Abstract
In this research, a comprehensive study of the solvent extraction of uranium (VI) from acidic sulfate solutions using Alamine 336 diluted in kerosene was scrutinized. The effects of the contact time between the phases, sulfuric acid concentration, extractant concentration, uranium (VI) concentration, organic/aqueous phase ratio, and temperature were investigated. An extraction efficiency of around 99.72% was attained at a sulfuric acid concentration of 0.15 mol L−1, using 0.05 mol L−1 Alamine 336 at 25 °C with an organic/aqueous phase ratio of 1:1. The findings indicated that the extraction of uranium (VI) was a rapid, exothermic, and spontaneous process. The estimated value of Log Kex was 5.92. Parametric variations in the processing parameters indicated a strong impact of the sulfuric acid concentration on the uranium extraction. Uranium (VI) stripping from the loaded organic phase was conducted using many salt and acid solutions in four steps, and the enthalpy change of the stripping reaction was obtained. 99.87% of uranium (VI) loaded in the organic phase was stripped using 0.5 mol L−1 (NH4)2CO3 in a single stripping step. Finally, uranium (VI) extraction from the leach liquor solution was performed under optimal conditions, and the recovery of the loaded organic phase was investigated with different agents. The results showed that uranium purification has a high selectivity coefficient.
Similar content being viewed by others
Introduction
An essential element of the nuclear industry is uranium1. The continued generation of electricity from nuclear power plants and other nuclear activities depends on the presence of uranium2. However, natural uranium reserves are depleting and attention is shifting to low-grade natural uranium resources3.
To date, many methods have been used to extract uranium (VI) from other elements in natural resources.To fulfill the nuclear industry’s current uranium requirements, uranium recovery from low-grade ores and other sources must be accomplished utilizing low-cost processes4,5.
To meet today’s demands, uranium must be recovered from low-grade ores and other sources using inexpensive methods. Solvent extraction6,7, ion exchange8,9, ultrafiltration10, adsorption11,12, and liquid membranes13,14,15 are useful methods for uranium recovery from various aqueous solutions. Solvent extraction (SX) techniques, also known as liquid-liquid extraction (LLE), have long been used to recover and separate metal ions from their sources. Compared with other methods, this procedure is somewhat easy, affordable, and potentially very selective16,17. Consequently, solvent extraction is one of the most effective methods for industrial metal extraction and purification.
In the solvent extraction process, the extractant plays an important role. Extractants greatly vary depending on the conditions and objectives of each project. Extractants such as TBP, D2EHPA, PC-88 A, and TOPO have been used for the solvent extraction of uranium (VI)18,19. Alamine 336 is a tertiary amine used to extract uranium (VI) from sulfate media20,21. The use of Alamine 336 extracts uranium from single-component solutions. In general, real solutions in the industry contain interfering elements that affect uranium extraction. Considering the importance of the purity of uranium samples, it is very valuable to study the extraction of uranium from real solutions. Also, after uranium is extracted with the organic phase, it must be recovered by the stripping phase. Therefore, it is necessary to conduct a comprehensive study on the optimization of uranium extraction from single-component sulfate media using Alamine 336 and to investigate the stripping reaction conditions and then complete the solvent extraction study using real samples.
The primary aim of this paper was to investigate the extraction of uranium (VI) from a sulfate solution using the Alamine 336 extractant. The exact species of uranium in the acidic solution and the conditions of the species of Alamine 336 adjacent to the sulfate solution were carefully analyzed. The effects of the contact time among the phases, sulfuric acid concentration, extractant concentration, uranium (VI) concentration, organic/aqueous phase ratio, and temperature on uranium (VI) extraction from the synthetic solution with Alamine 336 were studied, and consequently, the optimum conditions for solvent extraction and stripping were established.
Under optimal conditions, the uranium purification process was carried out. For this purpose, uranium (VI) was extracted from the liquor leach solution and the recovery of the organic phase loaded with the stripping agents was investigated.
Therefore, the innovation of this research was to investigate the extraction of uranium from real solutions with Alamine 336 and its recovery with different agents. The typology of uranium from the sulfate media was carefully performed and the thermodynamics of the recovery were also investigated.
