Abstract
Insecticide-based vector control, which comprises the use of insecticide-treated bed nets (ITNs) and indoor residual spraying (IRS), is the key method of malaria control in Ethiopia. However, its effectiveness is threatened as malaria vectors become resistant to insecticides. Thus, the aim of this study was to monitor the insecticide susceptibility status of malaria vectors. WHO susceptibility tests were used to detect knock-down and mortality rate in the wild malaria vectors collected in Gondar zuria woreda, Northwest Ethiopia. The WHO diagnostic doses of 0.75% permethrin, 0.05% deltamethrin, 0.05% alpha-cypermethrin, 0.1% propoxur and 0.25% pirimiphos-methyl were used. The major malaria vectors in Ethiopia, Anopheles gambiae s.l Anopheles funestus group, and Anopheles Pharoensis, were susceptible, to pirimiphos-methyl and propoxur. However, resistant to permethrin (mortality rate of 88.8%), alphacypermethrin (mortality rate of 67.5%), and deltamethrin (mortality rate of 73.8%). Although permethrin restoration was only 96% in permethrin resistant Anopheles mosquitoes, the efficacy of alphacypermethrin and deltamethrin was totally restored by pre-exposure to PBO. The susceptibility of malaria vectors to pirimiphos-methyl, propoxur, and PBO + pyrethroid insecticides is encouraging for successful malaria control. Further investigations are needed to better understand the molecular basis of pyrethroids insecticide resistant-malaria vectors.
Similar content being viewed by others
Introduction
Malaria is a vector-borne disease caused by five protozoan parasites (Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, Plasmodium ovale, and most recently implicated Plasmodium knowlesi) of the genus Plasmodium and transmitted by Anopheles mosquitoes biting1,2. There are over 460 and 140 described species of the genus Anopheles mosquitoes worldwide and in Africa, respectively. Only an estimated 30 to 40 species are regularly associated with plasmodium transmission to humans worldwide, and of those 140, eight species are known to be efficient vectors of malaria parasites3,4.
Anopheles gambiae complex includes at least eight different vector species, five of which are significant malaria vectors: Anopheles gambiae(s.l), Anopheles coluzzii, Anopheles arabiensis, Anopheles melus, and Anopheles merus 5. Anopheles funestus Giles complex consist of nine species: Anopheles parensis Gillies, Anopheles aruni Sobti, Anopheles confusus Evans and Leeson, Anopheles funestus, Anopheles vaneedeni Gillies and Coetzee, Anopheles rivulorum Leeson, Anopheles fuscivenosus Leeson, Anopheles leesoni Evans, and Anopheles Brucei, two of which, such as Anopheles funestus s.s and Anopheles parensis Gillies, mostly play a critical role in malaria parasite transmission6,7.
Anopheles nili group includes four closely related species: Anopheles nili sensu stricto, Anopheles somalicus, Anopheles carnevalei, and Anopheles ovengensis, of which Anopheles nili sensu stricto is highly anthropophilic and the group’s major malaria vector, and Anopheles stephensi was recently identified as a new malaria vector and widely distributed in Africa8,9. The primary malaria vector in Ethiopia is Anopheles gambae s.l., with secondary vectors including Anopheles pharoensis, Anopheles funestus, Anopheles nili, and the recently discovered Anopheles stephensi10,11.
The public health burden posed by Plasmodium vivax is not considered benign, as it causes severe morbidity and death. Nonetheless, Plasmodium falciparum remains the most serious threat to public health on a global scale, accounting for more than 90% of all malaria deaths in 2018. Children under the age of five are the most vulnerable group, accounting for 67% (272,000) of all malaria deaths worldwide1,12. For this reason WHO has established challenging global technical strategy targets for 2030, including the elimination of malaria in many countries and a reduction in incidence and mortality rates of at least 90%13,14. To achieve these goals, malaria parasites are targeted using anti-malarial drugs and vector control measures through the use of insecticides15.
There are two main methods of malaria vector control: indoor residual spraying (IRS) and insecticide-treated nets (ITNs). Permethrin, etofenprox, bifenthrin, deltamethrin, lambda-cyhalothrin, and alphacypermethrin, DDT (organochlorine), bendiocarb (carbamate), malathion, pirimiphos-methyl, fenitrothion (organophosphates), and pyriproxyfen are currently available insecticide products for malaria vector control, confined to four chemical classes. Pyrethroid insecticides are the only insecticides approved by the WHO to be used in treated bed nets because of their relatively low human toxicity, excite-repellent properties, rapid rate of knock-down, and killing effects16,17,18. Mosquitoes having resistance to pyrethroid insecticides can be controlled more effectively by using synergist PBO19.
In Ethiopia, IRS was first implemented in 1959 and continues to play an important role in malaria control. Despite the significant role played by insecticides in malaria control in Ethiopia, the disease remains endemic, with populations in some areas remaining at high risk of infection13,20. The emergence of insecticide resistance is one of the challenges to the main malarial controlling approaches21. However, the susceptibility status of malaria vectors to insecticides is largely unknown. Therefore, it is crucial to assess the insecticide susceptibility status of malaria vectors. Hence, the aim of this study was to determine the effectiveness of vector control tools in order to choose the most effective insecticides for vector control in the context of battling malaria.
