Abstract
Extracellular vesicles (EVs), particularly exosomes, are crucial mediators of intercellular communication that influence immune responses, cell proliferation, and angiogenesis. Their therapeutic potential has been explored for the treatment of chronic, non-healing wounds, especially in diabetic patients with chronic inflammation, impaired cellular proliferation, and poor angiogenesis. We investigated the role of exosomes derived from human embryonic kidney 293T (HEK293T) cells and human adipose-derived stem cells (hADSCs) on NIH3T3 fibroblast migration. Exosomes were characterized for size, concentration, and surface markers. Western blotting and scratch assays were used to assess ERK1/2 activation and cell migration. Characterization revealed that HEK293T exosomes were more abundant (3.1 × 10¹¹ ± 4.9 × 10⁹ particles/mL), larger (178 ± 4 nm), and exhibited stronger surface marker protein expression than exosomes from hADSCs (2.4 × 10⁹ ± 4.1 × 10⁸ particles/mL, 153 ± 5 nm). Both types of exosomes significantly enhanced NIH3T3 fibroblast migration with migration indices of 63.2 ± 4.3% and 48.9 ± 2.7% via ERK1/2 signaling, with HEK293T exosomes showing stronger effects because of their higher protein content. Western blot analysis revealed that robust ERK1/2 activation by both exosome types was crucial for fibroblast migration, with scratch assays highlighting their pro-migratory effects. Inhibition of the ERK1/2 pathway significantly reduced migration, with HEK293T exosomes showing a reduction to 9.6 ± 3.2% and hADSC exosomes to 2.6 ± 0.2% when treated with the MEK1/2 inhibitor PD98059. HEK293T exosomes induced more pronounced cell migration, and hADSC exosomes showed improved efficacy under stress, suggesting that both exosome types are promising candidates for targeted regenerative therapies in chronic wound healing.
Similar content being viewed by others
Introduction
Extracellular vesicles (EVs), particularly exosomes (30–150 nm), have emerged as pivotal mediators of intercellular communication and contribute significantly to various physiological and pathological processes1,2. As a specialized subset of EVs, exosomes are nanoscale, membrane-bound vesicles released by a wide range of cell types. They facilitate the transfer of bioactive molecules, such as proteins, lipids, and RNAs, to recipient cells, thereby modulating a multitude of cellular functions, including immune responses, cellular proliferation, and angiogenesis3,4,5. The involvement of exosomes in the progression of various diseases, including cancer and neurodegenerative disorders, has been extensively documented6,7. Because of their inherent capacity to alter the microenvironment of target cells, exosomes have recently gained significant attention as potential therapeutic agents8,9.
Exosomes are promising tools for delivering bioactive molecules and modulating immune responses, promising advances in targeted therapies. Chronic wounds affect 6.5 million patients in the U.S., costing over $25 billion annually due to prolonged care and complications10. These wounds, which are especially prevalent among diabetic patients, present significant clinical challenges due to impaired healing processes characterized by persistent inflammation, diminished cellular proliferation, and inadequate angiogenesis11,12,13. Chronic wounds are often associated with a heightened risk of infection and can lead to severe complications, including limb amputation14. Despite advancements in conventional treatments, such as dressings and wound debridement, their efficacy remains limited, underscoring the need for innovative therapeutic strategies15. Notably, exosomes derived from human adipose-derived stem cells (hADSCs) aid diabetic wound healing by enhancing vascularization and modulating immune responses through VEGF delivery16. Human embryonic kidney 293T (HEK293T)-derived exosomes are chosen for their high yield and efficiency in delivering bioactive molecules, which modulate cell survival, proliferation, and gene expression. Their consistent production and ease of manipulation make them ideal for studying intercellular communication and for therapeutic applications17. Stem cell-derived exosomes, particularly those derived from hADSCs, enhance wound healing by promoting cellular proliferation, stimulating angiogenesis, and attenuating inflammation18. Empirical studies have reported that hADSC-derived exosomes significantly accelerate wound closure and enhance vascularization in full-thickness mouse wound models, highlighting their therapeutic potential in chronic wound management16.
In the present study, we aimed to elucidate the molecular mechanisms by which exosomes modulate cellular behavior, with a particular focus on wound healing. We used exosomes derived from HEK293T cells and hADSCs to investigate their effects on NIH3T3 fibroblasts—key players in wound healing. We compared HEK293T and hADSC cells due to their distinct and complementary roles in EV research. HEK293T cells produce high yields of EVs and serve as a robust baseline for mechanistic studies, while hADSCs are clinically relevant for their regenerative potential, including angiogenesis, immune modulation, and wound healing. This comparison bridges experimental research and translational applications, enabling evaluation of both EV-mediated signaling mechanisms and their therapeutic potential in promoting fibroblast migration and wound repair. Western blot analysis was employed to assess receptor activation in NIH3T3 fibroblasts following treatment with exosomes from HEK293T cells and hADSCs, focusing on the level of phosphorylated ERK (p-ERK) as an indicator of enhanced fibroblast migration and proliferation. A deeper understanding of the molecular mechanisms underlying exosome-mediated wound healing may pave the way for the development of more effective therapies for chronic wounds.
