Abstract
Objective: To analyze and compare the incidence of adverse events (AEs) associated with different administration routes of colistin, with the aim of providing a reference for its safe and effective clinical use. Methods: Adverse event (AE) reports related to colistin were retrieved from the FDA Adverse Event Reporting System (FAERS) database. The reporting trends were analyzed, and the Reporting Odds Ratio (ROR) and Proportional Reporting Ratio (PRR) for colistin-associated AEs were calculated. A comparative analysis was conducted to examine the occurrence of AEs under different administration routes of colistin. Results: A total of 13,043 AE reports were extracted from the FAERS database. Further analysis of 176 key AEs associated with colistin indicated a significant increase in the number of reports after 2021. The year and country of the reports showed heterogeneity across different administration routes. Intravenous (IV) administration of colistin was associated with the highest proportion of AEs, and heterogeneity was also observed in the types of AEs reported for inhaled and oral (PO) administration routes. Conclusion: Compared to inhaled and PO administration routes, IV administration of colistin is more likely to result in AEs such as nephrotoxicity and drug ineffectiveness. Additionally, there are significant differences in the types of AEs associated with colistin across different administration routes.
Similar content being viewed by others
Introduction
Polymyxins are a class of non-ribosomal cyclic oligopeptide antibiotics discovered within the genus Bacillus in 19461. They are categorized into five distinct components, A, B, C, D, and E, based on structural differences2. However, only polymyxin B and polymyxin E have been extensively utilized in humans and animals. Polymyxin E is commonly known as colistin. Additionally, Both polymyxin B and E are produced in various salt derivatives, including sulfate and methanesulfonate. Polymyxin B is primarily available as a sulfate, while the methanesulfonate form is relatively rare. In clinical practice, both polymyxin E sulfate and methanesulfonate derivatives are widely used. Colistin methanesulfonate (CMS) is an inactive prodrug that is hydrolyzed into its active form with potent bactericidal effects upon entering the body3. Due to its strong antimicrobial activity against multidrug-resistant (MDR) bacteria, it is considered the last line of defense against infections caused by MDR Gram-negative bacteria. However, the use of colistin is limited due to potential adverse drug reactions, such as nephrotoxicity and neurotoxicity4.
Colistin exhibits varying therapeutic efficacies and safety profiles depending on the route of administration, with differences also observed in adverse drug reactions5,6,7,8. Currently, there are several clinical administration methods for colistin, with IV, inhaled and PO routes being the most commonly utilized. Inhalation (INH), as a frequently used method, has been shown to achieve effective bactericidal concentrations of colistin and aminoglycosides in the lungs of patients with ventilator-associated pneumonia (VAP) caused by MDR Gram-negative bacteria9. Moreover, INH of antimicrobials does not increase the risk of systemic toxicity or disrupt the gut microbiome10. Based on these findings, clinicians both domestically and internationally have attempted to use different administration methods for colistin to achieve similar therapeutic effects while reducing the incidence of adverse drug reactions. In 2014, Gu et al. conducted a meta-analysis comparing the clinical efficacy and safety of IV colistin alone versus a combination of aerosolized (AS) and IV colistin for treating VAP. The results indicated that the combination of AS and IV colistin was associated with better clinical outcomes compared to IV colistin alone. Moreover, there were no significant differences in microbiological eradication, ICU mortality, hospital mortality, or nephrotoxicity between the two treatment groups11. Furthermore, Boisson et al. utilized pharmacokinetic/pharmacodynamic (PK/PD) modeling to demonstrate that the antibacterial effect of CMS administered via AS was significantly greater than that of IV administration, This finding provides valuable support for using nebulized CMS to treat pulmonary infections in critically ill patients12. However, to date, there have been no reports on the differences in the incidence of major adverse drug events following different administration routes of colistin.
The U.S. FAERS is a globally accessible, open-source database widely used for post-marketing drug safety surveillance13. This study utilized FAERS data on AEs associated with different administration routes of colistin to analyze and reveal the potential types of AEs linked to each route. The analysis aims to provide a reference for detecting and preventing adverse reactions in clinical practice, thereby offering a theoretical foundation for the efficient, safe, and rational use of colistin.
Methods
Study design
A retrospective analysis was conducted using the colistin-related AEs reported in the FAERS database. We analyzed the reporting trends of colistin-related AEs and the administration routes, and further examined the occurrence of AEs under different administration methods.
Data source
In this study, data on AEs were sourced from the U.S. FAERS database. We collected colistin-related AE reports submitted to the FAERS database from the fourth quarter of 2003 (Q4 2003) to the third quarter of 2023 (Q3 2023). The data included basic patient information (gender, age, country), drug information (drug name, administration route), and AEs, all of which were used for retrospective analysis.