Methods
Study area
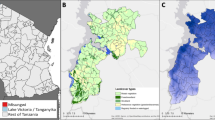
The study was carried out in the Gondar zuria district, Northwest Ethiopia, from March 1, 2022, to August 31, 2022. This district is 45 km away from Gondar, town, and 685 km away from Addis Ababa. It covers an area of 1108.53 km2 and has a population density of 188.4 people per km. Maksegnit town, located at 12 23′ 00’' latitudes and 37 33′ 00’' longitudes, is the capital of the Gondar zuria district and it receives 1047.6 mm of rain annually, with a mean maximum temperature of 27.4 °C, a mean minimum temperature of 14.7 °C, and a relative humidity of 45%22,23. It is malarious with significant malaria transmission for both P. falciparum and P. vivax malaria; Streams and irrigation water serve as permanent Anopheline breeding sites during dry seasons, and it has an altitude ranging between 1750 and 2600 m above sea level. The major malaria transmission season is from September through November, and the minor one is from April to May.
Study design, period, and population
A cross sectional study design was conducted from March 1, 2022, to August 31, 2022 at Gondar zuria district, Northwest Ethiopia. The study populations were adult female Anopheles mosquitoes that fulfilled the inclusion criteria.
Sample size determination
According to WHO guidelines, 120 adult female Anopheles mosquitoes of a given species were required to conduct a single set of WHO insecticide susceptibility test24. Of these, 80 were exposed to the insecticide that was being tested (in four replicates each of around 20 mosquitoes). The remaining 40 mosquitoes serve as “controls” (i.e., two replicates of each of around 20 mosquitoes). A total of 100 adult female Anopheles mosquitoes were required to run a test of the synergist assay.
The collection of larvae and processing
Larval sampling and rearing procedure
Anopheles mosquitoes larvae were collected using the standard dipping method (dipper) from the breeding site including road puddles, brick pits, marshes, and ditches. The larvae were placed in a plastic tin for transportation to the Maksegnit malaria research center. In the laboratory, the larvae were poured into the larvae tray and debris was removed from the water. Then larvae were placed in the larval trays with their natural water and larvae food.
The pupae were sorted and transferred with pipettes from the enamel trays to beakers with small amounts of water. Each beaker was placed inside a cage with a 10% sugar solution for rearing them in the cage. After two to three days, the pupae emerged to adults, and cages were put in a safe place and protected from contamination25,26. Temperature and relative humidity in testing rooms were within the range of 25 ± 2 °C and 80 ± 10%, respectively.
Insecticide susceptibility test for adult female Anopheles mosquitoes
WHO insecticide susceptibility tests were carried out following the standard World Health Organization27 protocol, using insecticide susceptibility test kits and insecticide-impregnated papers. 120 Anopheles mosquitoes were transferred from cages to six holding tubes with untreated papers for an hour. Batches of 20 mosquitoes in four replicates were exposed to insecticide-impregnated papers with discriminating concentrations of the most commonly used insecticides (permethrin (0.75%), deltamethrin (0.05%), alphacypermethrin (0.05%), pirimiphos-methyl (0.25%), and propoxur (0.1%) for 1 h in WHO test tubes.
The knockdown effects for all tested insecticides were recorded at 10, 15, 20, 30, 40, 50, and 60 min. A control in two replicates (40 female Anopheles mosquitoes were used for each insecticide), each with an equal number of mosquitoes, were exposed to papers impregnated with oil and run concurrently. After that, mosquitoes were placed in holding tubes made of untreated paper, where given 10% sucrose solution by using cotton wool. Finally, 24-h post-exposure mortality was noted, and each Anopheles mosquitoes were identified morphologically using a taxonomic key24. All WHO susceptibility tests were carried out in a room with 25 ± 2 °C temperatures and 80 ± 10% humidity.
Vector species composition
Anopheles mosquitoes were divided into three groups using a morphological key (Gillies and M. Coetzee,1987) after each test was done: the Anopheles gambiae (s,l,) the Anopheles funestus group, and the Anopheles pharoensis group28.
Synergist-insecticide bioassays
Synergist experiments were carried out using piperonylbutoxide (PBO), an inhibitor of oxidases including P450s, to determine the role of metabolic enzymes in the resistance profile, particularly cytochrome P450s. For these tests, batches of 25 non-exposed three-day-old adult female Anopheles mosquitoes were transferred to four (PBO-only, PBO + pyrethroids, insecticide only, and control labeled) holding tubes with untreated papers for an hour. After an hour, each of the batches in PBO-only and PBO + pyrethroids were transferred to two exposure tubes with 4%PBO-impregnated papers for one hour, and each of the batches of insecticide only, and control tubes were left in their holding tubes. Then, after 50 Anopheles mosquitoes from insecticide only and PBO + pyrethroids were transferred to the two insecticide-treated exposure tubes and exposed for 60 min again. Simultaneously the solvent control test mosquitoes were transferred to the oil-impregnated paper control tube and exposed for an hour. PBO only and control tubes were used as control.
The knockdown numbers for tested insecticides were recorded at 10, 15, 20, 30, 40, 50, and 60 min. Anopheles mosquitoes from insecticide only, control and PBO + pyrethroids tubes were transferred to holding tubes with cotton wool in sugar water after an hour exposure and maintained for 24 h. Mortality was recorded after 24 h and compared to those of insecticides with and without synergist exposure to evaluate the level of susceptibility restoration and the implication of P450s in resistance to the tested insecticide.
Standard definition
The results of the susceptibility tests were evaluated as recommended by WHO criteria: 98–100% mortality indicates susceptibility, 90–97% mortality indicates resistance candidate (more investigation is needed) and less than 90% mortality suggests resistance24.
