computed as R2 = 2 × MAF × (1 − MAF) × β222. All IVs included in our analysis demonstrated F values surpassing 10. During the harmonization process, ambiguous and palindromic SNPs were eliminated to ensure clarity and accuracy. To mitigate potential pleiotropy, the PhenoScanner V2 database (http://www.phenoscanner.medschl.cam.ac.uk/) was employed to exclude IVs associated with confounding factors or known risk factors for BMI. Ultimately, 423 SNPs for BMI1, 108 SNPs for BMI2, and 382 SNPs for PV were selected as IVs for subsequent MR analysis.
Mendelian randomization analyses
In our study, the inverse variance weighted (IVW) approach served as the primary analytical method, selected for its stability when dealing with balanced pleiotropy. To enhance our analysis, we also incorporated supplementary methods, including MR-Egger regression, the weighted median approach, and the MR-PRESSO test. These additional techniques were crucial in estimating the causal relationship under diverse conditions, as elaborated in references1,10,23,24,25,26,27,28,29,30,31,32,33. We reported our outcomes as odds ratios (OR) with 95% confidence intervals (CI), considering a P-value of less than 0.05 as indicative of statistical significance.
Study population
The trial was registered with the WHO International Clinical Trials Registry Platform Level 1 Registry (China Clinical Trials Registry, https://www.chictr.org.cn/index.html) under the registration number: ChiCTR1900024852. The clinical study was approved by the Research Ethics Committee of the University of Hong Kong Shenzhen Hospital and Union Shenzhen Hospital, Huazhong University of Science and Technology, and the Health Research Ethics Approval and Subject Consent Waiver [2019] 181) from various sub-centres of other hospitals involved in the study. and the study complied with the Declaration of Helsinki for research involving human participants. The clinical characteristics of the patients in our study were derived from the preliminary diagnostic information in the Multicentre Comprehensive Database of Chinese Patients with PsO. The patients in our study were clinically derived from patients with newly diagnosed plaque psoriasis with a preliminary diagnosis from the Multicentre Comprehensive Database of Chinese Patients with PsO. Exclusion criteria included psoriasis patients who had already been treated with any type of biologics.We finally studied 1,979 patients with diagnosed plaque psoriasis (from 31 October 2019 to 31 May 2022) from 12 hospitals across China.
Sensitivity analysis
In our sensitivity analyses, we focused on identifying and addressing potential horizontal pleiotropy, a scenario where the instrumental variable (IV) associated with the exposure influences the outcome through pathways not directly related to the exposure. To this end, we employed the MR-Egger intercept test. A significant intercept (p < 0.05) in this test suggests the presence of horizontal pleiotropy and requires careful interpretation of the findings. The results of the MR-Egger intercept test were visualized using scatterplots for better clarity. Additionally, we used Cochran’s Q statistics to evaluate heterogeneity among the included studies. Significant heterogeneity (p < 0.05) calls for cautious interpretation, and we represented these findings with funnel plots. To further address horizontal pleiotropy, the MR-PRESSO outlier test was utilized to detect and exclude anomalous SNPs (outliers), facilitating the estimation of corrected results. In our culling analysis, we sequentially removed each SNP for reanalysis, visualizing this process through forest plots to intuitively judge the robustness and reliability of our study’s results.
Cohort study between BMI and psoriasis
Our study involved an extensive, multicenter retrospective analysis, encompassing newly diagnosed PsO patients from 12 hospitals across China. The clinical characteristics of these patients were derived from initial diagnostic information, sourced from a comprehensive, multicentric database of PsO patients. We adhered to the guidelines of the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) statement for transparent reporting of multivariable prediction model development and validation. Ethical approval for health research and waivers of participant consent were obtained from the Ethics Committee of the University of Hong Kong-Shenzhen Hospital and the respective subcenters of the other hospitals involved. Informed consent was also obtained from all participating patients. The analysis included a total of 1,979 cases, recorded from October 31, 2019, to May 31, 2022. We categorized patients based on PASI (1,011 cases with PASI < 10 and 968 cases with PASI ≥ 10), BSA (725 cases with BSA < 10% and 1,254 cases with BSA ≥ 10%), and DLQI (767 cases with DIQI < 10 controls, 1,212 cases with DLQI ≥ 10).
