Abstract
Acute febrile illness (AFI) investigations are crucial for public health. They can provide data on disease prevalence, morbidity, and mortality, and improve treatment, management, control, and detection of outbreaks in areas with limited diagnostic tests. Current understanding of multiple causes of AFI in the paediatric population in Tanzania is limited. This study aimed to simultaneously detect 33 pathogens using TaqMan Array Card based real-time PCR. Whole blood samples were collected from a total of 247 children (2–59 months old) who presented with febrile illness at Dareda and Haydom hospitals in north-eastern Tanzania between November 2015 and March 2016. Overall, 50 (20.2%) and 8 (3.2%) of 247 children had at least one and more than one pathogen detected respectively. Bacterial zoonoses were frequently detected including Brucella spp. (n = 18, 7.3%), C. burnetii (n = 4, 1.6%), Bartonella spp. (n = 3, 1.2%), Rickettsia spp. (n = 3, 1.2%) and Leptospira spp. (n = 1, 0.4%). Dengue virus was detected in 14 (5.7%) individuals and Plasmodium spp. in 12 (4.9%) individuals. These findings reveal the potential clinical importance of zoonoses and arboviruses in febrile children in Tanzania and highlight the need to consider a broad range of pathogens in febrile illness diagnosis.
Similar content being viewed by others
Introduction
Acute febrile illness (AFI) is a leading cause of presentation at primary health facilities in the tropics 1 that contributes to high burdens of morbidity and mortality in sub-Saharan Africa and worldwide 2,3. Several infectious agents including viruses, parasites, and fungi can cause AFI, as well as non-infectious conditions like autoimmune diseases, inflammatory disorders, cancer, and drug reactions 4. The majority of deaths in children younger than five years (64.0%) are attributed to infectious causes 5. The infectious causes of AFI vary widely by age groups, immune status, geographic locations, ecology, and climate 6,7. Differential diagnoses are made by considering clinical presentations, patient history, results from diagnostic tests, and epidemiological factors. However, diagnosing acute febrile illness can be challenging because many diseases can present with similar symptoms and diagnostic tools for the full range of potential causes are often lacking 3,8,9.
AFI aetiology studies are crucial to inform empirical treatment and care, for generating estimates of prevalence, morbidity, death, and economic effect of multiple pathogens and to inform public health intervention development. Studies generating data on multiple pathogens can also detect novel and re-emerging pathogens and identify new outbreaks in contexts where there are few laboratory diagnostic tests available to identify causes of AFI 8.
TaqMan array cards (TAC) have been developed to enable simultaneous detection of multiple pathogens 10. TAC is a real-time PCR based system that is sensitive and specific for multiple pathogen detection and has been used for surveillance, emerging and re-emerging pathogen detection, and outbreak investigation in high and low malaria endemic contexts in Sub-Saharan Africa 9,10,11,12,13,14,15.
In Tanzania, there has been extensive research on AFI but the majority of studies have focused on individual pathogens. Only a small number of studies in Tanzania have included multi-pathogen diagnostic approaches 9,14,16,17,18,19. Of these, most have enrolled patients of all ages and provide data on AFI in adults and adolescents predominantly. There remains a lack of data on AFI aetiology in Tanzania overall and specifically in children under 5 years. This study aimed to simultaneously detect 33 agents that are associated with AFI in children under five years of age who sought medical care at Haydom and Dareda hospitals in Manyara Region, north-eastern Tanzania.
Methods
Study design, population, and sampling
A cross-sectional, hospital-based study was conducted to enrol children presenting for care with febrile illness to the outpatient or inpatient departments of Dareda and Haydom hospitals in the Manyara region of north-eastern Tanzania. These hospitals provide a range of healthcare services and serve predominantly agro-pastoral communities 20 in an area of low malaria endemicity 21.
Children aged 2–59 months with an axillary temperature of ≥ 38°C at the time of presentation were eligible for inclusion in the study. Children receiving anti-retroviral therapy, tuberculosis therapy, or who reported use of antibiotic therapies for three or more days in the two weeks before screening were excluded. Whole blood samples of up to 2.5mL volume were collected into EDTA vacutainers at the time of enrolment, stored temporarily at 2–8°C for up to three days then stored at -80°C, shipped to the Kilimanjaro Clinical Research Institute Biotechnology Laboratory and stored there at -80 °C freezer until TAC testing.