Data extraction and organization
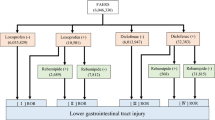
This study utilized OpenVigil2.1, a tool developed by Kiel University in Germany, for querying and extracting data from the FAERS. OpenVigil2.1 is a commonly used analytical tool for data extraction and analysis from the FAERS, known for its standardized and comprehensive information retrieval, and has been employed in numerous pharmacovigilance studies14,15. Using ‘colistin’ as the search term, we identified 13,043 AE reports related to the drug. We then extracted and analyzed the data from these reports, obtaining 918 distinct AEs along with their corresponding ROR and PRR. Subsequently, in an Excel spreadsheet, we filtered the data based on the drug names, retaining only AE data for the generic names ‘COLISTIN’, ‘COLISTIN SULFATE’, ‘COLY-MYCIN M Parenteral’, and ‘COLISTIMETHATE SODIUM’, totaling 249 records. After excluding reports with unclear administration routes, gender information, and duplicates, we focused on 176 colistin AE reports.
In the FAERS database, each AE is classified using the Medical Dictionary for Regulatory Activities (MedDRA) and encoded based on the Preferred Terms (PTs) within the MedDRA classification system. The MedDRA classification system is structured into five hierarchical levels: System Organ Class (SOC), High-Level Group Term (HLGT), High-Level Term (HLT), Preferred Term (PT), and Low-Level Term (LLT). Low-Level Terms (LLTs) are typically synonyms of PTs (including variations in spelling or terminology) or more specific terms. Each PT is linked to a specific HLT, HLGT, and a primary SOC; however, each HLT, HLGT, and SOC typically encompasses multiple PTs16,17,18. In this study, AEs were categorized and organized according to the SOC and PT levels from MedDRA version 27.1.
Data analysis
(1) Descriptive Analysis: We summarized the key data by the reporting year of AEs and the reporting region. A trend curve was plotted for different years, and pie charts were generated for different regions. The descriptive analysis of colistin AEs included the number of AE reports, administration routes, gender, age, reporting year, and the country of the reporter. Chi-square (χ2) tests and Fisher’s exact tests were performed to analyze the descriptive data across different administration routes.
(2) Signal Detection: AE risk signals for colistin were identified using a method based on the imbalance of reporting odds, with the statistical metrics including ROR and its corresponding 95% confidence interval (95% CI), PRR and the corresponding χ2 test results. The methods for screening AE signals are shown in Table 1, along with formulas (1) and (2).
In the analysis, a represents the number of reports for the AE(s) of interest associated with the drug of interest, b represents the number of reports for the same AE associated with all other drugs, c represents the number of reports for all other AEs associated with the drug of interest, d represents the number of reports for all other AEs associated with all other drugs. A signal of an adverse reaction is indicated if the following criteria are met: the number of reports n > 2; χ2 > 4; PRR > 2; and the lower bound of the 95% CI of the ROR > 1. The higher the PRR and ROR values, the stronger the signal intensity. A higher χ2 value suggests that the difference indicated by the PRR is more likely to be statistically significant.
Results
Analysis of signal detection for colistin AE reports in the FAERS database
An analysis of colistin-related AEs reported in the FAERS database identified a total of 918 AE reports. As shown in Fig. 1, these reports were associated with 27 distinct System Organ Classes (SOCs), with the top 3 being ‘infections and infestations’, ‘investigations’, and ‘general disorders and administration site conditions’. Further analysis of the most frequently reported PTs from Q4 2003 to Q3 2023 revealed the top 10 AEs. As shown in Table 2, these AEs were categorized into four SOCs: ‘infections and infestations’, ‘general disorders and administration site conditions’, ‘injury, poisoning and procedural complications’, and ‘renal and urinary disorders’. Among the reported AEs, the proportion of reports for drug ineffective was the highest (4.71%). Other AEs in the top 10 include off label use (2.35%), acute kidney injury (2.29%), septic shock (2.23%), sepsis (2.12%), drug resistance (1.71%), multiple organ dysfunction syndrome (1.52%), death (1.48%), condition aggravated (1.33%), and pathogen resistance (1.20%). Among these, the signal intensity for pathogen resistance was the highest, with an ROR = 104.13 (95% CI: 78.76, 137.66), PRR = 99.70 (χ2 = 4934.21). The next highest were for drug resistance (ROR = 57.57 (95% CI: 45.74, 72.46), PRR = 53.94, χ2 = 3982.21), septic shock (ROR = 48.20 (95% CI: 38.55, 60.26), PRR = 44.97, χ2 = 3513.61), multiple organ dysfunction syndrome (ROR = 27.81 (95% CI: 21.40, 36.14), PRR = 26.51, χ2 = 1421.55), sepsis (ROR = 15.65 (95% CI: 12.42, 19.72), PRR = 14.72, χ2 = 973.71), acute kidney injury (ROR = 10.60 (95% CI: 8.63, 13.02), PRR = 9.81, χ2 = 780.57). The signal intensity for condition aggravated, drug ineffective, and off label use was relatively weak (PRR < 5 & ROR < 5). Death had the weakest signal intensity (ROR = 1.16 (95% CI: 0.90, 1.49), PRR = 1.15, χ2 = 1.15), and did not even meet the criteria for generating an adverse reaction signal.