Materials and methods
Materials
HEK293T cells were kindly provided by Professor Akiyoshi Fukamizu of the Life Science Center for Survival Dynamics, University of Tsukuba, Japan. hADSCs were purchased from Lonza (PT-5006; Morrisville, NC, USA). NIH3T3 fibroblast were provided by the Health Science Research Resources Bank (JCRB0615; JCRB Cell Bank, Osaka, Japan).
For HEK293T cell culture and preparation of the exosome-contained solution, Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Waltham, MA, USA), fetal bovine serum (FBS; Gibco) and FreeStyle™ Expression Medium (Gibco) were used.
For hADSC culture and exosome isolation, ADSC Basal Medium (ADSC-BM; PT-3273; Lonza, Basel, Switzerland), ADSC-GM SingleQuots™ Supplement Kit (PT-4503; Lonza), DMEM/Ham’s F-12 with L-glutamine and sodium pyruvate (Wako Fujifilm, Osaka, Japan), insulin-transferrin-sodium selenite (ITS; Cosmo Bio, Tokyo, Japan), L-alanyl-L-glutamine (Wako Fujifilm) and Recombinant Human FGF basic/FGF2 (145 aa) GMP Protein (3718-GMP; R&D Systems, Minneapolis, MN, USA) were used.
For NIH3T3 fibroblast, newborn calf serum (NBCS; Gibco) was used.
For ultrafiltration of the exosome-containing solution, a Millex-GV syringe filter (Merck, Darmstadt, Germany), a Centrifugal Ultrafiltration Filter Unit 100,000 MWCO (CFU100K; AS ONE Corporation, Osaka, Japan), and Total Exosome Isolation Reagent (Thermo Scientific, Waltham, MA, USA) were used.
Reagents used for western blotting included a Micro BCA Protein Assay Kit (#23235; Thermo Scientific), RIPA buffer (Wako Fujifilm), beta-mercaptoethanol (Nacalai Tesque, Kyoto, Japan), Mini-PROTEAN Tetra Cell (BioRad, Hercules, CA, USA), Gel Doc XR + system (#1708195; BioRad), PVDF membrane (Merck), TBS-Tween Tablets (AS ONE Corporation), Blocking One (Nacalai Tesque), Clarity Western ECL Substrate (#1705060; BioRad, Hercules, CA, USA), Nitrocellulose membrane (Merck), Skim milk powder (Wako Fujifilm), Primary antibodies included anti-CD63, anti-CD81, anti-CD9 antibodies (EXOAB-CD63A-1, EXOAB-CD81A-1, EXOAB-CD9A-1, respectively; System Biosciences, Palo Alto, CA, USA) and phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (E10) Mouse mAb (#9106; Cell Signaling, Danvers, MA, USA). Secondary antibodies included goat anti-rabbit HRP secondary antibodies (EXOAB-CD63A-1, EXOAB-CD81A-1, EXOAB-CD9A-1; System Biosciences, Palo Alto, CA, USA) and HRP-conjugated Affinipure Goat Anti-Mouse IgG (H + L) (SA00001-1; Proteintech, Rosemont, IL, USA).
For the inhibition of ERK1/2 pathway, PD98059 (Merck), and U0126 (Wako Fujifilm) were used.
For the cell proliferation assay, the Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Mashiki, Japan) and Multiskan™ FC Microplate Photometer (51119050; Thermo Scientific, Waltham, MA, USA) were used.
For the morphology detection and labeling of exosomes, NanoSight LM10-HS (Malvern Panalytical, Worcestershire, UK), Excel support film on the Transmission Electron Microscopy (TEM) grids (No.649; Nisshin EM Co., Ltd., Tokyo, Japan), Tecnai G2 F20 (FEI Company Japan Ltd., Tokyo, Japan), DAPI (D9542; Merck), PKH67 Green Fluorescent Cell Linker Kit (Merck), and a basic fluorometer (DS-11 FX+; DeNovix, USA) were used.
All statistical analyses were performed using GraphPad Prism (version X.X; GraphPad Software, USA) and Microsoft Excel (Microsoft, USA). Data are expressed as mean ± standard deviation (SD). A two-tailed Student’s t-test was used for pairwise comparisons, and one-way ANOVA was used for multiple-group comparisons. P < 0.05 was considered statistically significant. These tools were used consistently throughout the study for data analysis.