Demographic and socio-economic data recorded for study participants included participant age in months, sex, the occupation of their mother and father, and if domestic animals were kept in their household. Clinical data recorded (for patients presenting to Haydom only) included the final diagnosis recorded in hospital records. Given variable information available to clinicians to inform this assessment and variable timing of field recording after initial presentation analyses of these data were limited to checks of the mention (or not) of pathogens (and corresponding disease names) detected in 10 or more individuals through TAC testing in the clinical diagnosis field.
Nucleic acid extraction and TaqMan array card testing
High pure viral nucleic acid large-volume kits (Roche, Germany) were used for nucleic acid extraction as per the manufacturer’s instructions. Briefly, 1.5 mL of EDTA blood was subjected to a lysate preparation process, followed by purification and elution of nucleic acid using spin columns, including a high pure extender assembly for an initial large volume. Extrinsic controls PhHV (Phocine Herpesvirus) for DNA targets and MS2 bacterial phage for RNA targets were added to each sample during the lysate preparation to evaluate extraction and amplification efficiency. For each batch of extractions, a blank was processed through the complete protocol and later tested on a real-time PCR to rule out contamination. The eluted nucleic acids were stored at 80 °C before testing.
A TAC system as described previously 10 was used to simultaneously detect 33 AFI-associated viral, bacterial, and parasitic pathogens. Target pathogens were Chikungunya virus, Dengue virus, Zika virus, Crimean Congo Haemorrhagic Fever (CCHF) virus, Bundibugyo virus, Ebola virus, Sudan virus, Hepatitis E virus, Lassa virus, Marburg virus, Measles virus, Nipah virus, O’nyong-nyong virus, Rift Valley fever virus, West Nile virus, Yellow fever virus, B. anthracis, Bartonella spp., Brucella spp., C. burnetii, Leptospira spp., N. meningitidis, Rickettsia spp., S. enterica Typhi, S. enterica Paratyphi A, S. aureus, S. pneumonia, Group A streptococcus, Y. pestis, Leishmania spp., Plasmodium spp., and T. brucei. Primers and probes at concentrations of 900 nM and 250 nM respectively were used 10. Test cards were loaded with a mixture of 75 µl of total nucleic acid and 25 µl of TaqMan fast virus one-step mastermix (Life Technologies; Thermo Fisher Scientific, Carlsbad, CA). No template, positive controls (for all target pathogens), and extrinsic controls (PhHV and MS2) were included in all test runs. Cards were run on the ViiA7 real-time PCR system (Life Technologies, Thermo Fisher Scientific, Carlsbad, CA) using the following PCR cycling conditions: reverse transcription step of 10 min at 50 °C and 1 denaturation cycle of 20 s at 95 °C followed by 40 two-step cycles of 3 s at 95 °C and 30 s at 60 °C. A run was considered valid if all positive controls were amplified, extrinsic controls were amplified for all samples, and no negative controls amplified. A sample was considered positive for a given target when it showed amplification with a cycle threshold (Ct) < 35. Samples showing amplification for a given target with Ct values ≥ 35 were considered borderline and were re-tested in a singleplex qPCR on the ViiA7 instrument using the individual target primers and probes 10 and identical cycling conditions. Samples with Ct < 40 in these confirmatory singleplex reactions were considered positive. Samples where amplification was not confirmed in the singleplex reaction were classified as negative. All other samples were classified as negative. All samples classified as positive and borderline for T.brucei in the TAC assay were re-tested in singleplex reactions and classified based on these singleplex reactions.
Samples that were Brucella spp. positive were additionally tested by quantitative PCR assay to distinguish between B. melitensis and B. abortus DNA. The Brucella speciation assay applied previously published primers, probes, and procedure 22. Briefly, duplicate reactions were performed on the Rotorgene platform (Qiagen) in 25 µL volumes, containing 25 µL TaqMan Universal Master Mix, 0.2 µM of each primer, 0.1 µM of each probe and 2.5 µL of template nucleic acid. Cycling conditions were as previously described (10 min at 95 °C followed by 45 cycles at 95 °C for 15 s and 57 °C for 1 min). Positive (B. abortus 544 and B. melitensis 16 M DNA) and negative (no template) controls were included in these assays. Samples were classified as positive for B. abortus and B. melitensis if a Ct < 40 was observed in either test well.