Statistical analysis
In our study, continuous variables were represented using mean ± standard deviation (mean ± SD). Specifically, the BMI group, a key categorical variable, was presented in terms of frequencies and percentages (%). To analyze the correlations between BMI and other variables, such as PASI, BSA, and DLQI, we employed both linear and logistic regression methods. When both the independent and dependent variables are continuous, standardization was applied to both. In cases where the independent variable is continuous and the dependent variable is categorical, only the independent variable was standardized. Multiple models were constructed, each adjusting for a different set of covariates to provide a nuanced understanding of how these covariates influence the observed association. Model 1 provided an unadjusted estimate, with no covariates included. In Model 2, adjustments were made for Age to account for potential confounding factors. In Model 3, adjustments were made for Sex to account for potential confounding factors. In Model 4, adjustments were made for combined diseases to account for potential confounding factors. In Model 5, adjustments were made for treatment effect to account for potential confounding factors. The results of each model were compared to assess the consistency of the effect estimates under varying levels of covariate adjustment, thereby providing insights into the robustness of the impact of BMI on PASI. All statistical analyses were performed using the TwoSampleMR (version 0.5.7), MendelianRandomization (version 0.8.0), and MRPRESSO package (version 1.0) within R Software 4.3.1, which is accessible at https://www.R-project.org.
Results
Mendelian randomization analyses
Effect of BMI on psoriasis
Finally, we further explored the relationship between BMI and PsO using MR study.
In MR analysis1, according to the IVW method, BMI was associated with a higher risk of PsO (OR 2.28, 95% CI 1.33–3.92, p = 0.003). The MR-PRESSO method yielded consistent significant results (OR 2.28, 95% CI 1.33–3.92, p = 0.003). Both MR-Egger (OR 2.40, 95% CI 0.57–10.19, p = 0.235). Weighted median (OR 1.54 95% CI 0.57–4.14, p = 0.390), and Weighted mode (OR 1.87, 95% CI 0.42–8.27, P = 0.407) showed consistent direction but insignificant results.
In MR analysis 2, the IVW method again confirmed a significant association between BMI and psoriasis risk (OR 2.37 95% CI 1.16–4.85, p = 0.018), with the MR-PRESSO method further supporting this finding (OR 2.37, 95% CI 0.16–4.85, p = 0.020) also showed significanct results. Both MR-Egger (OR 2.95, 95% CI 0.52–16.94, p = 0.227), Weighted median (OR 1.61 95% CI 0.52–5.00, p = 0.412), and Weighted mode (OR 2.11, 95% CI 0.52–8.51, p = 0.298) showed a consistent direction but not statistically significant results.
Taking together, the results from both MR analyses robustly highlight a significant causal effect of BMI on the development of psoriasis.
Effect of psoriasis on BMI
We also conducted a reverse MR analysis between psoriasis and BMI, but the IVW method did not reveal any reverse causal relationships.
Sensitivity analysis
Sensitivity analysis1: We conducted Cochran’s Q statistics and its funnel plots, the MR-Egger intercept test and its scatter plots, the leave-one-out analysis and its forest plots in the sensitivity analysis. Moreover, MR-PRESSO test was used to correct for heterogeneity. The analyses of BMI on PsO revealed no significant heterogeneity (Q = 386.46, p = 0.161 for IVW). There was no evidence of directional pleiotropy (intercept = − 0.0009, p = 0.941, Supplementary Table). After outliers were removed, the association was insignificant (T test: 388.48, p = 0.171). The leave-one-out analyses demonstrated the robustness of the findings.
Sensitivity analysis2: We conducted Cochran’s Q statistics and its funnel plots, the MR-Egger intercept test and its scatter plots, the leave-one-out analysis and its forest plots in the sensitivity analysis. Moreover, MR-PRESSO test was used to correct for heterogeneity. The analysis of BMI on PsO revealed no significant heterogeneity (Q = 118.70, p = 0.170 for IVW). There was no evidence of directional pleiotropy (intercept = − 0.006, p = 0.788). After outliers were removed, the association was significant (p = 0.182), and the value of Nb-Distribution was 10,000. The leave-one-out analyses demonstrated the robustness of the findings.
The funnel plots of the MR analysis’ Cochran’s Q statistics are displayed in the supplementary materials and depicted the scatter plots of the causal relationships between BMI and risk of PsO, as well as the forest plots of the leave-one-out analyses, respectively. Through MR -based research, we identified BMI as a crucial risk factor for PsO.
The IVW method in the MR analysis revealed no reverse causality between PsO and BMI. As a result, additional heterogeneity and pleiotropy testing was not required.
Cohort observational analysis between BMI and psoriasis
Logistic regression analysis (Fig. 2)
Restricted cubic strip chart (RCS) curves of the relationship between BMI and psoriatic disease. The upper row of graphs shows the logistic regression method, showing the relationship between BMI and PASI ≥ 10 (A), BSA ≥ 10% (B), and DLQI ≥ 10 (C), respectively. The coordinates are BMI and the vertical coordinate is the OR value with a reference value of 1. The next row is a linear regression approach showing the relationship between BMI and PASI (D), BSA (E), and DLQI (F), respectively. The horizontal coordinate is BMI and the vertical coordinate is the coefficient beta (95% CI) with a reference value of 0. PASI psoriasis area and severity index, BSA body surface area, DLQI dermatologic quality of life index, BMI body mass index.