Methods
Cell culture
HEK293T cells were cultured in DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin at 37 °C with 5% CO2 until reaching 80% confluence after 3 days. To collect exosomes, cells were washed twice with Phosphate-buffered saline (PBS, Wako Fujifilm) to remove FBS and cultured in FreeStyle™ Expression Medium. After 72 h, the culture medium was collected for exosome isolation.
hADSCs were cultured in ADSC-BM supplemented with the ADSC-GM SingleQuots™ Supplement Kit at 37 °C with 5% CO2 until confluence. To collect exosomes, cells were washed twice with PBS to remove the FBS and cultured in DMEM/Ham’s F-12 with L-glutamine and sodium pyruvate supplemented with ITS, FGF-2, and L-alanyl-L-glutamine. After 72 h, the culture medium was collected for exosome isolation.
NIH3T3 fibroblast were cultured in DMEM with 10% NBCS at 37 °C and 5% CO2 until confluence.
For the ERK1/2 pathway inhibition assay, we used MEK1/2 inhibitor PD98059 and U012619,20. For the detection of the inhibited phosphorylated ERK1/2 pathway, the inhibitors (PD98059:19 µM, U0126:10 µM) were added into culture medium and culture along with NIH3T3 fibroblasts for 30 min before the further exosome treatment.
Exosome isolation
The collected HEK293T and hADSC culture media were initially centrifuged at 200×g for 3 min at 25 °C to remove residual cells. The supernatant was then subjected to sequential centrifugation steps at 500×g for 30 min at 4 °C, followed by 2000×g for 30 min at 4 °C to remove cell debris. After filtration through a syringe filter (0.22 μm Millex-GV syringe filter) to eliminate larger vesicles, the filtrate was further concentrated by ultrafiltration at 5000×g for 60 min at 4 °C using a Centrifugal Ultrafiltration Filter with a 100 kDa molecular weight cutoff. The final exosome fraction was stored at – 30 °C until further analysis. The total protein concentration in this fraction was determined using a Micro BCA Protein Assay Kit, yielding approximately 2.5 mg/mL for the HEK293T fraction and 1 mg/mL for the hADSC fraction.
Size analysis of exosomes
For Nanoparticle Tracking Analysis (NTA), exosome samples were diluted to 10 µg/mL in PBS as a final concentration of total protein. Pre-analysis settings included a camera level of 16, a gain of 10, and a detection threshold of 4. These parameters were optimized to ensure accurate particle tracking and minimize background noise. NTA was performed under these controlled conditions to provide reproducible measurements of exosome size and concentration, allowing reliable comparison between HEK293T- and hADSC-derived exosomes.
Transmission electron microscopy (TEM)
The samples were prepared using a negative staining technique as described previously21. Briefly, a 10 µL exosome suspension (2 mg/mL) was placed on a thin film mesh grid (Excel support film on the TEM grids) for 30 s. After rinsing, the solution was replaced with a 1% phosphotungstic acid hydrate solution (150 µL in pure water) and exposed for 30 s. Excess solution was removed with filter paper, and the grid was air-dried. The prepared samples were examined using a Tecnai G2 F20 instrument at an acceleration voltage of 120 kV.
Quantification of microRNA (miRNA) concentration in exosomes
The miRNA concentration in the exosomes was quantified using Qubit™ microRNA Assay Kits (Invitrogen, Thermo Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. The miRNA-specific dye solution was diluted 200 times with HS buffer. The dye solution (198 µL HEK293T; 180 µL hADSC) was mixed with the exosome solution (2 µL HEK293T; 20 µL hADSC), vortexed for 3–5 s, and incubated for 2 min at room temperature. Fluorescence intensity of the solution was measured using a Qubit® 4.0 Fluorometer (Invitrogen, Thermo Scientific, Waltham, MA, USA).
Western blotting
To detect the representative exosome surface markers (CD63, CD81, and CD9), the fractions containing exosomes from hADSCs and HEK293T cells were adjusted to the same protein concentration (1 mg/mL for each) and incubated with RIPA buffer for 40 min on a shaker at 4 °C. The lysed samples were incubated for 10 min at 95 °C with loading buffers containing beta-mercaptoethanol. Mini-PROTEAN Tetra Cell was used for electrophoresis using 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE, 40 V for 40 min, 65 V for 3 h). The resulting gel was transferred to a PVDF membrane using a Semi-Dry Electrophoretic Transfer Cell (standard mode, 65 min) and blocked with 5× TBS-T (100 mL/Tablet) diluted blocking solution for 1 h at room temperature. The blocked membrane was incubated with primary antibodies (anti-CD63, anti-CD81, and anti-CD9; 1:1000 dilution in TBS-T containing 5% Bovine Serum Albumin, BSA) for 18 h at 4 °C. After washing three times with TBS-T for 5 min on a shaker at room temperature, the membrane was incubated with a secondary antibody (goat anti-rabbit HRP; 1:20000 in TBS-T) for 1 h at room temperature. The membranes were then treated with Clarity Western ECL Substrate and imaged using a Gel Doc XR + system.