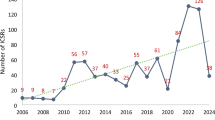
Trends and regional distribution of key colistin-related AE reports
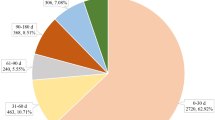
Figure 2A illustrates the trend in the number of reports for 176 key AEs associated with colistin. The trend chart reveals fluctuations in the number of colistin-related AE reports from 2003 to 2023. Notably, a significant increase in the number of colistin-related AE reports has been observed after 2021. Excluding 5 colistin-related AE reports with missing country information, the regional distribution of the remaining 171 colistin-related AE reports is shown in Fig. 2B. A total of 37 countries reported colistin-related AEs in the FAERS, with the majority from European countries (56.82%), followed by Asian countries (27.03%) and American countries (10.81%). Additionally, as shown in Fig. 2C, among the 176 colistin-related AE reports, Europe accounted for the largest proportion, at 56.82%, followed by the Americas (21.02%) and Asia (17.05%).
Trends in the annual total number of key colistin-related AEs and their regional distribution characteristics (Q4 2003 - Q3 2023). A: Annual distribution of AE reports; B: Geographical distribution of countries reporting colistin-related AEs; C: Geographical distribution of reported colistin-related AEs.
Reported characteristics of key Colistin-Related AEs
After summarizing the 176 key colistin-related AE reports, the findings are presented in Table 3. Among these, 89 reports were associated with IV administration of colistin, 45 reports were associated with INH administration, 20 reports were associated with PO administration, and a total of 22 reports were associated with ten other administration routes, including intramuscular, intracranial, transplacental, and topical administration. In the reporting of AEs associated with colistin administration via IV, INH, and PO routes, a comparison between any two of these administration methods revealed no statistically significant differences in the gender and age of patients (P > 0.05). However, significant differences were observed in the year and country of reporting for AEs associated with different administration routes of colistin. The statistical differences in the year and country of reporting for AEs associated with IV versus INH administration were both highly significant (P < 0.001). For IV versus PO administration, the statistical differences were P = 0.014 for the year and P = 0.002 for the country. For INH versus PO administration, the statistical differences in both the year and country of AE reporting were highly significant (P < 0.001). An in-depth analysis of the 176 carefully monitored AE reports associated with colistin reveals that IV administration is associated with the highest incidence of reported events (n = 89). Notably, although no statistically significant age-related differences in the occurrence of AEs were observed across the various administration routes, the age group of 18–59 years accounted for the highest proportion of reported AEs, irrespective of the administration method (IV: 48.31%, INH: 42.22%, PO: 50.00%).
Regarding the reporting year, the number of AE reports associated with the IV use of colistin increased significantly between 2019 and 2023 (n = 42), accounting for 47.19% of the total number of AE reports related to IV colistin in the FAERS database over the past two decades. Concurrently, a significant increase in the number of AE reports for colistin administered via the INH route has been observed since 2014–2018. From the perspective of regional distribution of AE reports, as shown in Fig. 3A, the majority of colistin-related AE reports originated from Europe, the Americas, and Asia. Notably, AE reports related to IV colistin administration were significantly higher than those for other administration routes, with INH being the second most reported administration route. Focusing on countries with at least 7 reported AEs related to colistin and at least 2 different administration routes, the results are presented in Fig. 3B. The majority of AE reports were from Western countries, with the USA having the highest number of reports (14.77%, n = 27), followed by the UK (n = 19). Further analysis of AE reports from individual countries, broken down by different colistin administration routes, is shown in Fig. 3C. With the exception of the UK, the Netherlands, and Spain, IV administration was the predominant route for colistin-related AEs in most countries.
