Abstract
This study explores the anticancer potential of Cirsium vulgare dry extracts in human colorectal adenocarcinoma (HT-29) and gastric carcinoma (KATO III) cell lines using both traditional 2D monolayer models and advanced 3D spheroid systems. Cell viability was assessed via the MTT assay, while the influence on cell migration was evaluated using a wound-healing assay. In 3D cultures, extract activity was further examined through magnetic 3D bioprinting to monitor spheroid growth dynamics, and viability of cells in spheroids was assessed by the WST-1 assay. Among the tested extracts, those derived from C. vulgare inflorescences (U1) and roots (U6, U7, U8, U9) demonstrated higher anticancer activity. The inflorescence extract (U1) exhibited the highest cytotoxic activity against both cancer cell lines, while root-derived extracts, particularly U7, showed potent suppression of HT-29 cell migration, achieving the most significant reduction in wound closure after 36 h (p < 0.05) at a concentration of 0.2 mg/mL. In spheroid models, U1 and U8 extracts reduced HT-29 cancer cell viability by 53.3–77.9% and 56.7–81.5%, respectively, and U1 emerged as the most effective inhibitor of spheroid growth, reducing diameter by 7–10%, compared to untreated controls. These findings underscore the promising anticancer activity of C. vulgare extracts, particularly U1 and U8, highlighting their potential as innovative therapeutic candidates for treating colorectal and gastric cancers. Further investigations are warranted to refine their application in oncological research.
Similar content being viewed by others
Introduction
The genus Cirsium, including Cirsium vulgare, is rich in phenolic compounds and exhibits significant pharmacological properties, including anticancer effects1. Several studies have investigated the antioxidant, antimicrobial, and anticancer potential of these plants, attributing much of their bioactivity to the presence of phenolic acids and flavonoids2,3,4,5,6,7,8,9. Cirsium vulgare has been noted for its strong antioxidant activity, mainly due to the presence of compounds like chlorogenic acid and apigenin-7-O-glucoside, which are common in the plant’s extracts across different phenological stages10,11.
Chlorogenic acid (CGA), a bioactive phenolic compound found in various plants, including C. vulgare, has shown notable antitumor and wound healing potential in several in vitro assays. CGA activates ERK1/2 signaling, leading to a reduction in cell proliferation12 and inhibits the proliferation of A549 lung cancer cells by targeting annexin A2, both in vitro and in vivo13. In melanoma cells, a hybrid of CGA with piperine was shown to reduce cell proliferation by modulating mitotic kinases14. Also, CGA induces mitochondrial apoptosis in hepatoma cells by suppressing noncanonical NF-κB signaling, suggesting its potential as an apoptosis inducer15 and in A498 human kidney cancer cells, CGA induces apoptosis through inactivation of the PI3K/Akt/mTOR signaling pathway, leading to cell death16. Furthermore, in pancreatic cancer cells, CGA inhibits cell proliferation, migration, and invasion through the AKT/GSK-3β/β-catenin signaling pathway17. CGA has shown a wound healing potential in addition to its anticancer properties. In studies on Parrotia persica, it was noted that CGA could aid in wound healing, highlighting its broad therapeutic scope12,13,14,15,16,17,18. CGA has been investigated for its effects on tumor cell behaviour in cancer cell spheroids19. Studies suggest that CGA can hinder tumor progression by affecting cell proliferation, migration, and inducing apoptosis in cancer cell lines, including HT-2920,21. The combination of these effects makes CGA a promising candidate in cancer treatment and wound care therapies18. Another study investigating the effects on KATO III and other cancer cell lines treated with polyphenols, including CGA, derived from plant leaf extracts, found that they suppressed cancer cell proliferation in a dose-dependent manner. Furthermore, a variation in sensitivity was observed between chlorogenic acid, caffeoylquinic acid derivatives and different types of cancer cells22. Studies with ASH (Acanthopanax senticosus HARMS) and its stem extracts showed an effect on the viability of KATO III cells by inhibiting growth and inducing apoptosis upon exposure to chlorogenic acid. The exposure of KATO III cells to ASH led to both growth inhibition and induction of apoptosis23.
Apigenin-7-O-glucoside (A7G), a flavonoid glycoside, has shown significant anticancer effects through various mechanisms. Studies indicate that A7G can inhibit cancer cell proliferation and induce apoptosis in several cell lines24. For instance, in the HepG2 liver cancer cell model, A7G reduced cell growth, promoted apoptosis via extrinsic pathways, and induced cell cycle arrest at the G2/M phase. Furthermore, it has demonstrated wound healing potential by enhancing migration in wound healing assays, making it a promising agent in both cancer therapy and tissue repair applications25. However, specific data regarding its effects on HT-29 and KATO III cells, as well as in 3D cell culture assays, would require further investigation.
Moreover, extracts from Cirsium species have shown cytotoxicity against various cancer cell lines. Comparing C. vulgare to other Cirsium species, the latter have also demonstrated anticancer potential. C. japonicum, for instance, contains unique flavonoids such as diosmetin, which are associated with potent antioxidant and anticancer activities. These compounds contribute to radical scavenging and have been shown to impact multiple cellular pathways relevant to cancer progression, including apoptosis and migration26. Also, C. japonicum has demonstrated inhibitory effects on breast cancer (MCF-7) and liver cancer (HepG2) cells. The anti-tumour efficacy of C. japonicum extracts is attributed mainly to specific flavonoids, such as pectolinarin and apigenin, which have shown pro-apoptotic and anti-angiogenic effects, as well as the ability to reduce cancer cell migration27. Likewise, C. setidens (Korean thistle) has shown strong cytotoxic effects on colorectal cancer (HT-29) and liver cancer (HepG2) cell lines. Cirsium setidens has been reported to induce apoptosis, mainly through activation of caspase signalling pathways, while also modulating the expression of key apoptosis-related proteins such as Bcl-2 and Bax. This modulation enhances the apoptotic response in cancer cells, while simultaneously reducing cell proliferation and migration28.