For the detection of the phosphorylated ERK1/2 pathway, the gel was transferred onto a nitrocellulose membrane and blocked with 5% skim milk in 0.1% TBS-T. Membranes were incubated with primary (Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (E10) mouse mAb; 1:1000 dilution in TBS-T containing 5% skim milk) and secondary antibodies (HRP-conjugated Affinipure Goat Anti-Mouse IgG (H + L)) as described previously.
Cell viability
The NIH3T3 fibroblasts were used for cell viability assays. Fibroblasts (1.5 × 105 cells/mL) were seeded in a 96-well microplate (100 µL/well; Iwaki, Shizuoka, Japan) in DMEM supplemented with 10% NBCS and incubated at 37 °C in 5% CO2 for 24 h. The medium was then replaced with DMEM containing 0.4% NBCS after a 24 h-starvation. The medium was then removed, and the fibroblasts were washed with PBS. DMEM was added to the control group and the inhibitor groups were treated for 1 h at different concentrations. Afterward, the CCK-8 reagent (10 µL) was added to each well and the plate was incubated at 37 °C with 5% CO2 for 2 h. The absorbance was measured at 450 nm with a Multiskan™ FC Microplate Photometer. The cell viability percentage was calculated using the following equation: Viability = (A_treatment – A_blank)/(A_control – A_blank) × 100%, where A is the absorbance.
Fluorescence labeling of the exosome membranes
The isolated exosomes (50 µL) were mixed with the PKH67 Green Fluorescent Cell Linker Kit (1:250 dilution) for 5 min. Total Exosome Isolation Reagent was mixed with the sample for 60 min at 4 °C. The mixture was then centrifuged at 10,000 ×g for 60 min at 4 °C, and the precipitate was collected as labeled exosomes. Fluorescence intensity of the PKH-labeled exosomes was measured using a DS-11 FX + basic fluorometer (DeNovix, USA). For the detection, the fluorometer was set to excitation wavelength of 470 nm, and emission was recorded in the range of 514–567 nm to capture the specific fluorescence signal from PKH67. The PKH labelling of HEK293T-derived exosomes mostly maintained their particle mean size and concentration, while the PKH labelling altered the mean particle size of hADSC-derived vesicles and affected the labelled populations (Fig. S7). Although we used those labelled exosomes for confocal microscopic observation, the PKH67 labeling might impact the cellular uptake of labelled exosome. However, the PKH67 was inserted into the hydrophobic bilayer membrane of exosomes, so that the exosome surface property could be remained intact. Therefore, we considered that the labelling is available for visualizing of the exosome.
Confocal microscopy
NIH3T3 fibroblast (1 × 105 cells/mL in 500 µL) were seeded onto a 35 mm glass-bottom dish and cultured for 24 h at 37 °C in 5% CO2. Labeled exosomes from HEK293T and hADSC were resuspended in 250 µL DMEM and co-cultured with NIH3T3 fibroblast for 30 min at 37 °C. After incubation, DAPI (1 mg/mL in 1µL) was used to stain the nuclei of NIH3T3 fibroblast for 20 min at 37 °C. After washing twice with PBS, the cells were observed using a confocal laser scanning microscope and compared with those in the PBS group.
Scratch assay
To observe NIH3T3 cell migration, scratch assays were performed on monolayers of NIH3T3 fibroblasts. Fibroblasts (1.5 × 105 cells/well) were seeded into 6-well plates and incubated overnight. A 200 µL pipette tip was used to create a linear wound. Serum-free medium containing exosomes from HEK293 cells or hADSCs was added after washing each well with PBS. An inverted microscope was used to capture images at 0 and 24 h. ImageJ (version 1.53a, NIH, USA) was used to measure the size of the migration area and calculate the migration rate as follows: Migration rate (%) = [area (0 h) – area (24 h)]/area (0 h) × 100%.
Results
Morphology and characterization of exosomes
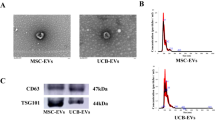
We observed the morphology of exosomes from HEK293T cells (Fig. 1a) and exosomes from hADSCs (Fig. 1b) using TEM. Revealing a spherical shape with diameters ranging from 50 to 200 nm, consistent with the size distribution and particle concentration measured by NanoSight analysis (Fig. 1c, d) The concentration of exosomes from hADSCs was determined to be 2.4 × 109 ± 4.1 × 108 particles/mL with a mean size of 153 ± 5 nm (P < 0.001, n = 5) ([total protein] = 1.0 mg/mL). In comparison, the concentration of exosomes from HEK293T cells was significantly higher at 3.1 × 1011 ± 4.9 × 109 particles/mL, with a mean size of 178 ± 4 nm (P < 0.001, n = 5) ([total protein] = 2.3 mg/mL). This result indicates that exosomes from HEK293T cells are more abundant and larger in size compared to exosomes from hADSCs.