The reporting of colistin-related AEs in different regions and countries based on administration routes. A: The reporting of colistin-related AEs for different administration routes across regions. B: The distribution of countries reporting colistin-related AEs; C: The reporting of colistin-related AEs for different administration routes across countries. IV: Intravenous; INH: Inhalation; PO: Oral.
Reported analysis of key colistin-related AEs
This study presents a categorical analysis of the 176 key colistin-associated AEs, with the results shown in Table 4. These AEs were stratified into three distinct categories based on predefined criteria: renal dysfunction, treatment failure, and other. Renal dysfunction refers to abnormalities in kidney-related functions and indicators, while treatment failure includes cases of drug resistance, pathogen resistance, disease exacerbation, and situations where the infection or condition remained uncontrolled. The ‘other’ category includes a diverse range of adverse reactions, such as hepatotoxicity, neurotoxicity, death, dermatological symptoms (including pruritus and rash), gastrointestinal disturbances (including nausea, vomiting, and abdominal discomfort), essentially covering all reported adverse reactions listed in the colistin product label. Analyzing the reports of AEs associated with colistin across different administration routes revealed that, regardless of the type of AE, IV administration routes had the highest number of AE reports. Specifically, renal dysfunction was reported in 26 cases, treatment failure in 33 cases, and other AEs in 30 cases. Notably, for IV administration, renal dysfunction and treatment failure together accounted for more than half of all reported AEs, reaching 66.29%. Additionally, statistical analysis using Fisher’s Exact Test and the Chi-squared test revealed that the types of AEs associated with IV colistin differed significantly from those associated with INH and PO administration (P = 0.047 for INH, P = 0.002 for PO). However, no significant difference in AE incidence was observed between INH and PO administration (P = 0.341).
Discussion
This study utilized the FAERS database to compare the reporting of AEs associated with colistin over a 20-year period, with a particular focus on comparing the reporting of AEs in 176 cases across different administration routes of colistin. Overall, the most frequently reported AE was drug ineffective, and the signal intensity for pathogen resistance was the highest. According to MedDRA, drug ineffective is similar to drug resistance, both of which are categorized under ‘general disorders and administration site conditions’ at the SOC level. The distinction between the two is minimal, and according to the LLT classification, both indicate that the drug did not exert its intended effect, leading to a lack of therapeutic outcome. This can be attributed to either incorrect drug administration or the development of clinical resistance in the patient. In contrast, pathogen resistance, classified under ‘infections and infestations’ at the SOC level, refers to cases where there is definitive microbiological evidence of resistance, leading to therapeutic failure. These observations suggest a gradual increase in colistin resistance and highlight the fact that, although colistin was initially considered the ‘last line of defense’ against infections caused by MDR Gram-negative bacteria19,20, the rapid emergence and spread of these pathogens have led to its extensive clinical use, consequently contributing to the rise in colistin resistance among Gram-negative bacteria21. In fact, certain Gram-negative bacteria, such as Proteus mirabilis, Moraxella catarrhalis, Helicobacter pylori, Serratia marcescens, Providencia spp., and Burkholderia cepacia, modify the lipopolysaccharides in their cell membranes with cationic groups, resulting in the repulsion of cationic colistin and thereby exhibiting intrinsic resistance to colistin22,23,24,25. In addition, some susceptible bacterial species can acquire colistin resistance genes through spontaneous chromosomal mutations and transposon/plasmid-mediated mechanisms26,27. The emergence of these resistance genes confers bacterial resistance to colistin, limiting its bactericidal effect. This highlights the importance of carefully considering the timing of colistin use and its antimicrobial spectrum in clinical practice. In addition, acute kidney injury is a common AE associated with colistin use, consistent with the warnings about potential adverse reactions in the product information. This substantiates the high incidence of nephrotoxicity among colistin’s adverse effects, with reports indicating that neuromuscular toxicity and nephrotoxicity are the most frequently reported AEs associated with colistin28. The incidence of acute kidney injury induced by colistin can range from 20–60%29, with nephrotoxicity being the most common side effect following IV administration of colistin32. This underscores the necessity of closely monitoring renal function in patients during colistin therapy to prevent adverse drug reactions.