The high quantities of chlorogenic acid found in C. vulgare may be responsible for its anticancer activity, particularly in inhibiting the growth and spread of gastric and colorectal cancer cells. Overall, C. vulgare and other Cirsium species stand out for their potent bioactive phenolic content, which not only combats oxidative stress but also has the potential to hinder the growth and spread of cancer cells. Research on C. vulgare extracts may provide valuable insights into its potential to induce apoptosis, inhibit migration, and exert cytotoxicity in cancer cells, thus supporting further exploration of its use in gastric and colorectal cancer treatment.
Materials and methods
Material and reagents
Extracts
Ethanolic extracts were made as described elsewhere11. After that, dry extracts were made after withdrawing ethanol with a vacuum and dry cabinet. The extracts after vacuum with rotation mechanism were left in a dry cabinet at a temperature of 50 °C for 4 days till all liquid phases evaporated.
Use of high-performance liquid chromatography for the quantitative and qualitative determination of phenolic compounds in extracts of C. vulgare
During the study, the ESC methodology for detecting phenolic compounds in C. vulgare plant extract was validated following the European Medicines Agency (EMA) ICH Q2(R2) guidelines. The validation assessed parameters including detection limit, quantification limit, linearity (R²), repeatability, and intermediate precision29. The qualitative and quantitative analysis of phenolic compounds was performed using a Waters Alliance 2695 liquid chromatograph with a Waters 996 photodiode array detector (PDA) and an ACE C18 (250 mm x 4.6 mm x 5 μm) column (Advanced Chromatography Technologies, Aberdeen, Scotland). The mobile phase consisted of solvent A (trifluoroacetic acid (0.1%)) and solvent B (acetonitrile). A gradient elution was applied: 95% A/5% B for 0 min, 85% A/15% B for 8 min, 80% A/20% B for 30 min, 60% A/40% B for 48 min, 50% A/50% B for 58–65 min, 5% A/95% B for 66–70 min, and 95% A/5% B for 71 min. The flow rate was 1 ml/min, the injection volume was 10 µL, and the column temperature was 25 °C. Absorbance was measured in the wavelength range of 330 nm to 400 nm. Phenolic compound identification was performed using standard solutions of the following compounds: CGA, apigenin-7-O-glucoside, apigenin, luteolin, hyperoside, neochlorogene, isoquercitrine, p-coumaric acid, caffeic acid, trans-ferulic acid, and 4-O-caffeoylquinic acid11. Eleven phenolic compounds were identified in the extract, with retention times ranging from 9.53 min to 47.79 min. The study determined that the linearity limits of the identified compounds ranged from 0.113 µg/mL to 176.7 µg/mL, covering all the phenolic compound concentrations detected during the study. The detection limit for all examined phenolic compounds was established when the signal-to-noise ratio (peak height to baseline noise) was 3:1, and the quantification limit was evaluated at a signal-to-noise ratio of 10:129. The detection limits for all analytes used in the study ranged from 0.03 µg/mL to 0.345 µg/mL, while the quantification limits ranged from 0.078 µg/mL to 1.104 µg/mL. The determination coefficient for all analytes was greater than 0.999, confirming the linearity of the ESC methodology29.
Cell culturing
The human colorectal adenocarcinoma cell line HT-29 and human gastric carcinoma cell line KATO III were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Human foreskin fibroblasts (HF) were originally obtained from ATCC and provided by Prof. Helder Santos (University of Helsinki, Finland). All cell lines were cultured in Dulbecco’s Modified Eagle’s GlutaMAX medium (Gibco (Carlsbad, CA, USA)), supplemented with 1% of 10,000 U/mL penicillin, 10 mg/mL streptomycin (Gibco), and 10% fetal bovine serum (Gibco). Cell cultures were grown at 37 °C in a humidified atmosphere containing 5% CO2.
Cell viability assay
The effect of dry extracts on cell viability was studied using 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich Co., St Louis, MO, USA) assay, as described elsewhere30. The cells were seeded in triplicates in 96-well plates (KATO III and H-29: 4 × 103 cells/well; HF: 5 × 103 cells/well). After 24 h of incubation, the cells were treated with serial dilutions of dry extracts (from 2.5 to 0.016 mg/mL) or chlorogenic acid (from 500 to 15.6 µM). After 72 h, the MTT reagent was added. After 3–4 h of incubation, the formazan crystals that had formed were dissolved in DMSO (Sigma-Aldrich Co., St. Louis, MO, USA). The absorbance was determined with a multi-detection microplate reader at 570 and 630 nm. Using the Hill equation, the EC50 values of extracts were calculated (the concentration of a compound/extract that results in a 50% reduction in the metabolic activity of cells).
‘Wound healing’ assay
‘Wound healing’ assay was employed to assess the ability of the most active dry extracts of C. vulgare U1, U6, U7, U8, and U9 to inhibit cell migration. The procedure was carried out as described elsewhere31. Briefly, HT-29 cancer cells were seeded at a density of 6 × 104 cells/well in 24-well plates. After 48 h of incubation, a 100 µL pipette tip was used to make the scratch in each well. Following a single PBS wash, the fresh medium containing 0.2 or 0.1 mg/mL of extracts U1, U6, U7, U8 and U9 was added to the cells. As a negative control, the medium without extracts was employed. Images of the ‘wound’ area were taken at 12, 24, and 36 h after scratching to track the progression of cell migration and ‘wound’ closure. The ‘wound’ area reduction over time is analyzed to assess cell migration rates.