Materials and methods
Materials
A potassium hydroxide (KOH) standard solution and ammonium oxalate ((NH4)2C2O4) obtained from Merck were used as the titrant and masking agents, respectively.
The extractant, Alamine 336 was purchased from NetSun. The organic phase was prepared by diluting Alamine 336 in kerosene (Merck Co). An aqueous phase containing uranium (VI) was obtained by dissolving an appropriate amount of UO2(C2H3O2)2-2H2O (Merck) in deionized water. H2SO4, HNO3, HClO4, HCl, H3PO4, NaCl, NH4Cl, NaNO3, NaF, Na2CO3, (NH4)2CO3, NaHCO3 (Merck), and H2O were used as stripping agents. All chemicals used were of analytical grade.
Analysis apparatuses
An X-Ray Fluorescence Spectrometer (XRF, OXFORD, ED 2000, England) was used to identify the chemical elements of the solid sample recovered from the evaporation of the leach liquor solution. The analysis of the metal ions was performed using an inductively coupled plasma-atomic emission spectrometer (ICP-AES, VARIAN, LIBERTY150AX TURBO, Australia). A Sartorius pH meter was used to measure the pH values.
Solvent extraction procedure
Alamine 336 was dissolved in kerosene that was saturated with deionized water in the proper proportions to produce the organic phase of the solvent extraction studies. Extraction (and stripping) experiments were performed by placing equal volumes of the organic and aqueous phase volumes with mechanical shaking (150 rpm). The constant parameters, including temperature, phase volumes, organic to aqueous volume ratio, and contact time of the two phases, were adjusted at 298 ± 1 K, 10 mL, 1:1, and 30 min, respectively. Following the equilibrium period between the two phases, they were segregated using a separation funnel, and the amounts of metal ions in the aqueous phases before and after the extraction and stripping operations were quantified using ICP-AES. A mass balance was used to determine the metal concentrations in the organic phase. Then, using Eqs. (1)–(3), the distribution coefficient (De), extraction percentage (E), and stripping percentage (S) were determined, respectively:
The uranium (VI) concentrations in the aqueous and organic phase, aqueous and organic volume, uranium (VI) concentrations in the stripping solution, and initial uranium (VI) concentrations in the organic phase are denoted by the following: [M]aq, [M]org, Vaq, Vorg, [M]aq.s, and [M]org, t.
Results and discussion
Characterization of the leach liquor solution
Several samples of the leach liquor solution were dried at a temperature of 70 oC, and the constituent elements were determined using XRF. ICP-AES was employed to quantify the concentrations of these elements. Table 1 illustrates the findings. The results indicated that the leach liquor solution contained Cu, Zn, Ni, Co, Th, Cr, and Mo elements with a concentration exceeding 100 mg L− 1. These elements can interfere with uranium extraction. Next, the concentration of sulfuric acid was determined using titration.
Considering the precipitation of cations in the leach liquor solution, it is not feasible to directly specify the sulfuric acid concentration using titration with the KOH standard solution. By the addition of ammonium oxalate (masking agent) to excess22, the cations in the leach liquor solution change to complex cations, and the hydroxide cations precipitate only in a highly alkaline medium. Consequently, the leach liquor solution was subjected to dilutions of two, ten, and fifteen times to ascertain the acid concentration. Next, the solution was supplemented with 0.1 mol L− 1 (NH4)2C2O4, and the sulfuric acid concentration was determined to be 0.109 mol L− 1 by titration with a 0.05 mol L− 1 KOH standard solution (Fig. S1). All the titrations were repeated three times, and the effect of the error resulting from the dilution on the results was found to be less than 3%.
Study of the uranyl ion species in sulfate medium
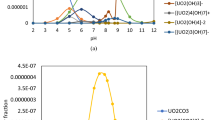
In the leaching processes using sulfuric acid, the uranium (VI) contained in the ore leads to the formation of complexes such as UO2SO4, UO2(SO4)22−, UO2(SO4)34− and UO22+. By taking the total uranium (VI) concentration, and the free sulfate concentration into consideration, the predominant species can be determined from Eqs. (S1) and (S4)23. Carl et al. reported the concentrations of free sulfate and bisulfate in a sulfuric acid solution as a function of the acid concentration24. Based on the presented results and Eqs. (S6) to (S8), the proportion of each species was determined in relation to the acid concentration. The species percentages are shown in (Table 2). These results showed that in 0.109 mol L− 1 H2SO4, UO2SO4 was the predominant species and constituting 74.8% of the whole uranyl species.