Initially, logistic regression analyses were conducted to examine the association between BMI—both as a continuous measure and in categorized groups—and the severity of psoriatic disease, (as measured by PASI score ≥ 10, BSA ≥ 10%, and DLQI ≥ 10, respectively). For analyses involving BMI groups, these were treated as categorical variables with group 2 (18.5 ≤ BMI < 24 kg/m2) serving as the reference.
When BMI was treated as a continuous variable, each one‐unit increase was significantly associated with higher odds of being classified in the PASI group. After adjusting for various covariates, the ORs ranged from 1.04 to 1.06 (p < 0.001 in all models, except model 3 where p = 0.003). Similarly, with standardized BMI, each one standard deviation increase was linked to a notable rise in the risk of being in the PASI group: an OR of 1.15 (95% confidence interval [CI] 1.05–1.27, p = 0.003) in model 3 and ORs of 1.25 (95% CI 1.14–1.37, p < 0.001) in the other models.
Regarding the BSA outcome, every one‐unit increase in continuous BMI conferred a 4%–6% higher risk (OR range: 1.04–1.06), with all models showing statistical significance (p < 0.001 or p = 0.005). For standardized BMI, each standard deviation increase was associated with an estimated 15%–24% increase in risk (OR range: 1.15–1.24) across different models (p < 0.001 or p = 0.005).
For the DLQI outcome, the results revealed a significant positive association with BMI. Specifically, each one‐unit increase in BMI resulted in 3% higher odds of having a DLQI score ≥ 10 (OR: 1.03; 95% CI 1.01–1.06, with p-values ranging from 0.008 to 0.024). When examined as a standardized variable, each standard deviation increases corresponded to 12% higher odds of a DLQI score ≥ 10 (OR range: 1.11–1.13; 95% CI 1.02–1.24, p-values ranging from 0.008 to 0.024). These associations remained consistent even after adjustments for age, sex, comorbidities, and treatment effects. Collectively, the findings indicate a robust and independent relationship between higher BMI and increased risk of severe psoriasis across the PASI, BSA, and DLQI outcomes.
Logistic regression analyses confirmed that—except for the underweight group (BMI group 1)—higher BMI categories were significantly associated with increased odds of adverse outcomes (PASI, BSA, and DLQI groups), demonstrating a significant dose–response trend (trend p < 0.001).
PASI: Compared to the reference group (18.5 ≤ BMI < 24 kg/m2), patients in BMI group 3 (24 ≤ BMI < 28 kg/m2) exhibited a 48% increase in odds (OR: 1.48, 95% CI 1.21–1.82, p < 0.001), those in BMI group 4 (28 ≤ BMI < 30 kg/m2) had a 59% increase (OR: 1.59, 95% CI 1.16–2.17, p = 0.004), and patients in BMI group 5 (BMI ≥ 30 kg/m2) showed a 104% increase (OR: 2.04, 95% CI 1.42–2.94, p < 0.001). In contrast, BMI group 1 showed no significant association with the outcomes (OR range: 1.22–1.40; all p > 0.05).
In this analysis, the BMI group was significantly associated with BSA group across multiple models. Using BMI group 2 as the reference, participants in BMI group 3 consistently exhibited increased odds of the outcome (OR range: 1.20–1.35; p ≤ 0.100), a finding that remained significant in most models after adjustment for covariates, including age, sex, comorbidities, and treatment effects. Similarly, individuals in BMI group 4 showed a marked increase in odds (OR range: 1.53–1.70; p ≤ 0.014) across all models, reinforcing the robust relationship with the outcome. BMI group 5 was also significantly associated with the outcome in most models (OR range: 1.36–1.59; p ≤ 0.117), although the effect size diminished slightly after covariate adjustment. In contrast, BMI group 1 did not exhibit a significant association with the outcome (OR range: 1.02–1.16; p ≥ 0.502). These results indicate that higher BMI categories are independently associated with increased odds of the outcome, even after controlling for potential confounders.
We evaluated the relationship between BMI group and DLQI group across different models. In Model 1, where no covariates were adjusted, Group 4 of BMI group (OR = 1.39, 95% CI 1.01–1.93, p = 0.047) and Group 5 (OR = 1.40, 95% CI 0.97–2.04, p = 0.078) exhibited a higher risk associated with DLQI group. Although the result of Group 5 was only close to the significance level. After further adjusting for age (Model 2), gender (Model 3), comorbidities (Model 4), and treatment effects (Model 5), the association of Group 4 remained significant. In addition, the associations of Group 1 (OR range: 0.87–0.94) and Group 3 (OR range: 1.14–1.18) were not statistically significant in all models (p > 0.05). These results indicate that a higher BMI group, especially Group 4, is significantly associated with an increased risk of DLQI group, and this association remains robust after adjusting for multiple covariates.