Data analysis
All data analyses, summaries, and graphs were performed using R version 4.3.2 23. Continuous variables were summarized using median and interquartile range (IQR). Categorical variables were summarized as frequencies and percentages. Binomial confidence intervals were calculated for all proportions.
Ethics declarations
This research was performed in accordance with relevant guidelines and regulations. Written consent was obtained from the parents or guardians of all participants before enrolment in the study. Ethical clearance for the collection and storage of participant samples was obtained from the College Research Ethics Review Committee (CRERC) of Kilimanjaro Christian Medical University College, certificate number 835. Additional clearance for testing for AFI pathogens was approved by the CRERC, certificate number PG 01/2022 and by the Medical Research National Health Research Ethics Review Committee (NIMR/HQ/R.8a/V0l.1X/2079).
Results
Socio-demographic distribution of participants
A total of 247 blood samples were obtained from febrile children who were recruited at Dareda and Haydom hospitals from November 2015 to March 2016. The demographic and clinical characteristics of these individuals are shown in Table 1. The majority of household respondents (82.2%) reported keeping domestic animals and farming was a dominant occupation for both mothers and fathers of the enrolled children (Table 1).
Pathogens detected by TAC real-time PCR
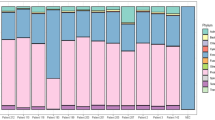
Of the 247 individuals tested, 50 (20.2%) individuals had one or more pathogens detected, including 8 bacteria, 1 protozoan, and 1 virus. The number and proportion of individuals positive for each pathogen is shown in Table 2; Fig. 1. The Ct values seen for each pathogen detected by TAC are shown in Fig. 2. A total of 8 (3.2%) individuals were positive for more than one pathogen. One individual had positive TAC results for three pathogens: C. burnetii, dengue, and group A Streptococcus. Seven individuals had two pathogens detected, including dengue and Brucella spp. (4 individuals), dengue and Rickettsia spp. (1 individual), Brucella spp. and S. aureus (1 individual), and Bartonella spp and Plasmodium (1 individual).
Cycle threshold (Ct) values observed for the pathogens detected by TAC in a population of febrile children (≤ 5 years, N = 247), presenting to hospitals in Haydom (orange points) and Dareda (blue points), northern Tanzania, 2015–2016. The dashed line at Ct = 35 indicates the threshold applied to differentiate samples classified as positive (Ct < 35) and borderline (Ct ≥ 35) by TAC.
T. brucei singleplex and Brucella speciation real-time PCR
None of the three samples initially positive for T. brucei by TAC were positive in the singleplex confirmatory test, so all were classified as negative for T. brucei. Of the 18 samples that were TAC positive for Brucella spp. five also amplified with B. abortus-specific primers. None amplified with B. melitensis-specific primers.
Clinical diagnosis
Clinical diagnoses were only recorded from participants enrolled in Haydom. Of the 133 enrolled in Haydom, 17 (12.8%) had malaria included in their clinical diagnosis. A range of pathogens were detected by TAC in this population of individuals with malaria included in the clinical diagnosis. One individual with a clinical diagnosis of malaria was TAC positive for Plasmodium. Other pathogens detected in this population of 17 individuals were Brucella spp. (n = 1), Coxiella burnetii (n = 1), dengue (n = 2), group A Streptococcus (n = 1) and Rickettsia spp. (n = 2). Neither brucellosis nor dengue were mentioned in the clinical diagnosis for any patients presenting to Haydom (Table 1).
Discussion
This study used TAC technology to detect eleven different pathogens associated with AFI in whole blood samples collected from children aged 2–59 months presenting with febrile illness from the rural agro-pastoral community of Manyara region in north-eastern Tanzania. Malaria was detected less frequently (n = 12 detections) than non-malaria pathogens combined (n = 45 detections). The two pathogens detected at highest frequency were Brucella spp. and dengue virus, both of which were detected more frequently than Plasmodium (Table 2; Fig. 1).
The overall proportion of children in whom one or more pathogens were detected by TAC was 20.2% in this study. This proportion is similar to the findings of a previous study using comparable blood TAC PCR diagnostics in Zanzibar (18.2%) 14. However, a similar study conducted in a high malaria endemicity region of Kilombero, Tanzania, found overall blood TAC PCR positivity of 49% in individuals aged one year and above 9. A study in Burkina Faso, Madagascar, and Sudan that used blood TAC with an expanded set of pathogens detected one or more pathogens in 62%, 24%, and 60% of febrile children and adolescents in these countries respectively 15. Although the target populations, methods for sampling and diagnostics are not all directly comparable, the consistent findings across these studies, that are also seen in this Tanzanian population, are the identification of zoonotic bacteria and arbovirus as prevalent pathogens among febrile patients 14,16,19.