Data quality assurance
The quality of data was assured by following WHO protocol: insecticide-impregnated papers were used only one times. Sterilized cages and 10% sucrose solution soaked in cotton pads were used. Three-day old adult female Anopheles mosquitoes were used. Temperature and relative humidity were within the range of 25 ± 2 °C and 80 ± 10%, respectively. Finally; each and everything was cross-checked and done carefully.
Data analysis
Data was entered and analyzed using SPSS version 26. The results of the susceptibility tests were evaluated as recommended by WHO criteria24 as follows: 98–100% mortality indicates susceptibility, 90–97% mortality indicates a resistance candidate (more investigation is needed), and less than 90% mortality suggests resistance.
Logit probit analysis was used to determine the KDT 50% and KDT 90% knockdown time of the insecticides (the time taken to knock down 50%, 90%, and 95% of mosquitoes, respectively). One-way ANOVA was used to compare the mean knock down rate of the Anopheles mosquitoes by insecticides to evaluate their effectiveness. An independent sample t-test was used to compare the mean knock down rate of the Anopheles mosquitoes by pyrethroid insecticides with and without PBO.
The Synergist assay was conducted by using PBO following WHO criteria29. Complete restoration of susceptibility following pre-exposure to PBO (i.e., ≥ 98% mean mortality) implies that a monooxygenase-based resistance mechanism fully accounts for the expression of the resistant phenotype in the Anopheles mosquitoes. Partial restoration of susceptibility following preexposure to PBO (i.e., mean mortality in the PBO followed by insecticide samples is greater than mean mortality in the insecticide-only samples but < 98%) implies that a monooxygenase-based resistance mechanism only partially accounts for expression of the resistant phenotype and that other resistance mechanism are likely to be present in the test population. No restoration of susceptibility following pre-exposure to PBO (mean mortality in the PBO followed by insecticide samples is equal to or lower than mean mortality in the insecticide-only samples) implies that the resistance phenotype detected is not based on mono-oxygenase-mediated detoxification.
Results
Anopheles species composition
A total of 900 Anopheles mosquitoes were collected, and three different species were identified: Anopheles gambiae s.l., Anopheles funestus s.l., and Anopheles pharoensis. Anopheles gambiae s.l. was the most abundant malaria vector species with 97.5%, followed by Anopheles funestus and Anopheles phronesis with 1.5% and 0.7%, respectively.
WHO insecticide susceptibility tests
Knockdown time
Permethrin and deltamethrin used 40 min, while propoxur was used 37 min to induce 50%knockdown of Anopheles mosquito, respectively. Pirimiphos-methyl and alphacypermethrin did not show 50% knockdown of Anopheles mosquito after an hour. 50% KDT for permethrin and deltamethrin was high in comparison to propoxur (Table 1). But, at each time interval, the knockdown number of Anopheles mosquitoes increased (Fig. 1).
The mean knockdown rate of the Anopheles mosquitoes by insecticides
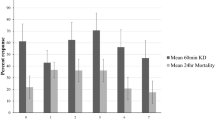
Mean knock down rate were 22.7% (95% CI 11.97–33.5) for alphacypermethrin, 27.8% (95% CI 8.7–47) for permethrin, 27% (95% CI 7–47) for deltamethrin, 3.3% (95% CI 0.55–7) for pirimiphos-methyl, and 29.7% (95% CI 3.2–56.2) for propoxur. The mean knock down rate of the Anopheles mosquitoes by permethrin and deltamethrin was the same while the mean knock down rate of the Anopheles mosquitoes by propoxur was high, but the mean knock down rate of the Anopheles mosquitoes by pirimiphos-methyl was small after an hour (Fig. 2).
However, there was no significant difference between average of the mean knockdown rates of the Anopheles mosquitoes by insecticides that being tested after 1 h (P > 0.092) (Table 2).
Phenotypic susceptibility levels of malaria vectors
According to WHO recommendations, phenotypic susceptibility tests on 600 adult female Anopheles mosquitoes were performed to evaluate the resistance profile against five insecticides, namely alphacypermethrin, permethrin, deltamethrin, pirimiphos-methyl, and propoxur. Malaria vectors were resistant to alpha-cypermethrin, permethrin, and deltamethrin, with mortality rates of 67.5%, 88.8%, and 73.75%, respectively. Complete susceptibility (100% death) of the Anopheles mosquitoes was observed with pirimiphos-methyl and propoxur (Table 3). The resistance intensity of alpha-cypermethrin was high when compared with permethrin and deltamethrin (Table 4).
The impact of piperonyl-butoxide in pyrethroid-resistant malaria vectors
The duration required to cause 50% knockdowns of the Anopheles mosquitoes using PBO + deltamethrin, PBO + permethrin, and PBO + alpha-cypermethrin was 22 min (95% CI 18.5–26.2), 29 min (95% CI 23–37), and 17 min (95% CI 13.2–21.4), respectively. When compared to the 50% knockdown time of the Anopheles mosquitoes by pyrethroid insecticides alone, the time required to induce 50% knockdown of the Anopheles mosquitoes by PBO + deltamethrin, PBO + permethrin, and PBO + alphacypermethrin decreased (Table-4). Additionally, PBO + deltamethrin and PBO + alpha-cypermethrin induced 90% knockdown of the Anopheles mosquito in 59 min (95% CI 46.9–86.2).