Linear regression (Fig. 2)
Subsequently, by linear regression, we observed the relationships between BMI and three psoriasis severity scores (PASI, BSA and DLQI), respectively.
When BMI was considered as a continuous variable, the results of the linear regression analysis showed a significant positive linear correlation between an increase in BMI and an increase in PASI (p < 0.001). Specifically, a one—unit increase in the continuous variable BMI was significantly associated with an increase in the PASI score. The β coefficients were 0.25 (95% CI: 0.14, 0.36, p < 0.001), 0.15 (95% CI 0.04, 0.27, p = 0.007), and 0.24–0.25 (95% CI 0.14, 0.35 and 0.15, 0.36 respectively, p < 0.001) in different models. For each standard—deviation increase in the standardized BMI, the change in the PASI score remained relatively stable across the models, ranging from 0.06 to 0.10, and all models showed statistical significance (p < 0.001 or p = 0.007). After adjusting for different covariates, the effect estimates of BMI on PASI were fairly consistent in most models. However, after adjusting for gender, the effect was slightly attenuated (β = 0.15, p = 0.007), suggesting that gender may have some influence on this relationship.
For BSA, unstandardized BMI, each 1-unit increase was linked to a beta coefficient of approximately 0.33–0.35 (p < 0.01) in models without sex adjustment, but this effect dropped to 0.20 (p = 0.072) once sex was included. Similarly, every 1-SD increase in standardized BMI produced a beta around 0.07 (p < 0.01) in most models, which declined to 0.04 (p = 0.072) with sex adjustment.
Multivariable regression analyses for DLQI revealed that each unit increase in BMI significantly raised DLQI scores, with beta coefficients between 0.12 and 0.15 (all p < 0.01). The standardized BMI analyses produced similar results, with beta coefficients ranging from 0.06 to 0.08 (all p < 0.01). These associations remained robust even after sequential adjustment for covariates including age, sex, comorbidities, and treatment effects.
In addition, when BMI was categorized, using BMI group 2 (18.5 ≤ BMI < 24 kg/m2) as the reference, subjects with BMI < 18.5 kg/m2 did not show statistically significant differences (p > 0.05). In contrast, higher BMI groups—BMI group 3 (24 ≤ BMI < 28 kg/m2), BMI_group 4 (28 ≤ BMI < 30 kg/m2), and BMI group 5 (≥ 30 kg/m2)—remained statistically significant even after sequentially adjusting for confounders such as age, sex, comorbidities, and treatment effects in PASI, BSA, and DLQI scores across models.
Discussion
Psoriasis is a systemic inflammatory condition that manifests through various presentations, with plaque psoriasis (psoriasis vulgaris) being its most common cutaneous manifestation. The rising incidence of psoriasis and obesity are pressing public health concerns, posing a significant challenge for dermatology clinicians. Understanding the interplay between these conditions is crucial for developing effective treatment strategies. Our study aimed to estimate the impact of BMI on the risk of severe psoriatic disease using a combined approach of genetic epidemiology and cohort studies, offering a comprehensive insight into this complex relationship.
The first analysis incorporated the largest up-to-date published BMI GWAS data to assess the direct causal effect of BMI on psoriatic disease (Fig. 1A). To further validate our findings, we re-examined the association using 2021 BMI data. Both MR analyses consistently indicated a causal link between increasing BMI and an elevated risk of developing PsO (p < 0.05 in the IVW approach).
In addition, we bolstered our findings with regression analyses by combining logistic regression and linear regression approaches on 1,979 patients with psoriasis from 12 multicenter cohorts in China. These analyses demonstrated that, for every 1-unit increase in BMI, the risk of severe psoriasis increased by 6% for PASI ≥ 10, by 6% for BSA ≥ 10%, and by 3% for DLQI ≥ 10. Together, the robust results from our two MR analyses, supported by the regression models, underscore the significant impact of BMI on the development and severity of psoriatic disease. In line with our results, a Korean cohort study of 399,461 psoriasis patients with abnormal versus normal BMI showed that patients with BMI > 30 kg/m2 had a higher risk of psoriasis than those with normal weight (HR 1.118; 95% CI 1.100–1.137)34. Particularly compelling evidence comes from interventional studies examining the impact of weight reduction through bariatric surgery. Egeberg et al.6 demonstrated in a Danish nationwide population-based cohort study that bariatric surgery, especially gastric bypass, showed protective effects across the psoriatic disease spectrum: reducing the risk of new-onset psoriasis (adjusted HR, 0.52; 95% CI 0.33–0.81; P = 0.004), progression to severe psoriasis (adjusted HR, 0.44), and development of psoriatic arthritis (adjusted HR, 0.29)6. Similarly, Maglio et al.35 observed in their longitudinal cohort study that surgical weight reduction led to a decreased incidence of psoriatic disease35. These interventional findings provide crucial support for our MR analyses by demonstrating that targeted BMI reduction can modify disease risk across multiple domains of psoriatic disease, strengthening the evidence for a causal relationship between obesity and psoriatic disease development and progression.