For the PKH-labeled exosomes used in confocal microscopy, the particle count was measured using NanoSight (Fig. S7). The particle concentration of HEK293T-derived exosomes post-labeling was 4.1 ± 0.2 × 108 particles/mL, and for hADSC-derived exosomes, it was 2.8 ± 0.2 × 108 particles/mL.
Western blot analysis (Fig. 1e) revealed the presence of representative exosomal surface markers CD9, CD63, and CD81 in exosomes from both HEK293T cells and hADSCs at the same total protein concentration of 2 mg/mL. We found that exosomes from HEK293T cells exhibited a stronger expression of these three markers than exosomes from hADSCs. Exosomes with the same protein concentration, rather than the same quantity of exosomes, served as a better horizontal comparison with existing studies. Consistent with the NanoSight data, we observed a higher number of HEK293T-derived exosomes than hADSC-derived exosomes with the same protein concentration, contributing to their higher expression and abundance of surface marker proteins. The total miRNA concentration was 34 ng/µL in HEK293T-derived exosomes and 0.24 ng/µL ([total protein] = 2.0 mg/mL) in hADSC-derived exosomes, suggesting that HEK293T-derived exosomes may be more effective for therapeutic applications at the same total protein concentration because of their higher quantity of bioactive components such as miRNAs.
Characterization of isolated exosomes. a TEM image of HEK293T exosomes. Scale bar: 100 nm b TEM image of hADSC exosomes. Analysis of c HEK293T and d hADSC exosome size distribution and particle number by NanoSight. e Characterization of exosomes using western blotting with representative surface markers (CD63, CD81, and CD9). The original data of (A, B) was shown in Fig. S2.
Internalization of exosomes by NIH3T3 fibroblasts
We then observed the interaction between PKH67-labelled exosomes and NIH3T3 fibroblast using confocal laser scanning microscopy (Fig. 2a, b). The labeled exosomes were detected in the cells, indicating their internalization into the cytoplasm of the NIH3T3 fibroblasts. Exosomes from HEK293T cells were more abundantly taken up by recipient cells than exosomes from hADSCs. The fluorescence intensity of the cells was measured for quantitative analysis (Fig. 2c). Compared to the PBS control group baseline fluorescence intensity (143.4 a.u.), the exosomes from the hADSC and HEK293T groups exhibited a higher fluorescence intensity (3035.9 a.u. and 3841.1 a.u., respectively), indicating the uptake of the exosomes by the fibroblasts.
Effects of exosomes on cell migrations
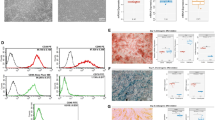
Next, scratch assays were performed with NIH3T3 fibroblast using exosomes from HEK293T cells. The control group showed minimal cell migration into the scratch area (Fig. 3), indicating baseline migration in the absence of exosome treatment. In contrast, exosomes from the HEK293T-treated group exhibited significant cell migration, where gap closure was noticeably more complete than that in the control group. This indicated the enhancement of cell migration activity and pro-migratory effects by the interaction of exosomes with cells. To study the effect of exosomes on cell migration, we focused on the ERK1/2 signaling pathway by using two inhibitors (PD98059 and U0126); ERK1/2 are ubiquitously expressed non-receptor proteins and participate in the regulation of cell adhesion, cell cycle progression, cell migration, proliferation, and transcription22. The activation of ERK1/2 promotes cell migration by interacting with cytoskeletal elements and regulating the activity of myosin light chain kinase23. Relative research on EVs derived from ADSCs found the alleviation of postoperative abdominal adhesion by activating the MAPK-ERK1/2 pathway24.
When NIH3T3 fibroblast were treated with exosomes from HEK293T cells in the presence of PD98059, cell migration was significantly reduced compared to that in the inhibitor-free group (Fig. 3). However, treatment with this inhibitor did not affect cell viability (Supplementary Fig. S1). After incubation for 24 h, the scratch area remained open, indicating that the inhibition of the ERK1/2 pathway was strongly involved in cell migration induced by exosomes. Similar results were obtained in a scratch assay using exosomes from hADSCs in the presence of PD98059 (Fig. 4). The migration of NIH3T3 fibroblast was activated more by exosomes from hADSC than in the control group, and migration was inhibited by the presence of the ERK1/2 inhibitor PD98059.