Compound activity in cell 3D cultures (spheroids)
The magnetic 3D Bioprinting method was utilized to form cancer cell spheroids as described elsewhere32. Briefly, the cells at 70% confluency were incubated with Nanoshuttle (n3D Biosciences, Inc., Houston, TX, USA) for 8 h. After that, the cells were trypsinized, centrifuged and seeded into ultra-low attachment 96-well plate at a ratio of 2 × 103 HT-29 cancer cells and 2 × 103 human fibroblasts/well. The plate was incubated for two days at 37 °C in a humidified atmosphere on a magnetic drive. Next, the medium with 0.35 mg/mL of selected extracts U1, U6 and U8 was added. Photos of spheroids were taken every two days using an Olympus IX73 inverted microscope (OLYMPUS CORPORATION, Tokyo, Japan), and analysis of spheroid size was performed using ImageJ, version 1.53o (National Institutes of Health, Bethesda, MD, USA) and Microsoft Office Excel 2016 software (Microsoft Corporation, Redmond, WA, USA). On the last day of the experiment, 10 µL of WST-1 reagent (Sigma-Aldrich Co, St. Louis, MO, USA) was added to each well. Following 10 h incubation, 50 µL of liquid from each well was moved to another 96-well plate and the absorbance was measured at 460 and 530 nm.
Data analysis
The results are presented as the mean of 3 repetitions ± standard deviation (SD). The data were processed using Microsoft Office Excel 2024 (“Microsoft”, WA, USA) and SPSS 25 (“IBM”, NY, USA) software. Significant differences between C. vulgare inflorescence, leaves, seeds, and root extracts were calculated using one-way ANOVA, followed by the Tukey post hoc comparison test.
Results and discussion
While the specific anticancer effects of C. vulgare (commonly known as bull thistle) have not been extensively studied, it is noteworthy that other species within the Cirsium genus, such as C. japonicum, contain CGA and have demonstrated resistance to oxidative stress33. This suggests potential anticancer properties that warrant further investigation.
Based on previous articles11, where quantitative and qualitative analyses of the composition of C. vulgare (bull thistle) extracts were conducted, and their antioxidant effects were determined using the CUPRAC method, it was decided to utilize these study results. For further anticancer research, 14 extracts with the highest determined CUPRAC values were selected (Table 1).
Cell viability
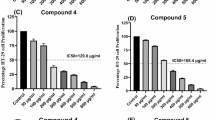
A study examining the effects of fourteen dry extracts of C. vulgare on cell viability demonstrated that all extracts were active across both tested cell lines. Extract U1 exhibited the highest activity, showing the most significant inhibition of KATO III cell line viability (EC50 0.19 ± 0.05 mg/mL). It also effectively reduced the HT-29 cell line viability (EC50 0.35 ± 0.08 mg/mL)). Respectively, extract U9 was the most effective at inhibiting the proliferation of HT-29 cells (EC50 0.29 ± 0.06 mg/mL) (Fig. 1). The least active extracts were 4 L, 8 L, 10 L, 6 L. In addition, they accumulated the highest amounts of CGA when comparing all extracts in terms of CGA content. In other studies, for colorectal cancer, CGA has been shown to arrest cells in the S phase and induce DNA damage in human colon cancer cell lines such as HCT116 and HT29. This effect is mediated by increased reactive oxygen species (ROS) production and the upregulation of phosphorylated p53, HO-1, and Nrf2. Additionally, CGA activates the mitochondrial apoptotic pathway in cancer cells, evidenced by DNA fragmentation, cleavage of pro-caspase-9 and PARP-1, and the upregulation of Bax and the Bax/Bcl-2 ratio34. Beyond its pro-apoptotic effects, CGA exhibits antioxidant properties that contribute to its anticancer activity. In models of oxidative stress, CGA reduced the production of reactive oxygen species (ROS) and increased the activity of antioxidant enzymes such as superoxide dismutase and mitochondrial glutathione. These effects help mitigate oxidative damage, which is a known contributor to cancer progression35.
Comparing the parts of the raw material from which the extracts were derived, the strongest effect on the HT-29 (human colorectal adenocarcinoma) cell line was observed in extracts made from the roots of thistle, except for extract U1, which was made from flowers. A trend was noted for extracts whose raw material was collected during mass flowering (U9) and at the end of the phenological dormant period (U7). Meanwhile, the KATO III cell line was most effectively inhibited by extracts made from the flowers of the plant. Specifically, extracts obtained during the seed maturity stage (U1) showed the highest activity in inhibiting cell line proliferation. The HF cell line was most effectively inhibited by extracts U5 and U8, which were derived from plant roots. The raw material for these extracts was collected at the end of the dormancy period. Gastric carcinoma cell lines, such as KATO III, are challenging to treat due to their complex tumor microenvironment and resistance to chemotherapy, making these findings promising36. Similarly, HT-29 is a colorectal adenocarcinoma model known for its resilience to conventional therapies37, thus, the observed activity of extracts U1 and U9 against these cells is noteworthy.