While the precise mechanisms by which obesity affects psoriatic disease remain unclear, emerging studies have shed light on the molecular parallels between obesity-induced inflammation and the inflammatory processes underlying psoriatic disease. Sivasami36, Bradley37, and Mrowietz38 have all demonstrated that obesity-associated inflammatory markers, such as TNF-α, IL-6, and adipokines, significantly overlap with those seen in psoriatic disease, suggesting a common inflammatory pathway. These insights provide a molecular basis for the observed association between obesity and psoriatic disease, reinforcing the need for targeted therapeutic interventions aimed at reducing obesity-induced inflammation. Some researchers believe that it may be due to the presence of large amounts of visceral fat in obese patients39. Visceral fat is the largest endocrine organ in the body and produces pro-inflammatory cytokines (e.g., TNF-α39, IL-639,40, IL-1740,41,42) and adipokines43, which have been implicated in the development of psoriasis42. Recent evidence has also demonstrated that these pro-inflammatory mediators, notably IL-17 and TNF-α, serve as critical molecular bridges linking obesity and increased psoriasis severity, thereby enhancing the inflammatory milieu in affected individuals36,44. Adipose tissue, particularly visceral fat, is an important source of these mediators, which contribute to the chronic low-grade inflammation observed in obese individuals. IL-17 has been shown to promote the inflammatory environment in the skin, exacerbating psoriasis symptoms40,41,42. Similarly, TNF-α, another cytokine elevated in obesity, has been implicated in driving systemic inflammation, which can exacerbate psoriatic disease39. Chiricozzi et al. highlighted that the crosstalk between skin inflammation and adipose tissue-derived products, such as adipokines and cytokines, provides pathogenic evidence connecting psoriasis to increased adiposity. These mediators not only perpetuate the inflammatory cascade in psoriatic skin but also contribute to the systemic inflammation associated with obesity. This aligns with clinical observations where blocking these factors, particularly IL-17, significantly alleviates psoriasis symptoms45. Boehncke and Schön further emphasized the role of IL-17 and TNF-α in the pathogenesis of psoriasis, underscoring their potential as therapeutic targets for managing both skin inflammation and obesity-related comorbidities46. This aligns with clinical observations where blocking these factors, particularly IL-17, significantly alleviates psoriasis symptoms25. Another aspect is that BMI shows a positive correlation with the severity of psoriasis. Some researchers have suggested that psoriasis is an immune-mediated chronic inflammatory disease exacerbated by obesity, and that dysfunction of adipose tissue in obese individuals aggravates the cardiovascular26,27 and metabolic complications of psoriasis10. Similar results have been found in other evidence; a very low-calorie ketogenic diet can reduce disease exacerbation and even block psoriasis triggers by producing ketone bodies. Combined with proper exercise management, it can lead to weight loss and systemic inflammation reduction, which can alleviate the severity of psoriasis and has been recommended as a potential first-line treatment for psoriasis patients1,28,29. Recent studies have further emphasized the clinical importance of BMI in psoriasis management, demonstrating its influence on the efficacy of certain small-molecule therapies. For instance, a study by Hagino et al. found that higher BMI was associated with reduced response rates to bimekizumab in patients with moderate-to-severe psoriasis30. Furthermore, recent publications highlight the impact of BMI on the efficacy of small-molecule therapies for psoriasis. These studies show that elevated BMI may reduce the effectiveness of certain treatments, underscoring the clinical importance of BMI not only in disease severity but also in therapeutic outcomes31,32. The authors discussed the potential mechanisms by which obesity may influence the efficacy of systemic treatments, including alterations in drug pharmacokinetics and pharmacodynamics, as well as the presence of a chronic inflammatory state. They emphasized the need for personalized treatment approaches that take into account patient-specific factors, such as BMI, to optimize therapeutic outcomes in psoriasis management.
In the regression analysis, we unexpectedly discovered that lower body weight does not necessarily equate to better health outcomes. Contrary to prevailing assumptions, our findings suggest that a BMI below 18.5 kg/m2 may not inherently reduce the risk of psoriasis, underscoring the intricate nature of this condition and the potential role of malnutrition. This revelation necessitates further investigation to comprehensively understand the full range of nutritional factors influencing psoriasis development33.