The mean knockdown rate of the Anopheles mosquitoes was significantly improved by PBO plus alpha-cypermethrin (P < 0.005) when compared to alpha-cypermethrin alone (Table 5). At each time interval, the knockdown number of the Anopheles mosquitoes by PBO + deltamethrin, PBO + permethrin, and PBO + alphacypermethrin was greater than deltamethrin, permethrin, and alphacypermethrin alone (Fig. 3).
Phenotypic susceptibility levels of malaria vectors to pyrethroids with PBO
Malaria vectors were resistant to alpha-cypermethrin and deltamethrin alone, but the efficacy of alpha-cypermethrin and deltamethrin was fully restored by pre-exposure to PBO from 68 to 100% and 72 to 100%, respectively. Anopheles mosquitoes were resistant to permethrin alone but partially restored by pre-exposure to PBO from 88 to 96% according to WHO guidelines (Fig. 4). Because the mortality rate in the controls and PBO-only was consistently lower than 5%, the mortality rate found in the experimental tests was not adjusted (Table 6).
Discussion
For many years, controlling and eradicating malaria has been hampered by insecticide resistance30,31. As a result of Anopheles mosquitoes becoming resistant to the majority of insecticides now used in public health32. For this reason, the World Health Organization27 published a Global Plan for insecticide resistance management in malaria vectors that outlines best-practice strategies (rotations of insecticides, use of interventions in combination, mosaic spraying, and use of mixtures) for preserving or prolonging the effectiveness of the insecticides used for malaria control, based on the best available evidence at the time30,33. However, geographically, there is a poor understanding of insecticide resistance status due to limited evidence or data to decide on appropriate insecticides34,35,36. Hence, this study has been demonstrated for the first time in Gondar zuria woreda regarding the resistance status of malaria vectors to insecticides used for malaria vector control, using standardized WHO protocols to reduce this gap24.
The results of the investigation shown that the Anopheles mosquitoes that transmit malaria parasites in the Gondar zuria woreda were identified along with their composition and density. The Anopheles gambae(s.l) complex was the most abundant malaria vector, followed by the Anopheles funestus group and the Anopheles pharoensis group. This outcome is in line with what Tilahun Adugna and his colleagues reported37. Also, this finding is similar with studies which reported Anopheles gambiae s. l. and Anopheles Pharoensis, which have been identified as primary and secondary vectors in south-western Ethiopia by different researchers, respectively38,39,40,41,42,43,44.
This indicates the presence of a probability to get different anopheles mosquito species from different malaria endemic areas that can be responsible for the transmission of malaria parasites if entomological studies are conducted in different endemic areas.
According to the findings of the investigation, the Gondar zuria woreda malaria vectors were susceptible to carbamate (propoxur) and organophosphate (pirimiphos-methyl) insecticides. However, they were resistant to all of the pyrethroid insecticides tested. Previous findings from Burkina Faso, Uganda, Mali, Rwanda, Kenya, and Tanzania agree with this one from 2005 to 201716,45,46,47,48,49,50,51.
According to Chanyalew T, Balkew M, Yared, and their colleagues, permethrin, alphacypermethrin, and deltamethrin all demonstrate comparable degrees of resistance of this study9,52,53. Additionally, reports from south-central Ethiopia and south-western Ethiopia demonstrated comparable pyrethroid resistance states of this study54,55. It also support by studies whch reports the resistance of deltamethrin and permethrin in Asian countries such as China’s Shandong province, Thailand, and Myanmar56,57. Suspected resistance to deltamethrin is reported from Madagascar and Badakhshan58,59. However, it needs confirmission according to WHO criteria24. But, Susceptible to pyrethroid insecticide is reported by Rana SM and his friends from pakistan60. Marcomba Sand and his colleagues’ report is inline with the report of Rana SM61. Similar findings are reported by Dhiman S and his colleagues62. This may be a product of environmental influences, in contrast to this study findings38.This all indicates the presence of resistance in malaria vectors to pyrethroid insecticides in different malaria-endemic areas.
Additionally, after 60 min, alpha-cypermethrin failed to knock down 50% of the malaria vectors. However, permethrin and deltamethrin KD 50% of the malaria vectors. According to Kinfe and his associate’s reports, permethrin and deltamethrin demonstrate comparable result63. This early detection indicates the low efficacy of alpha-cypermethrin and its higher resistance than permethrin and deltamethrin in malaria vectors. However, there was no significant difference between insecticides when comparing the mean knockdown rates of Anopheles mosquitoes (P > 0.092). This shows the absence of a significant difference regarding the efficacy of pyrethroids against Anopheles mosquitoes.
In this investigation, pirimiphos methyl, an insecticide from the class of organophosphates, was used to assess phenotypic levels of susceptibility. It has been completely (100%) toxic to malaria vectors according to WHO guidelines24. Even while primiphos methyl failed to knock down 50% of malaria vectors after 60 min, it miraculously caused full susceptibility after 24 h. This indicates a pro-insecticide called pirimiphos methyl needs mosquito cytochrome P450 enzymes to be activated in order to cause toxicity64. Hence, it takes a long time to be toxic to malaria vectors, so it should be used considering this. The outcome is in line with reports by Rakotoson JD and Soma DD with their colleagues50,58. Reports from Benin, Zambia, Madagascar, and Ghana are all in agreement with this study result58,65,66,67,68. The susceptibility of malaria vectors to primiphos methyl has also been documented from Gambella and other parts of the country52,69.