These results align with studies on other Cirsium species. For example, C. setidens, commonly used in East Asia for medicinal purposes, exhibited selective cytotoxicity against colorectal cancer cells, with significant effects observed at concentrations of 100–250 mcg/mL, where it induced apoptosis via caspase activation and Bcl-2 protein modulation, which are critical in cell survival38. Additionally, studies on C. japonicum found that 200–500 µg/mL concentrations of flavonoid-rich extracts exhibited cytotoxic effects in liver cancer (HepG2) and breast cancer (MCF-7) cell lines, largely attributed to flavonoids like pectolinarin and apigenin, which act on cancer-specific pathways, thus reducing off-target effects28,39. These concentrations are comparable to those used in our study, where C. vulgare extracts, particularly U1 and U9, showed selective anticancer effects, suggesting that certain phenological stages and plant parts contain bioactive compounds at concentrations sufficient to induce targeted cytotoxicity.
During the conducted research, it was found that C. vulgare sample extracts U4, U5, and U10 were non-selective in inhibiting the viability of non-cancerous human fibroblast (HF) cell lines, compared to the viability of KATO III and HT-29 cell lines (from 0.9 to 1.2 times). Meanwhile, the extracts of C. vulgare U1, U6, U7, U8, U9 samples had a lower effect on the viability of human fibroblast (HF) cell lines than on KATO III and HT-29 cancer cell lines (from 1.48 to 3.79 times). The interpretation of the results suggests that the extracts U1, U6, U7, U8, U9 are more selective towards KATO III and HT-29 cell lines than HF cells, compared to extracts U4, U5, U10 (Fig. 1).
In vivo studies have explored the anticancer effects of various natural compounds, including chlorogenic acid (CGA), apigenin, and luteolin, at different dosages. For example, in a study involving HCT116 colon cancer xenografts, intraperitoneal administration of 50 mg/kg per day of CGA resulted in significant tumor growth inhibition40. Similarly, a study using Huh7 hepatoma and H446 lung cancer xenograft models demonstrated that intraperitoneal administration of CGA at doses of 25 mg/kg/day, 50 mg/kg/day, and 200 mg/kg/day led to tumor volume reductions of 44.0%, 80.1%, and 83.1%, respectively, in Huh7 tumors, and 39.9%, 84.6%, and 86.3% in H446 tumors41. These findings suggest that CGA exhibits dose-dependent anticancer activity, with higher doses generally resulting in more significant tumor growth inhibition. In addition to CGA, other compounds like apigenin and luteolin have also shown anticancer potential. Apigenin, when administered at doses of 30–60 mg/kg in vivo, has been shown to prevent Helicobacter pylori-induced atrophic gastritis and carcinogenesis42. Luteolin, at a dose of 10 mg/kg, has demonstrated the ability to suppress tumor growth in various models43. These studies highlight the diverse anticancer effects of natural compounds, including CGA, apigenin, and luteolin, and suggest that they may have therapeutic potential for different types of cancers. However, optimal dosing regimens may vary depending on factors such as the specific cancer model and the administration route. Further research is necessary to refine these dosages and explore their full potential in cancer therapy.
The reduction in cell viability was also observed with chlorogenic acid, which was found at high levels in all the examined extracts. The yields of chlorogenic acid in these dry extracts ranged from 17.91 to 156.28 mg/g. However, no connection has been established between chlorogenic acid and its effects on cell lines. It is observed that the extracts showing significantly higher activity towards the HT-29 cell line and the KATO III cell line had relatively low yields of chlorogenic acid. The levels of chlorogenic acid in plant extracts varied greatly. The highest amounts of chlorogenic acid were found in extracts prepared from leaves during the late dormancy and peak regrowth stages of the plant’s first phenological year. However, in studies on cell viability and wound healing, the EC50 effects of these extracts were the weakest for both cancer cell cultures analyzed. Chlorogenic acid reduced the HF viability stronger compared to KATO III cancer cells and was highly non-selective against cancer cell lines (Fig. 1). Additionally, chlorogenic acid, which is prevalent in C. vulgare extracts, is well-documented for its anticancer properties. Concentrations of chlorogenic acid as low as 100 µM have been shown to induce apoptosis, enhance cell cycle arrest, and reduce proliferation in cancer cell lines44. In HT-29 cells, chlorogenic acid at 50–200 µM reduced proliferation and promoted apoptosis by modulating apoptosis-related proteins, suggesting that chlorogenic acid-rich C. vulgare extracts could achieve similar effects through apoptosis induction. The flower extract (U1) collected has a higher concentration of A7G at 27.62 mg/g, but it doesn’t contain a large concentration of the other compounds found in the root extract (U9). The root extract shows a lower A7g concentration at 4.48 mg/g, yet it contains several additional bioactive compounds, including cumaric acid (5.39 mg/g), neochlorogen (1.22 mg/g), and caffeic acid (2.13 mg/g). These compounds in the root extract suggest a broader spectrum of potential antioxidant and anti-inflammatory benefits compared to the flower extract45. Given that most active extracts (U1 and U9) contain high chlorogenic acid levels, these findings support their cytotoxic efficacy and potential mechanism via mitochondrial apoptosis pathways, which are especially relevant in colorectal and gastric cancer treatment.
‘Wound healing’ assay
After examining the effect of thistle extracts on cell viability, the most active extracts U1, U6, U7, U8, and U9 were selected for further studies. The effect of C. vulgare extracts (U1, U6, U7, U8, and U9) on the migration of HT-29 cancer cells was evaluated using the ‘wound healing’ method at 0.1 mg/mL and 0.2 mg/mL. It is generally known that the effect of tested substances on cell migration tested by ‘wound-healing’ assay, especially for a longer time, might depend on their effect on cell viability. Considering this, in our experiments, we decided to evaluate the effect on cell migration every 12 h only up to 36 h period, taking into account the established EC50 after 72 h. The chosen concentrations of tested extracts (0.1 and 0.2 mg/mL) did not reduce cell viability after the longest period of 36 h by more than 10%, and there were no statistically significant differences between the tested extracts U1, U6, U7, U8 and U9.