Limitations
Despite the substantial sample size employed in our study, the wide confidence intervals for single-sample estimates can be attributed to the comparatively limited number of cases within the population-based cohort. However, the congruence of our causal estimates with the most recent GWAS data from the EBI database and FinnGen reinforces the robustness of our findings. Consistent with expectations from a well-powered sample, our research corroborates the profound impact of obesity on the onset and progression of psoriasis, with severity escalating in tandem with higher BMI levels. To meticulously assess the relationship between various BMI ranges and psoriasis, we stratified BMI into five distinct categories. Intriguingly, our regression analyses indicate that lower body weight does not invariably correlate with more favorable outcomes. We chose to report both PASI and BSA as severity measures. While PASI is a more comprehensive tool that accounts for lesion size and severity, BSA is commonly used in clinical practice due to its simplicity. Although there is some overlap between the two, we believe that using both measures enhances the robustness of our study. Future studies should consider using more objective and less confounded assessment tools to accurately assess the relationship between obesity and psoriasis severity. Despite the limitations of the DLQI38, we still believe it has some applicability in this study. Specifically, DLQI is a validated instrument that integrates patients’ subjective perceptions and changes in quality of life. However, the DLQI may be confounded by depression, particularly in obese individuals, which can impact the accuracy of QoL assessments18,38. However, due to the lack of conclusive statistical evidence, we are unable to draw more definitive conclusions, emphasizing the necessity for further research in this domain.
Our MR studies and subsequent regression analyses provide compelling evidence for a causal relationship between increasing BMI and the development of psoriasis, confirming that the severity of psoriasis intensifies with higher BMI levels. These findings underscore the pivotal role of BMI in psoriasis, emphasizing the urgent need for effective obesity management strategies in both the general population and those affected by the condition. Clinical decision-makers are strongly advised to prioritize weight management and nutritional balance as integral components of psoriasis treatment protocols. Furthermore, providing patients with personalized nutritional counseling is highly recommended to address individual needs and optimize treatment outcomes.
Data availability
For this study, we obtained genome-wide association study (GWAS) data from two reputable sources: the European Bioinformatics Institute (EBI) OpenGWAS database (https://gwas.mrcieu.ac.uk/) and the FinnGen online database (https://www.finngen.fi/en/access_results). We accessed a broad spectrum of BMI-related genetic data from the EBI open database, including data1, (accession number: ebi-a-GCST90095039 for BMI1), and data2, (accession number: ebi-a-GCST90018947 for BMI2). Additionally, for PV, we employed data (finn-b-PSORI_STRICT, https://r8.risteys.finngen.fi/phenocode/PSORI_STRICT) from a meta-analysis, specifically using a stringent definition based on the International Classification of Diseases (ICD-10) criteria (L40-L400). The second part of the study was a multicenter retrospective analysis involving a sizable cohort of 1,979 patients newly diagnosed with psoriasis (Clinicaltrials.gov: ChiCTR1900024852). To support the clarity and consistency of the data, only records from the patient’s first visit were considered; any records of subsequent follow-up visits were systematically excluded. Data collection was carried out across 12 hospitals: Department of Dermatology, The University of Hong Kong-Shenzhen Hospital, Department of Dermatology, Xinjiang Uygur Autonomous Region People’s Hospital, Department of Dermatology, The First Affiliated Hospital of The Fourth Military Medical University, Department of Dermatology, Peking Union Medical College Hospital, Department of Dermatology, Dermatology Hospital of Southern Medical University, Department of Dermatology, Shanghai Skin Disease Hospital, Department of Dermatology, The Second Norman Bethune Hospital of Jilin University, Department of Dermatology, West China Hospital, Sichuan University, Department of Dermatology, Skin Disease Hospital of Tongji University, Department of Dermatology, Hebei Provincial Hospital of Traditional Chinese Medicine, Department of Dermatology, The First Affiliated Hospital of Guangxi Medical University, and Department of Dermatology, Shenzhen People’s Hospital. The datasets generated and/or analyzed in this study are not publicly available due to the individual patient sensitivity and patient case confidentiality of the data used in our study, and we regret to inform you that we are unable to share or disclose patients’ personal data with outside parties. However, it is available from the corresponding author upon reasonable request.
References
Nast, A. et al. Euroguiderm Guideline on the Systemic Treatment of Psoriasis Vulgaris – Part 2: Specific Clinical and Comorbid Situations. J. Eur. Acad. Dermatol. Venereol. 35, 281–317 (2021).
Griffiths, C. E. M., Armstrong, A. W., Gudjonsson, J. E. & Barker, J. N. W. N. Psoriasis. The Lancet. 397, 1301–1315 (2021).
Korman, N. J. Management of psoriasis as a systemic disease: What is the evidence?. Br. J. Dermatol. 182, 840–848 (2020).
Yanovski, J. A. Obesity: Trends in underweight and obesity - scale of the problem. Nat. Rev. Endocrinol. 14, 5–6 (2018).