Quantitative analysis of scratch assays
We quantitatively analyzed the results from the scratch assays using ImageJ software (Fig. 5), in which the migration index of NIH3T3 fibroblast was calculated under treatment conditions. The migration index of the exosomes from HEK293T and hADSC groups was 63.2 ± 4.3% and 48.9 ± 2.7%, respectively, while the control group, representing baseline cell migration, exhibited a migration index of 8.1 ± 2.3%. This demonstrated their ability to promote cell migration. Meanwhile, HEK293T exosomes exhibited a significantly higher migration index (63.2 ± 4.3%) compared to hADSC exosomes (48.9 ± 2.7%, P < 0.05), which was 29.2% higher, indicating the distinct migration-promoting capabilities of HEK293T and hADSC exosomes. HEK293T groups treatment with the inhibitor PD98059 significantly reduced the migration index to 9.6 ± 3.2%. This reduction was comparable to the baseline migration in the control group, indicating that PD98059 significantly inhibited exosome-induced migration activity induced by the exosomes. Similarly, the combination of hADSC exosomes and PD98059 resulted in a migration index of 2.6 ± 0.2%, showing an even more pronounced inhibitory effect. Additional inhibitors were also tested to elucidate the pathways involved in exosome-mediated cell migration. In the case of the MEK1/2 pathway inhibitor U0126, the migration index for exosomes from HEK293T cells was 5.6 ± 0.4%, while the combination with exosomes from hADSCs resulted in a migration index of 5.5 ± 1.8%. These results suggest that U0126 similarly inhibited the migration activity of exosomes, indicating the involvement of the ERK1/2 pathway.
ERK1/2 pathway activation by exosomes
We performed western blot analysis of p-ERK levels in NIH3T3 fibroblasts after treatment with exosomes from HEK293T cells and hADSCs (Fig. 6a). The control group (FBS (-)) showed weak p-ERK expression, whereas the NBCS group showed strong p-ERK expression as the positive group. Fibroblasts cultured with exosomes from HEK293T cells exhibited a significantly strong expression of p-ERK, indicating strong activation of the ERK1/2 pathway. This increase aligned with the observed enhancement in cell migration in scratch assays, suggesting that exosomes from HEK293T cells robustly activated ERK1/2 signaling. Similar results were obtained when treated with the exosomes from hADSCs. Thus, exosomes from both HEK293T cells and hADSCs promoted cell migration by activating the ERK1/2 pathway.
When fibroblasts were also incubated with PD98059, p-ERK levels were significantly reduced in the exosome groups, indicating inhibition of ERK pathway activation (Fig. 6b). However, in the U0126-treated groups, although p-ERK levels were significantly reduced in both HEK293T and hADSC exosome-treated groups, a faint band was still visible. This suggested that while U0126 effectively inhibits the ERK1/2 pathway, it may not completely inhibit ERK1/2 activation in the presence of exosomes. This suggests that multiple regulatory mechanisms are involved in exosome-mediated ERK1/2 activation.
Discussion
The differences in characteristics between HEK293T- and hADSC-derived exosomes could be related to the cells from which they are secreted. A previous study found a strong correlation between basal EV secretion rates and mitochondrial metabolism in human blood cells, with higher mitochondrial enzyme activity significantly increasing EV production25. The distinct EV secretion rates observed between HEK293T cells and hADSCs can be attributed to the differences in their mitochondrial enzyme activities, where higher mitochondrial activity corresponds to elevated EV production and total protein concentrations.
HEK293T-derived exosomes exhibited higher expression levels of tetraspanins, such as CD9 and CD81, as well as integrins and other adhesion molecules, which facilitate stronger and more specific interactions with recipient cell receptors. This likely contributed to the more efficient binding and internalization by NIH3T3 fibroblasts, which is consistent with the previous findings that receptor-ligand interactions and surface molecule expression significantly influence EV uptake26. hADSC exosomes also greatly enhanced the migration of NIH3T3 fibroblasts even with reduced surface protein expression. This may be due to the enrichment of specific miRNAs in hADSC exosomes, such as miR-21 and miR-146a, which are known for their anti-apoptotic and anti-inflammatory roles. Additionally, these exosomes likely contained higher levels of growth factors such as VEGF, HGF, and TGF-β, which are crucial for cell survival and proliferation27. The presence of surface receptor tyrosine kinases (RTKs) on hADSC exosomes may have also played a role in activating compensatory survival pathways such as PI3K/Akt, further enhancing cell viability25. These factors highlight the therapeutic potential of hADSC-derived exosomes in regenerative medicine, which is supported by research showing their regenerative properties and the ability to promote cell growth and survival. Our findings align with previous studies demonstrating that hADSC-derived exosomes promote tissue regeneration via MAPK-ERK1/2 and PI3K-Akt activation. Shi et al. reported enhanced peritoneal healing, while Liu et al. showed protection against ischemia-reperfusion injury through similar mechanisms22,23. These studies support our observations that hADSC exosomes facilitate fibroblast migration and survival. However, our study uniquely compares HEK293T- and hADSC-derived exosomes, revealing that while HEK293T exosomes exhibit stronger pro-migratory effects, hADSC exosomes provide greater cytoprotection under pathway inhibition. This distinction highlights their complementary therapeutic potential in regenerative medicine.