Bacterial pathogens dominate the pathogens identified in this population and the majority of pathogens identified are zoonoses. Of the bacterial pathogens, Brucella spp. was detected in 7.3% of the participants. Brucellosis diagnosis is very challenging, with confirmed brucellosis defined by culture positivity or demonstration of seroconversion in paired samples collected several weeks apart 24. These tools are rarely available in many brucellosis endemic contexts. Many serology based tests for brucellosis that are widely used in East Africa have very low accuracy 25 and consequently, brucellosis cases are often misdiagnosed. The proportion of febrile children with Brucella spp. detection identified in this study (6.9%) is comparable to the prevalence of acute brucellosis (6.1%) identified using culture and serological testing in a predominantly pastoralist population of children and adults sampled in northeastern Tanzania in 2016–2017 26. A study of febrile children and adults performed in north-eastern Kenya in 2011 classified 15.4% of individuals as probable brucellosis cases based on RT-PCR - based testing 27. Similarly, PCR based testing identified Brucella spp DNA in 27.6% individuals aged ≤ 20 years olds with clinically suspected brucellosis sampled at two predominantly pastoralist sites in Kenya 28. In livestock-keeping populations with endemic livestock brucellosis, children account for high proportions of acute cases 29. The interpretation of Brucella spp. DNA detection in human samples and use of DNA detection to define brucellosis disease status in endemic contexts is a topic of ongoing research. Test positivity based on PCR detection can persist for long periods after initial infection and is not always associated with active disease 30. However, in this population of under five year old children these detections are more likely associated with acute infections. The Brucella speciation assay revealed that five out of 18 individuals who were positive for Brucella spp had B. abortus detected while there were no detections for B. melitensis. The likely higher sensitivity of the TAC Brucella assay, which targets a multi-copy gene and the species-specific assays, which target single copy genes, may explain the low proportion of TAC positives that were Brucella species confirmed. Further investigation of the relative performance of these assays is needed. B. abortus and B. melitensis are typically associated with cattle or sheep and goats respectively and human infections are typically more severe with B. melitensis 29. Human brucellosis is typically acquired through close contact with livestock (cattle, sheep, and goats) or consumption of raw animal products 29. Consumption of purchased milk was associated with increased risk and boiling milk was associated with reduced risk of Brucella spp. DNA detection in a Kenyan population of febrile individuals 27. Infection via consumption of unpasteurized cattle milk or through contact with livestock in the peri-domestic environment may explain the prevalence of Brucella spp. DNA detection in this population of young children.
Dengue fever is a globally important mosquito-borne viral disease of considerable public health concern. Dengue virus is endemic and re-emerging in tropical and subtropical countries 31,32. Globally, severe dengue is more likely in children 33. Since 2010 Tanzania has experienced multiple outbreaks reported every two years in Dar es Salaam and coastal regions 34,35. Despite the favourable climate for Aedes mosquitoes there are limited data on dengue in paediatric populations in north-eastern Tanzania. In this study, blood TAC PCR has detected dengue virus in 5.7% of 247 febrile children. This finding is important as no previous studies have detected dengue at this prevalence in Tanzania. A fever study in the neighbouring Kilosa region detected active dengue infection at higher prevalence in children of five years of age and above than those below five years 36. These findings highlight the importance of using the TAC real-time PCR system. Real-time PCR is a sensitive and specific diagnostic tool that can detect acute phase viral infections 37. The identification of the dengue virus in this paediatric population suggests that dengue might be an under-recognized cause of fever in Tanzania. This underscores the need to increase awareness and improve diagnostic practices among healthcare providers. It is also important to understand the true burden and transmission dynamics of dengue in different age groups within the Tanzanian population through strengthening surveillance and epidemiological studies.