The ‘wound healing’ assay, which assesses cell migration as an indicator of metastatic potential, showed that the extract U7 showed the strongest effect on the HT-29 cell migration (p < 0.05) compared to the control. Even after 36 hours at a concentration of 0.2 mg/mL, the ‘wound’ remained open, with the ’wound’ area exceedingly more than 15% compared to control (Fig. 2). This is significant given the strong migratory behaviors of HT-29 cells, which are associated with metastatic progression. Anti-migratory effects at similar concentrations have been observed in studies on other Cirsium species. For instance, C. japonicum demonstrated migration inhibition in breast cancer cells (MCF-7) at concentrations of 100–300 mcg/mL, where its bioactive compounds affected cellular motility by altering cytoskeleton dynamics and downregulating matrix metalloproteinases (MMPs), crucial for metastasis39,46. This suggests that bioactive compounds in C. vulgare, such as CGA and apigenin derivatives, may inhibit migration in a concentration-dependent manner by targeting similar pathways.
Cirsium vulgare dry extract effect on HT-29 cell migration after 12, 24, 36 h of incubation with 0.2 and 0.1 mg/mL of Cirsium. (A) ‘Wound’ area (%) of HT29 cell monolayer. (B) Photos of ‘wound’ area in HT – 29. The asterisks (*) indicate that compared groups have sig. differences between groups p < 0.05. The scale bar indicates 100 μm, n = 3 (independent experiments).
Extracts U1, U6, and U8, showed a similar effect on the HT-29 cell line in the 2D migration model at both 0.2 mg/mL and 0.1 mg/mL concentrations. They statistically significantly decreased ‘the wound’ area after 24 h, compared with the control samples. The cancer cell ‘wound’ area remained visibly open after 24 h, with the ‘wound’ area exceeding 45%. Additionally, calculations indicated that, compared to the control group, the wound size remained more visible in the treated groups even after 36 h at a concentration of 0.2 mg/mL.
Chlorogenic acid has shown similar effects in other cancer models. Studies indicate that concentrations of 50–150 µM effectively downregulate MMPs and alter actin dynamics, reducing migration in gastric and colorectal cancers44. Thus, the anti-migratory effects of C. vulgare extracts observed in our study likely stem from the ability of CGA and flavonoid compounds to modulate cellular motility pathways, making them relevant for strategies aimed at reducing metastatic spread.
Extracts activity in cell 3D cultures (spheroids)
The 3D spheroid model, which closely resembles the in vivo tumor environment, revealed significant inhibition of spheroid growth and viability by C. vulgare extracts. It is widely recognized that assessing the effect of a compound solely by measuring changes in spheroid size is not entirely accurate, as it may not directly correlate with cell viability within the spheroids47. Since the spheroid-based method is a phenotypic approach suited for testing complex samples, utilizing a 3D system can effectively showcase the distinct chemical diversity of natural product libraries. This includes high-quality natural extracts and enriched fractions, across various models48. During the study, the effect of C. vulgare extracts on the HT-29 spheroid growth and viability was evaluated using the WST-1 assays (Fig. 3). KATO III cells did not form spheroids. Thus, this cell line was not used for extract activity evaluation in 3D cultures. HT-29 spheroids were composed of cancer cells combined with fibroblasts (HF) at a ratio of 1:1 to better represent the real tumor microenvironment. This approach considers the presence of stromal tissue in tumors, which plays a role in tumor growth and metastasis. At the start of the experiment, the spheroids had a diameter of 300–350 μm (Fig. 3A).
(A) Photos of HT – 29 (human colorectal adenocarcinoma) tumor spheroids at the start and the end of the experiment (after 8 days of incubation with 0.35 mg/mL of extracts); (B) HT – 29 spheroids viability at the end of the experiment; (C) HT – 29 spheroids size at the end of the experiment; Asterisks (*) indicate p < 0.05 compared to the control (untreated spheroids); (C) HT – 29 spheroids viability at the end of the experiment; Asterisks (*) indicate p < 0.05 compared to the control (untreated spheroids); crosses (×) represent mean values, inner dashes show medians, and whiskers indicate the maximum and minimum values; scale bars represent 200 μm.
HT-29 spheroids treated with U1 showed notable size and viability reductions after 8 days, decreasing spheroid diameter to 584.1–641.2 μm compared to control (643–690.1 μm, p < 0.05) (Fig. 3C). These findings mirror results from studies on C. japonicum, where concentrations of up to 100 µg/mL of flavonoid-rich extracts reduced HepG2 liver cancer spheroid growth by inducing apoptosis and hindering cell proliferation [28]. Given the similarity in effective concentrations, it appears that C. vulgare extracts may also influence cancer cell viability through apoptosis and anti-proliferative pathways, likely mediated by chlorogenic acid and flavonoids that disrupt signalling pathways essential for tumor growth.