Both the HEK293T- and hADSC-derived exosomes promoted cell migration, although their dependence on the ERK1/2 pathway varied. HEK293T exosomes demonstrated strong effects on cell migration, likely because of their high particle concentrations and surface protein levels, which enabled greater delivery of bioactive molecules. Valadi et al.28 emphasized that exosome-mediated transfer of mRNAs and miRNAs facilitates genetic exchange between cells, suggesting a mechanism for enhanced bioactivity. Higher levels of growth factors and cytokines in HEK293T exosomes activated the ERK1/2 pathway more effectively than those in hADSC exosomes. Additionally, Mulcahy et al.27 highlighted that uptake mechanisms involving receptor-ligand interactions, tetraspanins, and integrins significantly influence the cellular internalization of exosomes. The presence of RTKs on HEK293T exosomes, such as EGFR and VEGFR, can more efficiently trigger the downstream ERK1/2 signaling pathways29. Furthermore, the molecular composition of exosomes varies with the parent cell type, with HEK293T cells producing exosomes enriched in cargo that enhances cell migration and proliferation30. These findings align with previous research indicating a critical role of the ERK1/2 pathway in cell migration and wound healing, as its inhibition significantly diminishes the pro-migratory effects of exosomes.
All three inhibitors effectively reduced p-ERK levels induced by both HEK293T- and hADSC-derived exosomes. This reduction in p-ERK levels aligns with the observed decrease in cell migration in the presence of these inhibitors, indicating that the ERK1/2 pathway is crucial for mediating cell migration effects induced by exosomes. The differential effects of HEK293T and hADSC exosomes on ERK1/2 activation further underscore the importance of exosome source in determining the extent of ERK1/2 pathway activation and subsequent cellular responses. HEK293T exosomes induced stronger ERK1/2 activation than hADSC exosomes, which correlated with their more pronounced effects on cell migration.
This residual p-ERK signal could be due to several factors, including the potential presence of alternative signaling mechanisms or pathways in HEK293T exosomes that partially bypass U0126 inhibition, or a higher concentration of bioactive molecules within HEK293T exosomes that maintain minimal ERK1/2 activation despite U0126 treatment. The slight residual ERK1/2 activity in the U0126-treated HEK293T exosome group underscores the complexity of the cellular signaling pathways.
The differences between HEK293T- and hADSC-derived exosomes provide insights into their therapeutic potential. Exosomes with the same protein concentration, rather than the same quantity of exosomes, served as a better horizontal comparison with existing studies. HEK293T-derived exosomes had a higher number of cells with the same protein concentration compared with hADSC exosomes, contributing to their more robust enhancement of cell migration. This suggests that HEK293T-derived exosomes may be more effective for therapeutic applications because of their higher quantities of bioactive components such as miRNAs. Surface marker analysis revealed a higher protein abundance in HEK293T exosomes, presumably contributing to their more potent biological effects. However, hADSC exosomes offer better cytoprotective effects under ERK1/2 pathway inhibition, likely due to specific miRNAs and higher levels of growth factors like VEGF, HGF, and TGF-β. In scratch assays, HEK293T exosomes showed a more pronounced effect on cell migration, potentially because of their higher particle concentrations and protein contents. The faint residual p-ERK signal in the U0126-treated HEK293T exosome group suggests that HEK293T exosomes may activate alternative signaling pathways or contain higher concentrations of growth factors that partially bypass U0126 inhibition. Future research should explore these alternative pathways and the specific molecular cargos of both exosome types to optimize their therapeutic applications in wound healing and tissue repair.
This study faces limitations and challenges commonly reflected in the field. EV isolation may involve non-EV contaminants, and the heterogeneity of exosome populations was not fully addressed. Findings were based on in vitro assays, requiring in vivo validation. While ERK1/2 involvement was demonstrated, other pathways like PI3K/Akt remain unexplored. These challenges reflect broader difficulties in the field and underscore the need for further methodological advancements.
Conclusion
Both HEK293T- and hADSC-derived exosomes significantly enhanced NIH3T3 fibroblast migration by activating the ERK1/2 pathway, which align with present research trend supporting the regenerative potential of hADSC-derived exosomes via MAPK-ERK1/2 and PI3K-Akt activation. Meanwhile, HEK293T exosomes show stronger effects owing to their higher particle concentrations, exhibited a higher migration index (63.2 ± 4.3%) compared to hADSC exosomes (48.9 ± 2.7%, P < 0.05), which is consistent with their higher particle concentration (3.1 × 1011 ± 4.9 × 109) particles/mL vs. (2.4 × 109 ± 4.1 × 108) particles/mL, (P < 0.01) and higher miRNA content (33.5 ± 0.3 ng/µL vs. 0.25 ± 0.02 ng/µL, P < 0.01). While HEK293T exosomes showed stronger pro-migratory effects, hADSC exosomes offer potential advantages under inhibition conditions. Both exosome types demonstrate promising potential as candidates for targeted regenerative therapies for chronic wound healing.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Théry, C. et al. A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 7(1), 1535750 (2018).
Yáñez-Mó, M. et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 4(1), 27066 (2015).
Colombo, M., Raposo, G. & Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289 (2014).