In the analysis of 176 key AE reports associated with colistin, a significant increase in the number of reports for colistin-related AEs was observed in 2022. Between 2019 and 2023, reports of colistin-related AEs associated with IV and INH administration accounted for approximately half (48.51%) of the total number of reports for the same administration routes over the past 20 years (2003–2023). This increase reflects the growing clinical use of the drug during this period, likely driven by the rise in antimicrobial-resistant pathogens and the impact of the COVID-19 pandemic33,34. During the COVID-19 pandemic, the incidence of healthcare-associated infections caused by multidrug-resistant organisms (MDROs) increased by 20%, and the prevalence of MDR Gram-negative bacteria was significantly correlated with the surge of COVID-19 cases35. A study by Yang et al. also highlighted that MDROs were detected in 42.9% of COVID-19 patients, with carbapenem-resistant organisms (CROs) accounting for as much as 41.0%. Colistin, as the last line of defense against MDR Gram-negative bacterial infections, was administered to 28.5% of COVID-19 patients36. It is evident that the rise of MDR bacteria in nosocomial infections among COVID-19 patients has driven the increased clinical use of colistin. Additionally, we observed significant discrepancies in the frequency of AE reports associated with different administration routes of colistin, with IV administration recording the highest incidence (n = 89), followed by INH (n = 45) and PO administration (n = 20). The timing and national distribution of these reports also exhibited heterogeneity, reflecting a subtle shift in global colistin administration practices over the past two decades. Concurrently, the incidence of AEs appears to be correlated with the mode of administration. Traditionally, IV colistin administration has been the most common; however, an increasing number of recent studies suggest that AS colistin can assist in the treatment of MDR Gram-negative bacterial infections11,37,38,39. For pneumonia patients, INH administration delivers colistin directly to the site of infection, resulting in elevated colistin concentrations in lung tissue and reduced levels in plasma. This indicates that nebulization limits the systemic distribution of colistin, thereby minimizing systemic exposure40,41,42,43,44,45,46. Theoretically, INH administration of colistin could enhance its antimicrobial efficacy, reduce the development of bacterial resistance, and decrease adverse reactions by reducing the required dosage. Analysis of the geographical distribution of AE reports for colistin reveals notable discrepancies in reporting across different administration routes. IV administration is predominantly associated with reports from Europe and the USA, while INH administration reports are mainly concentrated in the UK, the USA, and Spain. PO administration reports are primarily identified in the Netherlands. This suggests a global variation in colistin prescription and use, but consistently highlights the higher frequency of AEs associated with IV colistin.
Utilizing the FAERS database, we analyzed the reporting frequency and signal intensity of AEs related to colistin. A stratified statistical analysis of 176 colistin-associated AEs revealed significant heterogeneity in AE reports between IV administration and other routes, such as INH and PO administration. Renal dysfunction and treatment failure are the predominant AEs associated with IV colistin administration. However, the combined reporting rate of renal dysfunction and treatment failure following inhaled colistin administration is similar to that of other AEs, while PO colistin administration is predominantly associated with a higher reporting rate of other AEs. Nonetheless, it is evident that renal dysfunction and treatment failure following IV colistin administration are the most frequently reported AEs across all administration routes. These findings highlight the heterogeneity in the incidence and types of AEs associated with the different administration routes of colistin. This observation mandates that clinicians exercise caution and tailor the choice of administration route of colistin to the specific needs of each patient. Moreover, it is imperative for medical professionals to be vigilant in anticipating and preventing AEs that are particularly associated with the chosen method of colistin delivery. This strategic approach to colistin administration is crucial for optimizing patient safety and therapeutic outcomes.
Of course, our study also has several limitations. For instance: (1) The FAERS database, being a voluntary reporting system, is susceptible to biases that may lead to underreporting or overreporting of AEs47, while our methodology excluded reports with identical characteristics and those lacking key details such as age, sex, and administration route, which may have resulted in the omission of certain AEs from our analysis. (2) Our analysis did not provide a detailed discussion of colistin dosing, as the FAERS database lacks comprehensive dosing data. This is a notable limitation, as emerging research suggests that suboptimal dosing may contribute to the development of colistin resistance, presenting a growing challenge in antimicrobial stewardship25. (3) Our study was unable to evaluate the impact of confounding factors such as patients’ medical histories and concomitant medications, as these factors were not recorded in the original data we obtained. In fact, colistin undergoes renal reabsorption and accumulates in renal proximal tubular cells, leading to nephrotoxicity48. Therefore, it is plausible that patients with pre-existing renal impairment or those concurrently receiving nephrotoxic drugs may be more susceptible to colistin-induced nephrotoxicity than those with normal renal function. This highlights the importance of renal function assessment in clinical decision-making processes regarding colistin therapy. (4) We were unable to determine the microbiological characteristics or the specific methods and guidelines used for antimicrobial susceptibility testing (AST) when colistin was administered, as this information was not available in the original data. Consequently, we were unable to analyze these subgroups, which may have affected the classification of AEs and introduced confounding factors when making comparisons between groups. While the FAERS database has inherent limitations, the substantial volume of data it contains enables robust statistical analysis of both commonly reported and novel AEs. The objective of this study is to elucidate the occurrence rates and risk signals associated with AEs following different administration routes of colistin. This information aims to offer insights that may guide clinical decisions regarding the optimal method of colistin administration and strategies for mitigating associated AEs.