Van der Schueren, B. et al. Obesity in people living with type 1 diabetes. The Lancet Diabetes Endocrinol. 9, 776–785 (2021).
Egeberg, A., Sørensen, J. A., Gislason, G. H., Knop, F. K. & Skov, L. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. Jama Surg. 152, 344–349 (2017).
Chen, H. et al. Does obesity Kuznets curve exist in developing economies? Evidence from 38 African countries based on heterogeneous panel data analysis on income-level classification. Front. Public Health. 11, 1200555 (2023).
Karlsson, T., Hadizadeh, F., Rask Andersen, M., Johansson, Å. & Ek, W. E. Body mass index and the risk of rheumatic disease: Linear and nonlinear mendelian randomization analyses. Arthritis Rheumatol. 75, 2027–2035 (2023).
Armstrong, A. W. & Read, C. Pathophysiology, clinical presentation, and treatment of psoriasis. Jama. 323, 1945 (2020).
Wu, J. J., Kavanaugh, A., Lebwohl, M. G., Gniadecki, R. & Merola, J. F. Psoriasis and metabolic syndrome: Implications for the management and treatment of psoriasis. J. Eur. Acad. Dermatol. Venereol. 36, 797–806 (2022).
Armstrong, A. W., Harskamp, C. T. & Armstrong, E. J. The association between psoriasis and obesity: A systematic review and meta-analysis of observational studies. Nutr. Diabetes. 2, e54 (2012).
Vata, D. et al. Update on obesity in psoriasis patients. Life. 13, 1947 (2023).
Budu-Aggrey, A. et al. Evidence of a causal relationship between body mass index and psoriasis: A mendelian randomization study. Plos Med. 16, e1002739 (2019).
Chalitsios, C. V. et al. Investigating modifiable pathways in psoriasis: A mendelian randomization study. J. Am. Acad. Dermatol. 88, 593–601 (2023).
Meurer, W. J. & Tolles, J. Logistic regression diagnostics. Jama. 317, 1068 (2017).
Tripepi, G., Jager, K. J., Dekker, F. W. & Zoccali, C. Linear and logistic regression analysis. Kidney Int. 73, 806–810 (2008).
Geale, K. & Schmitt Egenolf, M. Severity of psoriasis: Time to disentangle severity from symptom control. Br. J. Dermatol. 186, 1033-1034 (2022).
Mattei, P. L., Corey, K. C. & Kimball, A. B. Psoriasis area severity index (Pasi) and the dermatology life quality index (Dlqi): The correlation between disease severity and psychological burden in patients treated with biological therapies. J. Eur. Acad. Dermatol. Venereol. 28, 333–337 (2014).
Norden, A., Rekhtman, S., Strunk, A. & Garg, A. Risk of psoriasis according to body mass index: A retrospective cohort analysis. J. Am. Acad. Dermatol. 86, 1020–1026 (2022).
Wang, L. et al. Body-mass index and obesity in urban and rural China: Findings from consecutive nationally representative surveys during 2004–18. The Lancet. 398, 53–63 (2021).
Di Angelantonio, E. et al. Body-Mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. The Lancet. 388, 776–786 (2016).
Levin, M. G. et al. Genetics of height and risk of atrial fibrillation: A mendelian randomization study. Plos Med. 17, e1003288 (2020).
Burgess, S. & Thompson, S. G. Interpreting findings from mendelian randomization using the Mr-Egger method. Eur. J. Epidemiol. 32, 377–389 (2017).
Bowden, J., Davey, S. G. & Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525 (2015).
Menter, A. et al. Joint Aad-Npf guidelines of care for the management and treatment of psoriasis with biologics. J. Am. Acad. Dermatol. 80, 1029–1072 (2019).
Gao, N. et al. The association between psoriasis and risk of cardiovascular disease: A mendelian randomization analysis. Front. Immunol. 13, 918224 (2022).
Zhang, L., Wang, Y., Qiu, L. & Wu, J. Psoriasis and cardiovascular disease risk in European and East Asian populations: Evidence from meta-analysis and mendelian randomization analysis. BMC Med. 20, 1–421 (2022).
Barrea, L. et al. Very low-calorie ketogenic diet (Vlckd) in patients with psoriasis and obesity: An update for dermatologists and nutritionists. Crit. Rev. Food Sci. Nutr. 62, 398–414 (2022).
Barrea, L. et al. Clinical and nutritional management of very-low-calorie ketogenic diet (VLCKD) in patients with psoriasis and obesity: a practical guide for the nutritionist. Crit. Rev. Food Sci. Nutr. 63, 10775-10791 (2023).
Hagino, T., Onda, M., Saeki, H., Fujimoto, E. & Kanda, N. Predictive factors for responders to deucravacitinib treatment in patients with psoriasis. J. Dermatol. 52, 551-555 (2025).