Moreover, this study shows that extracts U1 and U8 reduced spheroid viability by up to 77.9% and 81.5%, respectively (Fig. 3B). This degree of viability reduction aligns with chlorogenic acid studies in spheroid models of HT-29 cell line, where 500 µM and above concentrations induce oxidative stress, activate caspase pathways, and disrupt cell survival signals in solid tumor models19. The activity observed in U1 and U8 treated spheroids suggests these extracts could bypass the resistance often found in tumor spheroids due to their hypoxic and nutrient-limited environment. Furthermore, the presence of fibroblasts in this 3D model allowed for the interaction of cancer cells with stromal components, which are key in tumor progression. Previous research on C. setidens shows that flavonoids within the plant can modulate cancer-stroma interactions, reducing tumor-promoting effects of fibroblasts49. This indicates that C. vulgare extracts may also interact within the tumor microenvironment to hinder both cancer cell growth and the supportive role of stroma, thus highlighting their potential in complex cancer models.
In anticancer in vitro activity testing, concentrations ranging from 0.1 mg/mL to 0.5 mg/mL are commonly used to evaluate the effects of plant extracts on cancer cell proliferation, apoptosis induction, and cell viability reduction. Studies have demonstrated that extracts from Artemisia absinthium exhibit cytotoxicity against cancer cells at concentrations as low as 0.1 mg/mL, with significant inhibition of cell growth observed at 0.5 mg/mL50. Similarly, Arnica montana extracts at comparable concentrations have shown the ability to inhibit cancer cell metabolic activity and induce apoptosis51. Ipomoea purpurea extracts have also demonstrated anticancer effects within this concentration range in lung and breast cancer cells52. Additionally, Mandragora autumnalis extracts exhibited significant antitumor activity in MCF-7 cells, with IC₅₀ values around 0.4 mg/mL53. These findings align with used of C. vulgare extracts at 0.1 mg/mL and 0.2 mg/mL, concentrations that are within the typical range for testing anticancer activity. Beyond in vitro studies, in vivo investigations have explored the anticancer potential of various natural compounds, including chlorogenic acid (CGA), apigenin, and luteolin, across different dosages and cancer models. For instance, in an HCT116 colon cancer xenograft model, intraperitoneal administration of CGA at 50 mg/kg per day significantly inhibited tumor growth40. Similarly, in Huh7 hepatoma and H446 lung cancer xenografts, CGA at doses of 25 mg/kg/day, 50 mg/kg/day, and 200 mg/kg/day led to tumor volume reductions of 44.0%, 80.1%, and 83.1%, respectively, in Huh7 tumors, and 39.9%, 84.6%, and 86.3% in H446 tumors41. These results highlight a dose-dependent anticancer effect, where higher CGA doses yield greater tumor suppression. In addition to CGA, apigenin and luteolin have also demonstrated promising anticancer activity in in vivo studies. Apigenin, when administered at 30–60 mg/kg, has been reported to prevent Helicobacter pylori-induced atrophic gastritis and carcinogenesis42. Similarly, luteolin, at a dose of 10 mg/kg, has exhibited tumor growth suppression across various cancer models43. Collectively, these findings underscore the therapeutic potential of natural compounds such as CGA, apigenin, and luteolin for cancer treatment. However, optimal dosing regimens remain dependent on factors such as cancer type and route of administration, necessitating further research to refine their clinical applications.
Conclusions
The process of anticancer drug development, which starts with the transformation of natural products from laboratory studies to clinical use, is complex and vital. This process involves preclinical investigations and subsequent clinical trials, with each stage being critical for converting natural substances into effective treatments54. The cytotoxicity observed in these extracts at concentrations comparable to those used in other Cirsium studies, along with their inhibition of migration and tumor spheroid growth, supports their potential for further investigation. The most active extract was obtained from the inflorescences collected at the seed maturity phenological stage during the first growing year. This extract, prepared using a heat reflux extraction method with a 50% ethanol-water solution as the solvent, holds potential for further investigation. Contrary to initial assumptions, the proportion of CGA in the extracts did not correlate with anticancer activity across different studies. Extracts with moderate levels of this compound exhibited greater activity than those with higher CGA content. These investigations warrant the isolation of specific bioactive compounds and the determination of their mechanisms of action, which could contribute to developing new treatment strategies for colorectal and gastric cancers.
Data availability
Authors agree to make data and materials supporting the results or analyses presented in their paper available upon reasonable request via e-mail: urte.griskeviciene@lsmu.lt.
References
Luo, W. et al. Recent research progress of Cirsium medicinal plants in China. J. Ethnopharmacol. 280, 114475 (2021).
Borawska, M. H. et al. Enhancement of antibacterial effects of extracts from Cirsium species using sodium picolinate and Estimation of their toxicity. Nat. Prod. Res. 24, 554–561 (2010).
Nalewajko-Sieliwoniuk, E., Malejko, J., Mozolewska, M., Wołyniec, E. & Nazaruk, J. Determination of polyphenolic compounds in Cirsium palustre (L.) extracts by high performance liquid chromatography with chemiluminescence detection. Talanta 133, 38–44 (2015).
Nazaruk, J. & Gudej, J. Flavonoid compounds from the flowers of Cirsium rivulare (Jacq.) all. Acta Pol. Pharm. 60, 87–89 (2003).
Nazaruk, J. Antioxidant activity and total phenolic content in Cirsium five species from north–east region of Poland. Fitoterapia 79, 194–196 (2008).
Aydın Kurç, M. et al. Antimicrobial and antioxidant efficacy of the lipophilic extract of Cirsium vulgare. Molecules 28, 7177 (2023).
Sabudak, T. et al. Investigation of some antibacterial and antioxidant properties of wild Cirsium vulgare from Turkey. Indian J. Pharm. Educ. Res. 51, s363–s367 (2017).