Study of the generation of preferable species of Alamine 336
By increasing the sulfuric acid concentration, Alamine 336 can be convert into two other species, namely, (R3NH)2SO4 and R3NHHSO4 (Reactions 4 and 5)25. Reaction (6) indicates that UO2SO4 is removed with (R3NH)2SO4 due to the creation of the ion-associated complex26. Conversely, it is evident that in reaction (7), the extraction percentage of uranium (VI) diminishes in the presence of R3NHHSO4 owing to the generation of acid. Therefore, to maximize uranium (VI) extraction, the H2SO4 concentration must be chosen in such a way that it causes the (R3NH)2SO4 formation in the organic phase.
To determine the optimum sulfuric acid concentration in the feed solution to produce (R3NH)2SO4, aqueous solutions at different sulfuric acid concentrations and 0.05 mol L− 1 Alamine 336 were employed. On a mechanical shaker, the two phases were agitated and then split using a separating funnel. Titration using the KOH solution helped to determine the remaining sulfuric acid content in the aqueous phase. Then, the acid concentration in the organic phase was determined by mass balance. Therefore, 0.15 mol L− 1 H2SO4 was the optimum concentration of sulfuric acid for producing (R3NH)2SO4 (Table S1).
Consequently, the maximum transformation of Alamine 336 to (R3NH)2SO4 occurs in conjunction with 0.15 mol L− 1 H2SO4. Based on the above discussion, it is anticipated that the maximum uranium(VI) extraction from the sulfuric acid medium will be obtained at 0.15 mol L− 1 H2SO4. Otherwise, whether greater or lower, the extraction percentage begins to drop at any acid concentration other than the ideal concentration. At lower concentrations, the decline in the extraction percentage resulted from the preponderance of the form of the free amine (R3N), at higher concentrations, it resulted from the predominance of the form of R3NHHSO4 in the organic phase.
Effect of phase contact time
The extraction of uranium (VI) from sulfuric acid solutions (0.15 and 0.5 mol L−1) by Alamine 336 (0.0125 and 0.05 mol L−1) was studied by changing the contact time from 1 to 120 min. The results are shown in (Fig. 1). The experimental results demonstrated that equilibrium arises after 10 min. However, a phase contact time of 30 min was pursued to ensure the complete extraction equilibrium.
Effect of sulfuric acid concentration
The concentration of sulfuric acid is a very critical parameter in the uranium (VI) solvent extraction process. The impact of the sulfuric acid concentration in the range 0.00025–6.0 mol L−1 on the uranium (VI) extraction percentage was scrutinized by 0.0125, 0.05, and 0.1 mol L−1 Alamine 336. The results are demonstrated in (Fig. 2).
At 0.00025 mol L−1 H2SO4, a significant part of Alamine 336 is a free amine (R3N). Therefore, the extraction percentage of uranium (VI) is less than 20%. Additionally, the extraction percentage experienced a significant increase in the range of 0.00025 to 0.15 mol L− 1 H2SO4, as the concentration of sulfuric acid increased, a specific quantity of R3N is converted to (R3NH)2SO4 (Reaction 4). The occurrence of the maximum uranium(VI) extraction percentage (99.72%) corresponded to the concentration of 0.15 mol L− 1 at which nearly the entire Alamine 336 was transformed into the (R3NH)2SO4. The extraction percentage began to slowly decrease at concentrations higher than 0.15 mol L− 1 the reason for which is the gradual transformation of (R3NH)2SO4 into R3NHHSO4(Reactions 5 and 7). When the concentration of sulfuric acid (H2SO4) increases, the equilibrium reaction (7) shifts to the left and the extraction decreases. Hence, the concentration of 0.15 mol L− 1 was selected as the optimum concentration of sulfuric acid for uranium (VI) extraction.
Furthermore, experimental data for sulfuric acid concentrations above 4 mol L− 1 demonstrated the formation of red sediment particles at the interface of the organic and aqueous phases, seemingly resulting from the degradation of Alamine 336.