This study found that 4.9% of febrile children had Plasmodium spp. detected. The prevalence obtained from this study using real-time PCR is higher than expected based on findings of the Tanzania demographic health malaria indicator survey that malaria prevalence in children of less than five years of age has been reduced to < 1% in Manyara region since 2016 38. Comparing the TAC results for Plasmodium spp. with the clinical classification of the febrile children at Haydom only one individual with a clinical diagnosis of malaria was TAC positive for Plasmodium. The higher prevalence detected by TAC may indicate enhanced detection of asymptomatic or subclinical malaria. Previous research indicates poor correspondence between clinical and laboratory-based diagnoses of malaria16 and that the use of sensitive PCR diagnostic tests may overestimate the rate of undiagnosed malaria 39.
This study has some limitations. TAC cards were used to detect 33 pathogens, and although a wide range of AFI pathogens was explored, not all infections known to cause fever in this or similar populations were fully covered. AFI studies conducted elsewhere have included a greater variety of pathogens and revealed pathogens at high prevalence, such as a recent AFI study in Senegal that highlights the role of Borrelia spp. in AFI 40. The blood samples used in this study had been stored for several years. Although samples were stored at -80 °C and a sensitive detection technique was used, there is the possibility that some nucleic acid might have been degraded leading to a lack of detection or higher detection Ct values than would have been observed if samples were tested immediately. The exclusion of children who reported using antibiotic therapies for three or more days in the two weeks before screening may have resulted in error in estimates of the prevalence of several bacterial pathogens in the tested population compared to the total population seeking care. Due to the lack of control participants and limited clinical outcome data for this population, it is challenging to determine the clinical relevance of pathogen detections and to make inferences about the clinical impact of the detected infections.
Conclusion
Findings from this study suggest that dengue virus and zoonoses such as brucellosis, bartonellosis, Q fever, rickettsiosis, and leptospirosis are important causes of acute febrile illness in children presenting from agro-pastoral communities in Manyara north-eastern Tanzania. Testing for several aetiologies of fever can help inform development of fever management and treatment guidance aimed at improving patient outcomes. Utilizing the TaqMan Array Card Real-time PCR systems for periodic surveillance provides a powerful and efficient tool for the detection of pathogens in diverse samples, contributing to early detection of pathogens, identification of context specific pathogen burdens, development of effective responses targeting multiple pathogens, and integrated health monitoring.
Data availability
The datasets generated and analysed for this study are available in the Enlighten research data repository of the University of Glasgow - https://doi.org/10.5525/gla.researchdata.1700.
References
Feikin, D. R. et al. The burden of common infectious disease syndromes at the clinic and household level from population-based surveillance in rural and urban Kenya. PLoS ONE 6, e16085. https://doi.org/10.1371/journal.pone.0016085 (2011).
Tam, P. Y. I., Obaro, S. K. & Storch, G. Challenges in the etiology and diagnosis of acute febrile illness in children in low- and middle-income countries. J. Pediat. Inf. Dis. Soc. 5, 190–205. https://doi.org/10.1093/jpids/piw016 (2016).
Maze, M. J. et al. The epidemiology of febrile illness in sub-Saharan Africa: Implications for diagnosis and management. Clin. Microbiol. Infect. 24, 808–814. https://doi.org/10.1016/j.cmi.2018.02.011 (2018).
Barathan, M. From fever to action: Diagnosis, treatment, and prevention of acute undifferentiated febrile illnesses. Pathog. Dis. https://doi.org/10.1093/femspd/ftae006 (2024).
Liu, L. et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 379, 2151–2161. https://doi.org/10.1016/S0140-6736(12)60560-1 (2012).
Kasper, M. R. et al. Infectious etiologies of acute febrile illness among patients seeking health care in south-central Cambodia. Am. J. Trop. Med. Hyg. 86, 246–253. https://doi.org/10.4269/ajtmh.2012.11-0409 (2012).
World Health Organization. WHO informal consultation on fever management in peripheral health care settings: a global review of evidence and practice. (2013).
D’Acremont, V., Lengeler, C. & Genton, B. Reduction in the proportion of fevers associated with Plasmodium falciparum parasitaemia in Africa: A systematic review. Malar J 9, 240. https://doi.org/10.1186/1475-2875-9-240 (2010).
Hercik, C. et al. A diagnostic and epidemiologic investigation of acute febrile illness (AFI) in Kilombero. Tanzania. PLoS One 12, e0189712. https://doi.org/10.1371/journal.pone.0189712 (2017).
Liu, J. et al. Development of a TaqMan array card for acute-febrile-illness outbreak investigation and surveillance of emerging pathogens Including Ebola Virus. J. Clin. Microbiol. 54, 49–58. https://doi.org/10.1128/JCM.02257-15 (2016).