Fernández-Martínez, E. et al. Hepatoprotective effects of nonpolar extracts from inflorescences of thistles Cirsium vulgare and Cirsium ehrenbergii on acute liver damage in rat. Pharmacogn Mag. 13, S860–S867 (2018).
Kozyra, M. & Głowniak, K. Phenolic acids in extracts obtained from the flowering herbs of cirsium vulgare (Savi) ten. Growing in Poland. Acta Soc. Bot. Pol. 82, 325–329 (2013).
Aggarwal, G. et al. Traditional uses, phytochemical composition, Pharmacological properties, and the biodiscovery potential of the genus cirsium. Chem. (Easton). 4, 1161–1192 (2022).
Griskeviciene, U. et al. Effect of the phenological stage on the phenolic composition, and antioxidant and antimicrobial properties of Cirsium vulgare (Savi) ten. Extracts Life. 14, 1191 (2024).
Sapio, L. et al. Chlorogenic acid activates ERK1/2 and inhibits proliferation of osteosarcoma cells. J. Cell. Physiol. 235, 3741–3752 (2020).
Wang, L., Du, H. & Chen, P. Chlorogenic acid inhibits the proliferation of human lung cancer A549 cell lines by targeting Annexin A2 in vitro and in vivo. Biomed. Pharmacother. 131, 110673 (2020).
Pressete, C. G. et al. Piperine-Chlorogenic acid hybrid inhibits the proliferation of the SK-MEL-147 melanoma cells by modulating mitotic kinases. Pharmaceuticals (Basel) ;16. (2023).
Jiang, Y. et al. Chlorogenic acid inhibits proliferation in human hepatoma cells by suppressing noncanonical NF-κB signaling pathway and triggering mitochondrial apoptosis. Mol. Biol. Rep. 48, 2351–2364 (2021).
Wang, X. et al. Chlorogenic acid inhibits proliferation and induces apoptosis in A498 human kidney cancer cells via inactivating PI3K/Akt/mTOR signalling pathway. J. Pharm. Pharmacol. 71, 1100–1109 (2019).
Chen, X. et al. Chlorogenic acid inhibits proliferation, migration and invasion of pancreatic cancer cells via AKT/GSK-3β/β-catenin signaling pathway. Recent. Pat. Anticancer Drug Discov. 19, 146–153 (2024).
Moghadam, S. et al. Wound healing potential of chlorogenic acid and Myricetin-3-O-β-Rhamnoside isolated from Parrotia persica. Molecules 22, 1501 (2017).
Vélez, M. D., Pedroza-Díaz, J. & Santa-González, G. A. Data on the cytotoxicity of chlorogenic acid in 3D cultures of HT-29 cells. Data Brief. 50, 109527 (2023).
Li, W. et al. Chlorogenic acid regulates the proliferation and migration of high-grade serous ovarian cancer cells through modulating the miR199a5p/DDR1 axis. Acta Biochim. Pol. (2022).
In vitro Evaluation of the Antioxidant and Anticancer Activities of Chlorogenic Acid on Human Colon Cancer (HT-29) Cells. Tropical Journal of Natural Product Research ;8. (2024).
Kurata, R., Adachi, M., Yamakawa, O. & Yoshimoto, M. Growth suppression of human cancer cells by polyphenolics from Sweetpotato (Ipomoea Batatas L.) leaves. J. Agric. Food Chem. 55, 185–190 (2007).
Hibasami, H. et al. Induction of apoptosis by Acanthopanax senticosus HARMS and its component, sesamin in human stomach cancer KATO III cells. Oncol Rep. (2000).
Bhosale, P. B. et al. Apigetrin promotes TNFα-Induced apoptosis, necroptosis, G2/M phase cell cycle arrest, and ROS generation through Inhibition of NF-κB pathway in Hep3B liver cancer cells. Cells 11, 2734 (2022).
Bhosale, P. B. et al. Inhibition of cell proliferation and cell death by apigetrin through death Receptor-Mediated pathway in hepatocellular cancer cells. Biomolecules 13, 1131 (2023).
Zhao, Z-W. et al. Antioxidant effects and phytochemical properties of seven Taiwanese cirsium species extracts. Molecules 26, 3935 (2021).
Zhu, L. et al. Glucagon-like peptide-1 receptor expression and its functions are regulated by androgen. Biomed. Pharmacother. 120, 109555 (2019).
Jung, H. A. et al. Protective effects of flavonoids isolated from Korean milk Thistle cirsium Japonicum Var. Maackii (Maxim.) Matsum on Tert -butyl hydroperoxide-induced hepatotoxicity in HepG2 cells. J. Ethnopharmacol. 209, 62–72 (2017).
European Medicines Agency. ICH Topic Q 2 (R1. ) Validation of Analytical Procedures: Text and Methodology. Https://WwwEmaEuropaEu/En/Documents/Scientific-Guideline/Ich-Guideline-Q2r1-Validation-Analytical-Procedures-Text-Methodology-Step-5-First-Version_enPdf (2006).
Zubrickė, I., Jonuškienė, I., Kantminienė, K., Tumosienė, I. & Petrikaitė, V. Synthesis and in vitro evaluation as potential anticancer and antioxidant agents of Diphenylamine-Pyrrolidin-2-one-Hydrazone derivatives. Int. J. Mol. Sci. 24, 16804 (2023).
Dabkeviciute, G., Maccioni, E. & Petrikaite, V. Effect of Sunitinib derivatives on glioblastoma single-cell migration and 3D cell cultures. Am. J. Cancer Res. 13, 1377–1386 (2023).