Propoxur from the carbamate class was used to evaluate the susceptibility status of malaria vectors. It caused a 50% knockdown of malaria vectors within 37 min. But it couldn’t cause 90% knockdown at 60 min. This report is in agreement with reports from different regions in Ethiopia70. According to WHO standards24, malaria vectors have been verified to be completely susceptible (100%) to propoxur. A report from Nigeria is in line with this71. Similar to this, comparable findings from Ethiopian regions have been reported38,52,63,69. By Alemayehu and his colleagues, the susceptibility of malaria vectors is reported in line with this72. These different comparable results may indicate the effectiveness of propoxur.
The synergistic effect of piperonyl-butoxide (PBO) with pyrethroid insecticides (alpha-cypermethrin, permethrin, and deltamethrin) was observed by exposing Anopheles mosquitoes to synergists before insecticides to check the presence of enzymes that can be controlled by PBO. Permethrin and deltamethrin alone had a knockdown time of 34 and 47 min to knock down 50% of malaria vectors, respectively, but when combined with PBO, the time was lowered to 29 and 22 min to knock down 50% of malaria vectors at 60 min, respectively. After 60 min, among pyrethroid class insecticides that have been assessed, only alpha-cypermethrin was unable to knock down 50% of malaria vectors. However, when they were exposed to PBO prior to insecticides, 50% and 90% knockdown of malaria vectors were observed.
Deltamethrin also could not knock down 90% of malaria vectors after 60 min. But it induced knock down 90% after exposure of Anopheles mosquitoes to PBO, followed by deltamethrin. This report is in line with outcomes from various locations in Africa73,74,75,76,77. The knockdown and mortality rates of the Anopheles mosquitoes have increased by PBO pre-exposure followed by pyrethroid insecticide. It is comparable with other reports that have been mentioned above. The reduction of knockdown time after malaria vectors are exposed to PBO indicates the presence of metabolizing enzymes in the malaria vectors that can metabolize the insecticides.
After 24 h, the mortality rates of malaria vectors by PBO + pyrethroid insecticide and insecticide alone were calculated and compared. Complete restoration to deltamethrin and alpha-cypermethrin-resistant malaria vectors, as well as partial restoration to permethrin-resistant malaria vectors, have been achieved in accordance with WHO guidelines24.
Mortality rates of malaria vectors by deltamethrin, alpha-cypermethrin, and permethrin alone were 72%, 68%, and 88%, respectively, but after being pre-exposed to PBO, mortality rates of malaria vectors were 100%, 100%, and 96%,respectively. This is in line with outcomes from various researches conducted in Africa73,74,75,76,77. This suggests that detoxifying enzymes had a part in the development of resistance. Complete restoration of susceptibility suggests that the resistant phenotype in the malaria vectors is entirely explained by a monooxygenase-based resistance mechanism, whereas partial restoration of susceptibility indicates that additional resistance mechanisms are probably present in the malaria vectors and that a monooxygenase-based resistance mechanism only partially accounts for the manifestation of the resistant phenotype.
An independent sample t-test reveals the absence of a significant difference between the mean knockdown rates of the malaria vectors by permethrin and PBO + permethrin, as well as between deltamethrin and PBO + deltamethrin. However, there was a significant difference between alphacypermethrin and PBO + alphacypermethrin. This implies that the detoxifying enzymes produced by malaria vectors may metabolize alphacypermethrin rather than permethrin and deltamethrin.
The results of this study are not unexpected because several investigations conducted in various locations of Ethiopia have consistently shown the existence of resistance to the pyrethroid insecticides as well as the malaria-causing vectors in this study area is consistent with those identified in different locations of Ethiopia. In general, these data are critical for selecting the most effective insecticides for vector control in order to reduce malaria.
Conclusion
This study demonstrated that malaria vectors are susceptible to pirimiphos-methyl, propoxur, and PBO + pyrethroid insecticides, but resistant to pyrethroids insecticide. Anopheles gambae s.l. Anopheles funestus, and Anopheles pharoensis are malaria vectors in Gondar zuria woreda with Anopheles gambae complex predominating. Thus, the incidence rate of malaria and malaria vectors can be controlled in this area using alphacypermethrin, deltamethrin, and permethrin-impregnated mosquito nets integrated with PBO and/or through indoor residual spraying of sprayable human dwellings with pirimiphos-methyl and propoxur. Based on the findings of this study, we recommend that the use of pyrethroids integrated with bed nets should not provide important protection in the future; rather, it is better to use pyrethroids-impregnated mosquito nets with PBO and/or the use of propoxur and pirimiphos-methyl should continue for effective control of malaria by indoor spraying technique in this area. Since Anopheles mosquitoes could develop various resistant mechanisms with various pathways, more research should be done on molecular confirmation of the presence of the knockdown resistance alleles in this area.
Data availability
We confirmed that all the data for this manuscript are available in the manuscript , if someone wants to request the data can contact Silesh Barasa.
Abbreviations
- ANOVA:
-
Analysis of variance
- DDT:
-
Dichlorodiphenyltrichloroethane
- FMH:
-
Federal Minister of Health
- IRS:
-
Indoor residual spraying
- ITNs:
-
Insecticide treated nets
- LLINs:
-
Long-lasting insecticidal nets
- KD:
-
Knock down
- PQ:
-
Prequalification
- PBO:
-
Piperonylbutoxide
- UoG:
-
University of Gondar
- WHO:
-
World Health Organization
References
Snow, R. W. Global malaria eradication and the importance of Plasmodium falciparum epidemiology in Africa. BMC Med. 13(1), 1–3 (2015).
Sabbatani, S., Fiorino, S. & Manfredi, R. The emerging of the fifth malaria parasite (Plasmodium knowlesi). A public health concern?. Braz. J. Infect. Dis. 14(3), 299–309 (2010).