Liu, J. et al. A laboratory-developed TaqMan array card for simultaneous detection of 19 enteropathogens. J. Clin. Microbiol. 51, 472–480. https://doi.org/10.1128/JCM.02658-12 (2013).
Abade, A. et al. Use of TaqMan array cards to screen outbreak specimens for causes of febrile illness in Tanzania. Am. J. Trop. Med. Hyg. 98, 1640–1642. https://doi.org/10.4269/ajtmh.18-0071 (2018).
Moore, C. C. et al. Etiology of sepsis in uganda using a quantitative polymerase chain reaction-based TaqMan array card. Clin. Infect. Dis. 68, 266–272. https://doi.org/10.1093/cid/ciy472 (2019).
Ali, M. A. et al. Etiologic agents of fever of unknown origin among patients attending Mnazi Mmoja hospital Zanzibar. J. Commun. Health 45, 1073–1080. https://doi.org/10.1007/s10900-020-00832-w (2020).
Marks, F. et al. Pathogens that cause acute febrile illness among children and adolescents in Burkina Faso, Madagascar, and Sudan. Clin. Infect. Dis. 73, 1338–1345. https://doi.org/10.1093/cid/ciab289 (2021).
Crump, J. A. et al. Etiology of severe non-malaria febrile illness in Northern Tanzania: A prospective cohort study. PLoS Negl. Trop. Dis. 7, e2324. https://doi.org/10.1371/journal.pntd.0002324 (2013).
D’Acremont, V. et al. Beyond malaria–causes of fever in outpatient Tanzanian children. N. Engl. J. Med. 370, 809–817. https://doi.org/10.1056/NEJMoa1214482 (2014).
Chipwaza, B. et al. Dengue and Chikungunya fever among viral diseases in outpatient febrile children in Kilosa district hospital Tanzania. PLoS Negl. Trop. Dis. 8, e3335. https://doi.org/10.1371/journal.pntd.0003335 (2014).
Chipwaza, B. et al. Prevalence of bacterial febrile illnesses in children in Kilosa district Tanzania. PLoS Negl. Trop. Dis. 9, e0003750. https://doi.org/10.1371/journal.pntd.0003750 (2015).
de Glanville, W. A. et al. Classification and characterisation of livestock production systems in northern Tanzania. PLoS ONE 15, e0229478. https://doi.org/10.1371/journal.pone.0229478 (2020).
Ministry of Health (MoH) Tanzania Mainland, Ministry of Health (MoH) Zanzibar, National Bureau of Statistics (NBS), Office of the Chief Government Statistician (OCGS) & ICF. Tanzania Demographic and Health Survey and Malaria Indicator Survey 2022 Key Indicators Report. ( Dodoma, Tanzania, and Rockville, Maryland, USA, 2023).
Probert, W. S., Schrader, K. N., Khuong, N. Y., Bystrom, S. L. & Graves, M. H. Real-time multiplex PCR assay for detection of Brucella spp., B. abortus and B. melitensis. J. Clin. Microbiol. 42, 1290–1293 (2004).
R Core Team. R: A Language and Environment for Statistical Computing, http://www.R-project.org (2023).
Centers for Disease Control and Prevention. Brucellosis (Brucella spp.) 2010 Case Definition, https://wwwn.cdc.gov/nndss/conditions/brucellosis/case-definition/2010/ (2010).
Lukambagire, A. S. et al. Performance characteristics and costs of serological tests for brucellosis in a pastoralist community of northern Tanzania. Sci. Rep. 11, 5480. https://doi.org/10.1038/s41598-021-82906-w (2021).
Bodenham, R. F. et al. Prevalence and speciation of brucellosis in febrile patients from a pastoralist community of Tanzania. Sci. Rep. 10, 7081. https://doi.org/10.1038/s41598-020-62849-4 (2020).
Kiambi, S. G., Fevre, E. M., Omolo, J., Oundo, J. & de Glanville, W. A. Risk factors for acute human brucellosis in Ijara, north-eastern Kenya. PLoS Negl. Trop. Dis. 14, e0008108. https://doi.org/10.1371/journal.pntd.0008108 (2020).
Akoko, J. M. et al. Molecular epidemiology of Brucella species in mixed livestock-human ecosystems in Kenya. Sci. Rep. 11, 8881. https://doi.org/10.1038/s41598-021-88327-z (2021).