Šermukšnytė, A., Kantminienė, K., Jonuškienė, I., Tumosienė, I. & Petrikaitė, V. The effect of 1,2,4-Triazole-3-thiol derivatives bearing hydrazone moiety on cancer cell migration and growth of melanoma, breast, and pancreatic cancer spheroids. Pharmaceuticals 15, 1026 (2022).
Cho, M., Kim, Y., You, S., Hwang, D. Y. & Jang, M. Chlorogenic acid of cirsium Japonicum resists oxidative stress caused by aging and prolongs healthspan via SKN-1/Nrf2 and DAF-16/FOXO in caenorhabditis elegans. Metabolites 13, 224 (2023).
Nguyen, V., Taine, E. G., Meng, D., Cui, T. & Tan, W. Chlorogenic acid: A systematic review on the biological functions, mechanistic actions, and therapeutic potentials. Nutrients 16, 924 (2024).
Singh, S. et al. Neuroprotective effect of chlorogenic acid on mitochondrial Dysfunction-Mediated apoptotic death of DA neurons in a parkinsonian mouse model. Oxid. Med. Cell. Longev. 2020, 1–14 (2020).
Shah, S., Pocard, M. & Mirshahi, M. Targeting the differentiation of gastric cancer cells (KATOIII) downregulates epithelialmesenchymal and cancer stem cell markers. Oncol. Rep. (2019).
Hwang, J-T. et al. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 247, 115–121 (2007).
Lee, J. H. et al. Antioxidant effects of cirsium setidens extract on oxidative stress in human mesenchymal stem cells. Mol. Med. Rep. 14, 3777–3784 (2016).
Ghil Cirsium Japonicum extract induces apoptosis and anti-proliferation in the human breast cancer cell line MCF-7. Mol. Med. Rep. ;3. (2010).
Zhou, Y. et al. Natural polyphenols for prevention and treatment of cancer. Nutrients 8, 515 (2016).
Huang, S. et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics 9, 6745–6763 (2019).
Kuo, C-H. et al. Apigenin has anti-atrophic gastritis and anti-gastric cancer progression effects in Helicobacter pylori -infected Mongolian gerbils. J. Ethnopharmacol. 151, 1031–1039 (2014).
Lu, J. et al. Luteolin exerts a marked antitumor effect in cMet-overexpressing patient-derived tumor xenograft models of gastric cancer. J. Transl Med. 13, 42 (2015).
Ranjbary, A. G. et al. Chlorogenic acid induces apoptosis and cell-cycle arrest in colorectal cancer cells. Mol. Biol. Rep. 50, 9845–9857 (2023).
Alam, M. et al. Therapeutic implications of caffeic acid in cancer and neurological diseases. Front. Oncol. ;12. (2022).
Cho, C. et al. Cirsium Japonicum Var. Maackii and apigenin block Hif-2α‐induced Osteoarthritic cartilage destruction. J. Cell. Mol. Med. 23, 5369–5379 (2019).
Sonju, J. J. et al. Assessment of antitumor and antiproliferative efficacy and detection of Protein-Protein interactions in cancer cells from 3D tumor spheroids. Curr. Protoc. 2, e569 (2022).
Silvestri, A., Vicente, F., Vicent, M. J., Stechmann, B. & Fecke, W. Academic collaborative models fostering the translation of physiological in vitro systems from basic research into drug discovery. Drug Discov Today. 26, 1369–1381 (2021).
Song, J-H. et al. Hydroethanolic extract of cirsium setidens ameliorates doxorubicin-induced cardiotoxicity by AMPK-PGC-1α-SOD-mediated mitochondrial protection. Phytomedicine 129, 155633 (2024).
UĞUR, D., GÜNEŞ, H. & GÜNEŞ, F. Cytotoxic activities of certain medicinal plants on different cancer cell lines. Turk. J. Pharm. Sci. 14, 222–230 (2017).
Žitek, T., Postružnik, V., Knez, Ž., Golle, A. & Dariš, B. Knez Marevci M. Arnica Montana L. Supercritical extraction optimization for antibiotic and anticancer activity. Front. Bioeng. Biotechnol. ;10. (2022).
Beheshti, F. et al. Anticancer activity of Ipomoea purpurea leaves extracts in monolayer and Three-Dimensional cell culture. Evidence-Based Complement. Altern. Med. 2021, 1–14 (2021).
Mahmod, A. I. & Talib, W. H. Anticancer activity of Mandragora autumnalis: an in vitro and in vivo study. Pharmacia 68, 827–835 (2021).
Naeem, A. et al. Natural products as anticancer agents: current status and future perspectives. Molecules 27, 8367 (2022).
Funding
Science Foundation of Lithuania University of Health Sciences project ‘Evaluation of the Biological Effects of Phytochemical Extracts and Accumulated Phenolic Compounds of Naturally Growing Spear Thistle (Cirsium vulgare) in Lithuania, In Vitro,’ 2022.
Author information
Authors and Affiliations
Contributions
Conceptualization, U.G. (chemical experiments) and V.P. (biological experiments); methodology, U.G. and V.P.; formal analysis, V.P., and U.G.; investigation, U.G., and V.P.; writing—original draft preparation, U.G. and V.P.; writing—review and editing, V.P., L.I. and U.G.; visualization, U.G. and. V.P.; supervision, V.P and L.I. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Griškevičienė, U., Ivanauskas, L. & Petrikaitė, V. Anticancer properties of Cirsium vulgare (Savi) ten. Dry extracts from different plant parts and phenological stages of Raw material collection. Sci Rep 15, 12105 (2025). https://doi.org/10.1038/s41598-025-96329-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-96329-4