Stevenson, J. C. & Norris, D. E. Implicating cryptic and novel anophelines as malaria vectors in Africa. Insects. 8(1), 1 (2016).
Okara, R. M. et al. Distribution of the main malaria vectors in Kenya. Malar. J. 9(1), 1–11 (2010).
Hancock, P. A. et al. Modelling spatiotemporal trends in the frequency of genetic mutations conferring insecticide target-site resistance in African mosquito malaria vector species. BMC Biol. 20(1), 1–17 (2022).
Burke, A. et al. Anopheles parensis contributes to residual malaria transmission in South Africa. Malar. J. 18(1), 1–7 (2019).
Kweka, E. J. et al. A first report of Anopheles funestus sibling species in western Kenya highlands. Acta Trop. 128(1), 158–161 (2013).
Ndo, C. et al. Cryptic genetic diversity within the Anopheles nili group of malaria vectors in the equatorial forest area of Cameroon (Central Africa). PLoS ONE 8(3), e58862 (2013).
Yared, S. et al. Insecticide resistance in Anopheles stephensi in Somali Region, eastern Ethiopia. Malar. J. 19(1), 1–7 (2020).
Messenger, L. A. et al. Insecticide resistance in Anopheles arabiensis from Ethiopia (2012–2016): A nationwide study for insecticide resistance monitoring. Malar. J. 16(1), 1–14 (2017).
Taffese, H. S. et al. Malaria epidemiology and interventions in Ethiopia from 2001 to 2016. Infect. Dis. Poverty 7(06), 1–9 (2018).
Abbasher Hussien Mohamed Ahmed, K. et al. Clinical characteristics, complications, and predictors of outcome of hospitalized adult Sudanese patients with COVID-19 and malaria coinfection in Sudan: A multicenter retrospective cross-sectional study. J. Med. Virol. 94, 3685–3697 (2022).
World Health Organization. World Malaria Report 2015 (World Health Organization, 2016).
Nasir, S., Amarasekara, S., Wickremasinghe, R., Fernando, D. & Udagama, P. Prevention of re-establishment of malaria: Historical perspective and future prospects. Malar. J. 19(1), 1–16 (2020).
World Health Organization. Global technical strategy for malaria 2016-2030 (World Health Organization, 2015).
Ondeto, B. M. et al. Current status of insecticide resistance among malaria vectors in Kenya. Parasit. Vectors 10(1), 1–13 (2017).
Messenger, L. A. et al. A whole transcriptomic approach provides novel insights into the molecular basis of organophosphate and pyrethroid resistance in Anopheles arabiensis from Ethiopia. Insect Biochem. Mol. Biol. 139, 103655 (2021).
Ahadji-Dabla, K. M. et al. Susceptibility of a malaria vector Anopheles gambiae sl (Diptera: Culicidae) to WHO recommended insecticides in Togo (West Africa). J. Entomol. Zool. Stud. 3, 75–79 (2015).
Pennetier, C. et al. Efficacy of Olyset(R) Plus, a new long-lasting insecticidal net incorporating permethrin and piperonyl-butoxide against multi-resistant malaria vectors [corrected]. PLoS ONE 8(10), e75134 (2013).
Raviglione, M. & Maher, D. Ending infectious diseases in the era of the sustainable development goals. Porto Biomed. J. 2(5), 140–142 (2017).
Oxborough, R. M. et al. Susceptibility testing of Anopheles malaria vectors with the neonicotinoid insecticide clothianidin; results from 16 African countries, in preparation for indoor residual spraying with new insecticide formulations. Malar. J. 18(1), 1–11 (2019).
Lemma, W. Description of malaria epidemics and normal transmissions using rainfall variability in Gondar Zuria highland District, Ethiopia. Heliyon 7(8), e07653 (2021).
Minwuyelet, A., Eshetu, T., Milikit, D. & Aschale, Y. Prevalence and risk factors of asymptomatic Plasmodium infection in Gondar Zuria District, Northwest Ethiopia. Infect. Drug Res. 13, 3969 (2020).
World Health Organization. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes (World Health Organization, 2015).
Soleimani-Ahmadi, M. et al. Environmental characteristics of anopheline mosquito larval habitats in a malaria endemic area in Iran. Asian Pac. J. Trop. Med. 6(7), 510–515 (2013).
Oliver, S. V. & Brooke, B. D. The effect of elevated temperatures on the life history and insecticide resistance phenotype of the major malaria vector Anopheles arabiensis (Diptera: Culicidae). Malar. J. 16(1), 1–13 (2017).
WHO GS. World malaria report, 2013. (2014).
Coetzee, M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar. J. 19(1), 1–20 (2020).
World Health Organization. Standard Operating Procedure for Determining the Ability of PBO to Restore Susceptibility of Adult Mosquitoes to Pyrethroid Insecticides in WHO Tube Tests (World Health Organization, 2022).
Riveron, J. M. et al. Insecticide Resistance in Malaria Vectors: An Update at a Global Scale. Towards Malaria Elimination-a Leap Forward (IntechOpen, 2018).
World Health Organization. Global Report on Insecticide Resistance in Malaria Vectors: 2010–2016 (World Health Organization, 2018).
Ranson, H. & Lissenden, N. Insecticide resistance in African Anopheles mosquitoes: A worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 32(3), 187–196 (2016).
World Health Organization. Global Plan for Insecticide Resistance Management in Malaria Vectors (World Health Organization, 2012).