Conclusion
This study revealed that, compared to inhaled and PO administration routes, IV administration of colistin is more likely to result in AEs such as nephrotoxicity and drug ineffectiveness. Additionally, significant differences in the types of AEs associated with colistin were observed depending on the different routes of administration. This study supports previous literature on the association between AS colistin and nephrotoxicity, while further extending the knowledge by comparing the differential incidence of AEs across various administration routes. These findings underscore the clinical importance of personalizing colistin administration based on individual patient characteristics and prioritizing the prevention and management of AEs most likely to occur with each administration route.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Ainsworth, G. C., Brown, A. M. & Brownlee, G. Aerosporin, an antibiotic produced by Bacillus aerosporus Greer. Nature 159, 263. https://doi.org/10.1038/160263a0 (1947).
Stansly, P. G. & Brownlee, G. Nomenclature of polymyxin antibiotics. Nature 163, 611. https://doi.org/10.1038/163611a0 (1949).
Nation, R. L., Velkov, T. & Li, J. Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clin. Infect. Diseases: Official Publication Infect. Dis. Soc. Am. 59, 88–94. https://doi.org/10.1093/cid/ciu213 (2014).
Biswas, S., Brunel, J. M., Dubus, J. C., Reynaud-Gaubert, M. & Rolain, J. M. Colistin: an update on the antibiotic of the 21st century. Expert Rev. anti-infective Therapy. 10, 917–934. https://doi.org/10.1586/eri.12.78 (2012).
Vardakas, K. Z., Voulgaris, G. L., Samonis, G. & Falagas, M. E. Inhaled colistin monotherapy for respiratory tract infections in adults without cystic fibrosis: a systematic review and meta-analysis. Int. J. Antimicrob. Agents. 51, 1–9. https://doi.org/10.1016/j.ijantimicag.2017.05.016 (2018).
Valachis, A., Samonis, G. & Kofteridis, D. P. The role of aerosolized colistin in the treatment of ventilator-associated pneumonia: a systematic review and metaanalysis. Crit. Care Med. 43, 527–533. https://doi.org/10.1097/CCM.0000000000000771 (2015).
Sole-Lleonart, C. et al. Nebulization of antiinfective agents in invasively mechanically ventilated adults: A systematic review and Meta-analysis. Anesthesiology 126, 890–908. https://doi.org/10.1097/ALN.0000000000001570 (2017).
Zhou, Y. et al. Efficacy and safety of different polymyxin-containing regimens for the treatment of pneumonia caused by multidrug-resistant gram-negative bacteria: a systematic review and network meta-analysis. Crit. Care. 28, 239. https://doi.org/10.1186/s13054-024-05031-w (2024).
Wood, G. C. & Swanson, J. M. An update on aerosolized antibiotics for treating Hospital-Acquired and Ventilator-Associated pneumonia in adults. Annals Pharmacotherapy. 51, 1112–1121. https://doi.org/10.1177/1060028017723934 (2017).
Palmer, L. B. Aerosolized antibiotics in critically ill ventilated patients. Curr. Opin. Crit. Care. 15, 413–418. https://doi.org/10.1097/MCC.0b013e328330abcf (2009).
Gu, W. J., Wang, F., Tang, L., Bakker, J. & Liu, J. C. Colistin for the treatment of ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria: a systematic review and meta-analysis. Int. J. Antimicrob. Agents. 44, 477–485. https://doi.org/10.1016/j.ijantimicag.2014.07.004 (2014).
Boisson, M. et al. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob. Agents Chemother. 58, 7331–7339. https://doi.org/10.1128/AAC.03510-14 (2014).
Dauner, D. G. et al. Performance of subgrouped proportional reporting ratios in the US food and drug administration (FDA) adverse event reporting system. Exp. Opin. Drug Saf. 22, 589–597. https://doi.org/10.1080/14740338.2023.2182289 (2023).