Hagino, T., Saeki, H., Fujimoto, E. & Kanda, N. Effectiveness of long-term bimekizumab treatment and predictive factors for responders in moderate-to-severe psoriasis: A 52-week real-world study. J. Dermatol. 52, 317-328 (2025).
Hagino, T., Saeki, H., Fujimoto, E. & Kanda, N. Real-world effectiveness and safety of Bimekizumab in Japanese patients with psoriasis: A single-center retrospective study. J. Dermatol. 51, 649–658 (2024).
Zuccotti, E. et al. Nutritional strategies for psoriasis: Current scientific evidence in clinical trials. Eur. Rev. Med. Pharmacol. Sci. 22, 8537–8551 (2018).
Han, J. H. et al. Increased risk of psoriasis in subjects with abdominal obesity: A nationwide population-based study. J. Dermatol. 46, 695–701 (2019).
Maglio, C., Peltonen, M., Rudin, A. & Carlsson, L. M. S. Bariatric surgery and the incidence of psoriasis and psoriatic arthritis in the swedish obese subjects study. Obesity (Silver Spring, Md.). 25, 2068–2073 (2017).
Sivasami, P. et al. Obesity-induced dysregulation of skin-resident Pparγ (+) Treg cells promotes Il-17a-mediated psoriatic inflammation. Immunity. 56, 1844–1861 (2023).
Bradley, D., Deng, T., Shantaram, D. & Hsueh, W. A. Orchestration of the adipose tissue immune landscape by adipocytes. Annu. Rev. Physiol. 86, 199–223 (2024).
Mrowietz, U. et al. Efficacy and safety of apremilast in patients with limited skin involvement, plaque psoriasis in special areas and impaired quality of life: Results from the embrace randomized trial. J. Eur. Acad. Dermatol. Venereol. Jeadv. 37, 348–355 (2023).
Wong, Y., Nakamizo, S., Tan, K. J. & Kabashima, K. An update on the role of adipose tissues in psoriasis. Front. Immunol. 10, 1507 (2019).
Park, S. H. et al. Impact of Obesity on the Il-6 Immune Marker and Th17 Immune Cells in C57Bl/6 Mice Models with Imiquimod-Induced Psoriasis. Int. J. Mol. Sci. 24, 5592 (2023).
Sivasami, P. et al. Obesity-induced dysregulation of skin-resident Pparγ + Treg cells promotes Il-17a-mediated psoriatic inflammation. Immunity (Cambridge, Mass.). 56, 1844–1861 (2023).
Shi, Z. et al. Short-term exposure to a western diet induces psoriasiform dermatitis by promoting accumulation of Il-17a–producing Γδ T cells. J. Invest. Dermatol. 140, 1815–1823 (2020).
Goolam, M. N. et al. Relationship of visceral fat and adipokines with cardiometabolic diseases in psoriasis. Acta. Derm. Venereol. 99, 1218–1223 (2019).
Wang, Y. et al. Bcat2-mediated branched-chain amino acid catabolism is linked to the aggravated inflammation in obese with psoriasis mice. Mol. Nutr. Food Res. 68, e2300720 (2024).
Chiricozzi, A. et al. Crosstalk between skin inflammation and adipose tissue-derived products: Pathogenic evidence linking psoriasis to increased adiposity. Expert Rev. Clin. Immunol. 12, 1299–1308 (2016).
Boehncke, W. & Schön, M. P. Psoriasis. Lancet (London, England). 386, 983–994 (2015).
Acknowledgements
The authors would like to express their sincere gratitude to all the coordinators, dermatologists and researchers involved in this multicenter clinical study.
Funding
This work was supported by Sanming Projectof Medicine in Shenzhen (No.SZSM202103014), Shenzhen Stable Support for General Projects (20231122162117001), High Level-Hospital Program, Health Commission of Guangdong Province, China (HKUSZH201906001) and HaiYa Young Scientist Foundation of Shenzhen University General Hospital (2024-HY004).
Author information
Authors and Affiliations
Contributions
Huiwei Wang contributed to all aspects of study design, conduct, data interpretation, and the writing of the manuscript. Suchun Hou, Xiaojing Kang, Chen Yu, Bin Yang, Yuling Shi, Fuqiu Li, Wei Li, Jun Gu, Mingjun Lei, Youkun Lin, Gang Wang, Hongzhong Jin, Xiaoming Liu contributed to eligibility screening, data extraction from eligible studies, and data analysis and interpretation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
IRB approval status
Reviewed and approved by HKU-SZH Medical Ethics Committee [2019]181.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, H., Hou, S., Kang, X. et al. BMI matters: understanding the link between weight and severe psoriasis. Sci Rep 15, 11158 (2025). https://doi.org/10.1038/s41598-025-94505-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-94505-0