Bhatt, S. et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526(7572), 207–211 (2015).
Moyes, C. L. et al. Analysis-ready datasets for insecticide resistance phenotype and genotype frequency in African malaria vectors. Scientific Data 6(1), 1–11 (2019).
Mnzava, A. P. et al. Implementation of the global plan for insecticide resistance management in malaria vectors: Progress, challenges and the way forward. Malar. J. 14(1), 1–9 (2015).
Adugna, T., Getu, E. & Yewhalaw, D. Species diversity and distribution of Anopheles mosquitoes in Bure district, Northwestern Ethiopia. Heliyon 6(10), e05063 (2020).
Abraham, M., Massebo, F. & Lindtjørn, B. High entomological inoculation rate of malaria vectors in area of high coverage of interventions in southwest Ethiopia: Implication for residual malaria transmission. Parasite Epidemiol. Control 2(2), 61–69 (2017).
Getachew, D., Balkew, M. & Tekie, H. Anopheles larval species composition and characterization of breeding habitats in two localities in the Ghibe River Basin, southwestern Ethiopia. Malar. J. 19(1), 1–13 (2020).
Demissew, A. et al. Impact of sugarcane irrigation on malaria vector Anopheles mosquito fauna, abundance and seasonality in Arjo-Didessa, Ethiopia. Malar. J. 19(1), 1–8 (2020).
Chanyalew, T., Natea, G., Amenu, D., Yewhalaw, D. & Simma, E. A. Composition of mosquito fauna and insecticide resistance status of Anopheles gambiae sensu lato in Itang special district, Gambella, Southwestern Ethiopia. Malar. J. 21(1), 1–10 (2022).
Chanyalew, T., Yewhalaw, D., Simma, E. A. Composition of Mosquito Fauna and Insecticide Resistance Status of Anopheles gambiae complex in Itang special woreda, Gambella, Southwestern Ethiopia. (2021).
Behailu, T., Mohammed, S. & Adugna, G. Entomological study on species composition, behavior, longevity and probability of surviving sporogony of Anopheles mosquitoes in Lare District, Ethiopia. J. Parasitol. Vector Biol. 9(9), 137–145 (2017).
Lelisa, K., Asale, A., Taye, B., Emana, D. & Yewhalaw, D. Anopheline mosquitoes behaviour and entomological monitoring in southwestern Ethiopia. J. Vector Borne Dis. 54(3), 240 (2017).
Echodu, R., Anena, J., Iwiru, T., Mireji, P., Malinga, G. M., Opiyo, E. A. et al. High level of resistance in Anopheles arabiensis mosquito to pyrethroid insecticides from low malaria transmission zone of Moroto district, Karamoja region, Uganda: Implication for malaria vector control. (2020).
Hakizimana, E. et al. Susceptibility of Anopheles gambiae to insecticides used for malaria vector control in Rwanda. Malar. J. 15(1), 1–11 (2016).
Hancock, P. A. et al. Mapping trends in insecticide resistance phenotypes in African malaria vectors. PLoS Biol. 18(6), e3000633 (2020).
Keita, M. et al. Susceptibility status of Anopheles gambiae sensu lato to insecticides commonly used for malaria control in Mali (1990). Bull. Soc. Pathol. Exot. 109(1), 39–45 (2016).
Moyes, C. L. et al. Evaluating insecticide resistance across African districts to aid malaria control decisions. Proc. Natl. Acad. Sci. 117(36), 22042–22050 (2020).
Soma, D. D. et al. Insecticide resistance status of malaria vectors Anopheles gambiae (sl) of southwest Burkina Faso and residual efficacy of indoor residual spraying with microencapsulated pirimiphos-methyl insecticide. Parasit. Vectors. 14(1), 1–9 (2021).
Ochomo, E. et al. Pyrethroid susceptibility of malaria vectors in four Districts of western Kenya. Parasit. Vectors 7(1), 1–9 (2014).
Chanyalew, T., Natea, G., Amenu, D., Yewhalaw, D. & Simma, E. A. Mosquito Species Composition And Insecticide Resistance Status of Anopheles arabiensis In Itang Special Woreda, Gambella, Southwestern Ethiopia. (2021).
Balkew, M. et al. Insecticide resistance in Anopheles arabiensis (Diptera: Culicidae) from villages in central, northern and south west Ethiopia and detection of kdr mutation. Parasit. Vectors 3(1), 1–6 (2010).
Gari, T. et al. Malaria incidence and entomological findings in an area targeted for a cluster-randomized controlled trial to prevent malaria in Ethiopia: Results from a pilot study. Malar. J. 15(1), 1–13 (2016).
Massebo, F., Balkew, M., Gebre-Michael, T. & Lindtjørn, B. Blood meal origins and insecticide susceptibility of Anopheles arabiensis from Chano in South-West Ethiopia. Parasit. Vectors 6(1), 1–10 (2013).
Dai, Y. et al. Development of insecticide resistance in malaria vector Anopheles sinensis populations from Shandong province in China. Malar. J. 14(1), 1–5 (2015).
Chaumeau, V. et al. Insecticide resistance in malaria vectors along the Thailand-Myanmar border. Parasit. Vectors 10(1), 1–11 (2017).
Rakotoson, J.-D. et al. Insecticide resistance status of three malaria vectors, Anopheles gambiae (sl), An. funestus and An. mascarensis, from the south, central and east coasts of Madagascar. Parasit. Vectors. 10(1), 1–17 (2017).