Tian, X. et al. Cardiac disorder-related adverse events for aryl hydrocarbon receptor agonists: A safety review. Exp. Opin. Drug Saf. 21, 1505–1510 (2022). https://doi.org/10.1080/14740338.2022.2078301
Jiao, X. F. et al. Ovary and uterus related adverse events associated with statin use: An analysis of the FDA adverse event reporting system. Sci. Rep. 10, 11955 (2020). https://doi.org/10.1038/s41598-020-68906-2
Bousquet, C., Sadou, E., Souvignet, J., Jaulent, M. C. & Declerck, G. Formalizing MedDRA to support semantic reasoning on adverse drug reaction terms. J. Biomed. Inform. 49, 282–291. https://doi.org/10.1016/j.jbi.2014.03.012 (2014).
Xiong, R. et al. Post-marketing safety surveillance of Dalfampridine for multiple sclerosis using FDA adverse event reporting system. Front. Pharmacol. 14. https://doi.org/10.3389/fphar.2023.1226086 (2023).
Brown, E. G., Wood, L. & Wood, S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 20, 109–117. https://doi.org/10.2165/00002018-199920020-00002 (1999).
Davies, M. & Walsh, T. R. A colistin crisis in India. Lancet Infect. Dis. 18, 256–257. https://doi.org/10.1016/S1473-3099(18)30072-0 (2018).
Vaara, M. Polymyxins and their potential next generation as therapeutic antibiotics. Front. Microbiol. 10. https://doi.org/10.3389/fmicb.2019.01689 (2019).
Nang, S. C., Azad, M. A. K., Velkov, T., Zhou, Q. T. & Li, J. Rescuing the Last-Line polymyxins: achievements and challenges. Pharmacol. Rev. 73, 679–728. https://doi.org/10.1124/pharmrev.120.000020 (2021).
Raetz, C. R., Reynolds, C. M., Trent, M. S. & Bishop, R. E. Lipid A modification systems in gram-negative bacteria. Annu. Rev. Biochem. 76, 295–329. https://doi.org/10.1146/annurev.biochem.76.010307.145803 (2007).
Olaitan, A. O., Morand, S. & Rolain, J. M. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front. Microbiol. 5, 643. https://doi.org/10.3389/fmicb.2014.00643 (2014).
Simpson, B. W. & Trent, M. S. Pushing the envelope: LPS modifications and their consequences. Nat. Rev. Microbiol. 17, 403–416. https://doi.org/10.1038/s41579-019-0201-x (2019).
El-Sayed Ahmed, M. A. E. et al. Colistin and its role in the era of antibiotic resistance: An extended review (2000–2019). Emerg. Microb. Infect. 9, 868–885 (2020). https://doi.org/10.1080/22221751.2020.1754133
Poirel, L., Jayol, A. & Nordmann, P. Polymyxins: Antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin. Microbiol. Rev. 30, 557–596. https://doi.org/10.1128/CMR.00064-16 (2017).
Mohapatra, S. S., Dwibedy, S. K. & Padhy, I. Polymyxins, the last-resort antibiotics: mode of action, resistance emergence, and potential solutions. J. Biosci. 46. https://doi.org/10.1007/s12038-021-00209-8 (2021).
Falagas, M. E. et al. Toxicity after prolonged (more than four weeks) administration of intravenous colistin. BMC Infect. Dis. 5, 1. https://doi.org/10.1186/1471-2334-5-1 (2005).
Kim, E. J. & Kim, E. S. Exploring new predictors of Colistin-Associated nephrotoxicity. Infect. Chemother. 50, 283–285. https://doi.org/10.3947/ic.2018.50.3.283 (2018).
Tsuji, B. T. et al. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American college of clinical pharmacy (ACCP), European society of clinical microbiology and infectious diseases (ESCMID), infectious diseases society of America (IDSA), international society for Anti-infective Pharmacology (ISAP), society of critical care medicine (SCCM), and society of infectious diseases pharmacists (SIDP). Pharmacotherapy 39, 10–39. https://doi.org/10.1002/phar.2209 (2019).
Nation, R. L., Rigatto, M. H. P., Falci, D. R. & Zavascki, A. P. Polymyxin acute kidney injury: Dosing and other strategies to reduce toxicity. Antibiotics 8. https://doi.org/10.3390/antibiotics8010024 (2019).
Zavascki, A. P. & Nation, R. L. Nephrotoxicity of polymyxins: Is there any difference between colistimethate and polymyxin B? Antimicrob. Agents Chemother. 61. https://doi.org/10.1128/AAC.02319-16 (2017).
Gajic, I. et al. A comprehensive overview of antibacterial agents for combating Multidrug-Resistant bacteria: the current landscape, development, future opportunities, and challenges. Antibiotics 14, 221. https://doi.org/10.3390/antibiotics14030221 (2025).