Corbel, M. J., World Health Organization, Food and Agriculture Organization of the United Nations & World Organisation for Animal Health. Brucellosis in humans and animals. (World Health Organization, Geneva, 2006).
Di Bonaventura, G., Angeletti, S., Ianni, A., Petitti, T. & Gherardi, G. Microbiological laboratory diagnosis of human brucellosis: An overview. Pathogens https://doi.org/10.3390/pathogens10121623 (2021).
Wright, W. F. & Pritt, B. S. Update: The diagnosis and management of dengue virus infection in North America. Diagn Microbiol Infect Dis 73, 215–220. https://doi.org/10.1016/j.diagmicrobio.2012.03.021 (2012).
Eshetu, D. et al. Seropositivity to dengue and associated risk factors among non-malarias acute febrile patients in Arba Minch districts, southern Ethiopia. BMC Infect. Dis. 20, 639. https://doi.org/10.1186/s12879-020-05370-3 (2020).
Tsheten, T. et al. Clinical predictors of severe dengue: a systematic review and meta-analysis. Infect. Dis. Poverty 10, 123. https://doi.org/10.1186/s40249-021-00908-2 (2021).
Mwanyika, G. O. et al. Circulation of dengue serotype 1 viruses during the 2019 outbreak in Dar es Salaam Tanzania. Pathog. Glob. Health 115, 467–475. https://doi.org/10.1080/20477724.2021.1905302 (2021).
Mustafa, U. K., Sauli, E., Brinkel, J. & Kreppel, K. S. Health professionals’ knowledge on dengue and health facility preparedness for case detection: A cross-sectional study in Dar es Salaam Tanzania. PLoS Negl. Trop. Dis. 17, e0011761. https://doi.org/10.1371/journal.pntd.0011761 (2023).
Chipwaza, B. et al. Occurrence of 4 dengue virus serotypes and chikungunya virus in Kilombero Valley, Tanzania, during the dengue outbreak in 2018. Open Forum Infect. Dis. https://doi.org/10.1093/ofid/ofaa626 (2021).
Siva Raghavendhar, B. et al. Virus load and clinical features during the acute phase of Chikungunya infection in children. PLoS ONE 14, e0211036. https://doi.org/10.1371/journal.pone.0211036 (2019).
Ministry of Health, Community Development, Gender, Elderly and Children, Dar es Salaam,, Ministry of Health, Zanzibar, National Bureau of Statistics, Dar es Salaam, Office of the Chief Government Statistician, Zanzibar & ICF. Tanzania Malaria Indicator Survey 2017 (2018).
Faucher, J. F. et al. What would PCR assessment change in the management of fevers in a malaria endemic area? A school-based study in Benin in children with and without fever. Malar. J. 9, 224. https://doi.org/10.1186/1475-2875-9-224 (2010).
Levine, Z. C. et al. Investigating the etiologies of non-malarial febrile illness in Senegal using metagenomic sequencing. Nat. Commun. 15, 747. https://doi.org/10.1038/s41467-024-44800-7 (2024).
Acknowledgements
We gratefully acknowledge the contribution of the hospital staff and patients attending the sampled health facilities, as well as communities in the sites visited.
Funding
This work was supported by the Biotechnology and Biological Sciences Research Council project: African Skills Training for Health Research and Learning (ASTRAL) grant number BB/R020280/1, A University of Glasgow One Health training program for Tanzania, by a Leverhulme - Royal Society Africa Award (grant number AA130131; https://www.leverhulme.ac.uk, https://royalsociety.org) and by the National Institutes of Health under award number D43TW008270.
Author information
Authors and Affiliations
Contributions
A.M., A.H.S.L., B.T.M., J.G. & E.R.H. conceived the experiment. A.H.S.L., B.T.M., S.I.M, R.A.K. & J.E.B.H. supervised the project. A.H.S.L. collected samples and primary data. A.M., J.L., N.A., & J.N. developed or conducted laboratory testing. A.M., A.H.S.L. & J.E.B.H analysed the results. A.M. & J.E.B.H drafted the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maro, A., Lukambagire, A.S., Mmbaga, B.T. et al. Detection of pathogens associated with acute febrile illness in children under five years of age in rural Tanzania. Sci Rep 15, 11520 (2025). https://doi.org/10.1038/s41598-025-96190-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-96190-5