Ahmad, M. et al. Status of insecticide resistance in high-risk malaria provinces in Afghanistan. Malar. J. 15(1), 1–9 (2016).
Rana, S. M., Khan, E. A., Yaqoob, A., Latif, A. A. & Abbasi, M. M. Susceptibility and irritability of adult forms of main malaria vectors against insecticides used in the indoor residual sprays in Muzaffargarh district, Pakistan: A field survey. J. Med. Entomol. 51(2), 387–391 (2014).
Marcombe, S. et al. Insecticide resistance status of malaria vectors in Lao PDR. PLoS ONE 12(4), e0175984 (2017).
Dhiman, S. et al. Evaluation of insecticides susceptibility and malaria vector potential of Anopheles annularis sl and Anopheles vagus in Assam, India. PLoS ONE 11(3), e0151786 (2016).
Kinfe, E. et al. Susceptibility of Anopheles gambiae sl, and Anopheles funestus sl to seven insecticides in southern Ethiopia. Ethiop. J. Public Health Nutr. 4(2), 153–159 (2021).
Syme, T. et al. Pyrethroid-piperonyl butoxide (PBO) nets reduce the efficacy of indoor residual spraying with pirimiphos-methyl against pyrethroid-resistant malaria vectors. Sci. Rep. 12(1), 1–13 (2022).
Fuseini, G., Ebsworth, P., Jones, S. & Knight, D. The efficacy of ACTELLIC 50 EC, pirimiphos methyl, for indoor residual spraying in Ahafo, Ghana: Area of high vector resistance to pyrethroids and organochlorines. J. Med. Entomol. 48(2), 437–440 (2011).
Chanda, J. et al. Pyrethroid and carbamate resistance in Anopheles funestus giles along Lake Kariba in southern Zambia. Am. J. Trop. Med. Hyg. 103(2 Suppl), 90 (2020).
Aïkpon, R., Sèzonlin, M., Ossè, R. & Akogbéto, M. Evidence of multiple mechanisms providing carbamate and organophosphate resistance in field An. gambiae population from Atacora in Benin. Parasit. vectors. 7(1), 1–7 (2014).
Aïkpon, R. et al. Bendiocarb resistance in Anopheles gambiae sl populations from Atacora department in Benin, West Africa: A threat for malaria vector control. Parasit. Vectors 6(1), 1–7 (2013).
Balkew, M. et al. Insecticide resistance: A challenge to malaria vector control in Ethiopia. Malar. J. 11(1), 1–2 (2012).
Ejeta, D. & Tefera, T. Larvae density, breeding habitat preference and insecticide susceptibility status of Anopheles gambiae (Diptera: Culicidae) in Assosa district, western Ethiopia. (2022).
Umar, A. et al. Susceptibility test of female anopheles mosquitoes to ten insecticides for indoor residual spraying (IRS) baseline data collection in Northeastern Nigeria. J. Entomol. Nematol. 6(7), 98–103 (2014).
Alemayehu, E. et al. Mapping insecticide resistance and characterization of resistance mechanisms in Anopheles arabiensis (Diptera: Culicidae) in Ethiopia. Parasit. Vectors 10(1), 1–11 (2017).
Kusimo, M. O. et al. Pyrethroid resistance in the New World malaria vector Anopheles albimanus is mediated by cytochrome P450 CYP6P5. Pestic. Biochem. Physiol. 183, 105061 (2022).
Djègbè, I. et al. Dynamics of insecticide resistance in malaria vectors in Benin: First evidence of the presence of L1014S kdr mutation in Anopheles gambiae from West Africa. Malar. J. 10(1), 1–11 (2011).
Machani, M. G. et al. Phenotypic, genotypic and biochemical changes during pyrethroid resistance selection in Anopheles gambiae mosquitoes. Sci. Rep. 10(1), 1–8 (2020).
Omotayo, A. I. et al. Multiple insecticide resistance mechanisms in urban population of Anopheles coluzzii (Diptera: Culicidae) from Lagos, South-West Nigeria. Acta Trop. 227, 106291 (2022).
Dadzie, S. K. et al. Evaluation of piperonyl butoxide in enhancing the efficacy of pyrethroid insecticides against resistant Anopheles gambiae sl in Ghana. Malar. J. 16(1), 1–11 (2017).
Acknowledgements
We acknowledge our colleagues, Dr. Meshesha Balkew for his material inputs and Mr. Muluken Assefa for his support in the morphological identification of the Anopheles mosquito.
Funding
This study was financially supported by the East African Consortium for Clinical Research (EACCR3) funded through the European & developing countries Clinical Trials Partnership (EDCTP3) which is part of EACCR3-malaria node project hosted by KEMRI-Welcome Trust Research Program (KWTTP).
Author information
Authors and Affiliations
Contributions
S. B was conceived, designs the study, interpretation of the data, and wrote original draft of the manuscript. A.J.Z was participated in the design of the study and edited the draft of the manuscript. M. A was participated in the design of the study and edited the draft of the manuscript. D. W, W.A, D.D, B.A, and E.T were participated in data analysis. Finally all the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical consideration
This study was conducted after ethical approval was obtained from research and ethics review committee of the school of biomedical and health sciences.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Barasa, S., Aemro, M., Abebe, W. et al. Assessing insecticide susceptibility status of Anopheles mosquitoes in Gondar zuria district, Northwest Ethiopia. Sci Rep 15, 14452 (2025). https://doi.org/10.1038/s41598-025-96370-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-96370-3