Darby, E. M. et al. Molecular mechanisms of antibiotic resistance revisited. Nat. Rev. Microbiol. 21, 280–295. https://doi.org/10.1038/s41579-022-00820-y (2023).
Baker, M. A. et al. The impact of coronavirus disease 2019 (COVID-19) on Healthcare-Associated infections. Clin. Infect. Diseases: Official Publication Infect. Dis. Soc. Am. 74, 1748–1754. https://doi.org/10.1093/cid/ciab688 (2022).
Yang, X. et al. Global antimicrobial resistance and antibiotic use in COVID-19 patients within health facilities: A systematic review and meta-analysis of aggregated participant data. J. Infect. 89, 106183. https://doi.org/10.1016/j.jinf.2024.106183 (2024).
Zhu, Y. et al. Nebulized colistin in ventilator-associated pneumonia and tracheobronchitis: Historical background, pharmacokinetics and perspectives. Microorganisms 9 (2021). https://doi.org/10.3390/microorganisms9061154
Choe, J. et al. Inhalation with intravenous loading dose of colistin in critically ill patients with pneumonia caused by carbapenem-resistant gram-negative bacteria. Ther. Adv. Respir. Dis. 13, 1753466619885529. https://doi.org/10.1177/1753466619885529 (2019).
De Pascale, G. et al. Use of high-dose nebulized colistimethate in patients with colistin-only susceptible Acinetobacter baumannii VAP: Clinical, pharmacokinetic and microbiome features. Antibiotics 12. https://doi.org/10.3390/antibiotics12010125 (2023).
Boisson, M. et al. Pharmacokinetics of nebulized colistin methanesulfonate in critically ill patients. J. Antimicrob. Chemother. 72, 2607–2612. https://doi.org/10.1093/jac/dkx167 (2017).
Lu, Q. et al. Nebulized and intravenous colistin in experimental pneumonia caused by Pseudomonas aeruginosa. Intensive Care Med. 36, 1147–1155. https://doi.org/10.1007/s00134-010-1879-4 (2010).
Rouby, J. J., Monsel, A., Ehrmann, S., Bougle, A. & Laterre, P. F. The INHALE trial: multiple reasons for a negative result. Lancet Infect. Dis. 20, 778–779. https://doi.org/10.1016/S1473-3099(20)30481-3 (2020).
Benitez-Cano, A. et al. Systemic pharmacokinetics and safety of high doses of nebulized colistimethate sodium in critically ill patients with hospital-acquired and ventilator-associated pneumonia. J. Antimicrob. Chemother. 74, 3268–3273. https://doi.org/10.1093/jac/dkz356 (2019).
Lu, Q. et al. Efficacy of high-dose nebulized colistin in ventilator-associated pneumonia caused by multidrug-resistant Pseudomonas aeruginosa and acinetobacter baumannii. Anesthesiology 117, 1335–1347. https://doi.org/10.1097/ALN.0b013e31827515de (2012).
Athanassa, Z. E. et al. Pharmacokinetics of inhaled colistimethate sodium (CMS) in mechanically ventilated critically ill patients. Intensive Care Med. 38, 1779–1786. https://doi.org/10.1007/s00134-012-2628-7 (2012).
Tang, T. et al. Optimization of polymyxin B regimens for the treatment of carbapenem-resistant organism nosocomial pneumonia: a real-world prospective study. Crit. Care. 27, 164. https://doi.org/10.1186/s13054-023-04448-z (2023).
Ghosh, P. & Dewanji, A. Effect of reporting bias in the analysis of spontaneous reporting data. Pharm. Stat. 14, 20–25. https://doi.org/10.1002/pst.1657 (2015).
He, X. et al. Risk factor analysis for the occurrence of colistin-related nephrotoxicity: A retrospective observational cohort study. Int. J. Antimicrob. Agents. 64, 107232. https://doi.org/10.1016/j.ijantimicag.2024.107232 (2024).
Funding
The work was supported by Beijing Health and Medical Public Welfare Foundation Medical Science Research Fund (YWJKJJHKYJJ-BXS1-22002).
Author information
Authors and Affiliations
Contributions
PTX and LLX conceived and designed the study. All authors contributed to material preparation and data collection. PTX conducted the statistical analysis. PTX and LLX prepared the first draft of the manuscript. All authors critically revised the manuscript for important intellectual content. ZHT supervised the study. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, P., Xu, L., Ji, H. et al. Analysis and comparison of adverse events of colistin administered by different routes based on the FAERS database. Sci Rep 15, 10384 (2025). https://doi.org/10.1038/s41598-025-94947-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-94947-6