Tkach, M. & Théry, C. Communication by extracellular vesicles: where we are and where we need to go. Cell 164(6), 1226–1232 (2016).
El Andaloussi, S., Mäger, I., Breakefield, X. O. & Wood, M. J. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 12(5), 347–357 (2013).
Kalluri, R. & LeBleu, V. S. The biology, function, and biomedical applications of exosomes. Science 367(6478), eaau6977 (2020).
Fevrier, B. & Raposo, G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr. Opin. Cell Biol. 16(4), 415–421 (2004).
Vader, P., Breakefield, X. O. & Wood, M. J. Extracellular vesicles: emerging targets for cancer therapy. Trends Mol. Med. 20(7), 385–393 (2014).
Barile, L. & Vassalli, G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 174, 63–78 (2017).
Sen, C. K. et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair. Regen.. 17(6), 763–771 (2009).
Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 366(9498), 1736–1743 (2005).
Guo, S. & DiPietro, L. A. Factors affecting wound healing. J. Dent. Res. 89(3), 219–229 (2010).
Gurtner, G. C., Werner, S., Barrandon, Y. & Longaker, M. T. Wound repair and regeneration. Nature 453(7193), 314–321 (2008).
Armstrong, D. G., Boulton, A. J. & Bus, S. A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 376(24), 2367–2375 (2017).
Reinke, J. M. & Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 49(1), 35–43 (2012).
Liu, R., Dong, R., Chang, M., Liang, X. & Wang, H. C. Adipose-derived stem cells for the treatment of diabetic wound: from basic study to clinical application. Front. Endocrinol. 13, 882469 (2022).
Kowal, J., Tkach, M. & Théry, C. Biogenesis and secretion of exosomes and extracellular vesicles. Curr. Opin. Cell Biol. 29, 116–125 (2016).
Mathivanan, S. & Simpson, R. J. ExoCarta: a compendium of exosomal proteins and RNA. Proteomics 9(21), 4997–5000 (2009).
Hashimoto, Y. et al. Inflammatory cytokine-induced HIF-1 activation promotes epithelial-mesenchymal transition in endometrial epithelial cells. Biomedicines 11(1), 210 (2023).
Ichikawa, M. K. et al. Ets family proteins regulate the EMT transcription factors snail and ZEB in cancer cells. FEBS Open. Bio. 12(7), 1353–1364 (2022).
Huang, T. et al. Surface modulation of extracellular vesicles with cell-penetrating peptide-conjugated lipids for improvement of intracellular delivery to endothelial cells. Regen. Therapy. 22, 90–98 (2023).
Roskoski, R. Jr ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol. Res. 66(2), 105–143 (2012).
Wortzel, I. & Seger, R. The ERK cascade: distinct functions within various subcellular organelles. Genes cancer. 2(3), 195–209 (2011).
Shi, M. et al. Extracellular vesicles derived from adipose mesenchymal stem cells promote peritoneal healing by activating MAPK-ERK1/2 and PI3K‐Akt to alleviate postoperative abdominal adhesion. Stem Cells Int. 2022(1), 1940761 (2022).
Auber, M. & Svenningsen, P. An estimate of extracellular vesicle secretion rates of human blood cells. J. Extracell. Biol., 1(6), e46 (2022).
Mulcahy, L. A., Pink, R. C. & Carter, D. R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles. 3(1), 24641 (2014).
Zhang, Z. J. et al. MiRNA expression profile during osteogenic differentiation of human adipose-derived stem cells. J. Cell. Biochem. 113(3), 888–898 (2012).
Li, J. et al. Microvesicle-mediated transfer of microRNA-150 from monocytes to endothelial cells promotes angiogenesis. J. Biol. Chem. 291(46), 24090–24100 (2016).
Kosaka, N. et al. Secretory mechanisms and intercellular transfer of MicroRNAs in living cells. J. Biol. Chem. 285(23), 17442–17452 (2010).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9(6), 654–659 (2007).
Acknowledgements
Authors thank Akiko Kuramochi, Yoshio Ohba, Takeyuki Uchida and Yuya Sato for technical support, Dr Masahiro Asada for inhibitors and Dr. Masamune Morita for measurement with NanoSight.
Funding
This research was supported in part by a Grant-in-Aid for Scientific Research (B) (22H03966) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan.
Author information
Authors and Affiliations
Contributions
W.Z.: conceptualization, design, and execution of the experiments, methodology, data collection and analysis, manuscript preparation, and writing. S.O.: data collection and analysis. Y.T.: conceptualization, design of the experiments, data analysis and interpretation, manuscript preparation, and final approval of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have read and agreed to the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, W., Obuchi, S. & Teramura, Y. Analysis of cellular responses following interaction with extracellular vesicles derived from HEK293T and human adipose derived stem cells. Sci Rep 15, 11835 (2025). https://doi.org/10.1038/s41598-025-95559-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-95559-w