Effect of Alamine 336 concentration
The impact of the extractant concentration in the range 0.001–0.1 mol L−1 Alamine 336 was studied on the extraction of uranium (VI) from 0.15, 0.75, and 2.0 mol L−1 H2SO4. The results are depicted in (Fig. 3). As expected, uranium (VI) extraction was enhanced with an increase in the extractant concentration. The increase in uranium (VI) extraction percentage were 11.49–99.91, 21.58–94.25 and 13.71–39.09% for 0.15, 0.75 and 2.0 mol L−1 H2SO4, respectively. Above 0.05 mol L− 1 Alamine 336, uranium (VI) extraction percentage did not practically change very significantly.
Consequently, the concentration of 0.05 mol L− 1 was selected to be the optimum concentration of Alamine 336. The other experiments were conducted at this optimum concentration. The extraction percentage changes about sulfuric acid concentrations of 0.75 and 2 mol L−1 owing to R3NHHSO4 formation. The concentration of sulfuric acid increases, resulting in a uranium(VI) extraction curve with a lower plateau. For example, the curve plateaus associated with 2 and 0.75 mol L− 1 H2SO4 are lower than those of 0.15 mol L− 1 H2SO4. Based on reaction (6) and (Table 2), the relation between the apparent equilibrium constant of uranium(VI) extraction and the distribution ratio at 0.15 mol L− 1 H2SO4 is expressed in the form of Eq. (8) and Eq. (9):
Given Eq. (9), a linear diagram with a slope of 2.01 was obtained by plotting the log D as a function of log (R3NH)2SO4 concentration at the optimum concentration of sulfuric acid (0.15 mol L−1). The slope associated with this diagram proved the suggested mechanism in Eq. (6). Under these conditions, the logarithm of apparent equilibrium constant for the extraction reaction was obtained to be 5.92.
Effect of organic phase saturation on uranium (VI) extraction
Effect of organic phase saturation on uranium (VI) extraction was studied. At first, the impact of uranium (VI) concentration in the range 150–750 mg L−1 on the extraction of uranium (VI) was investigated from 0.15 mol L−1 H2SO4 utilizing 0.0125 and 0.05 mol L−1 Alamine 336. The findings are shown in (Fig. 4). The obtained findings revealed that the uranium (VI) extraction percentage was reduced from 98.72 to 73.24% and remained almost constant for 0.0125 and 0.05 mol L−1 Alamine 336, respectively. The results show that 0.05 mol L−1 Alamine 336 is suitable for uranium extraction from low-concentration solution.
Moreover, the effect of organic/aqueous phase ratio (O/A) in the range of 0.1–1.5 was studied on the extraction of uranium(VI) from 0.15 mol L−1 H2SO4. The results indicated that 85.48 to 99.72% uranium (VI) was extracted by the raise of the O/A ratio from 0.1 to 1. A further increase in the O/A ratio did not cause another rise in the uranium (VI) extraction percentage.
Thermodynamic study of extraction
Using 0.05 mol L−1 Alamine 336, the effect of temperature in 20–60 oC range on the uranium (VI) extraction percentage was examined from 0.15 mol L−1 H2SO4. The findings indicated that the extraction of uranium using Alamine 336 diminishes with rising temperature. According to van’t Hoff’s equation, the logarithmic plot of the distribution ratio vs. the reciprocal of temperature (1/T) yields a linear graph from which enthalpy changes may be derived from the slope of the graph Eq. (10). Furthermore, the Gibbs free energy changes, as well as entropy changes, can be calculated from Eqs. (11) and Eq. (12), respectively, using the logarithm of apparent equilibrium constant about the extraction reaction26,27.
Equation (10) to Eq. (12) yielded the results for the thermodynamic parameters for Alamine 336 at the 0.05 mol L− 1 concentration as follows: ΔH=-11.308 kJ mol−1, ΔG=-33.797 kJ mol−1, ΔS = +75.428 J K−1 mol−1. The results showed that uranium (VI) extraction with Alamine 336 is an exothermic reaction, and the extraction percentage decreases with increasing temperature. Moreover, the negative value of ΔG indicated that the extraction reaction takes place spontaneously28.
Effect of the stages number in the uranium (VI) stripping
Uranium (VI) extraction from the organic phase was accomplished using a variety of stripping agents. To achieve this, the laden organic phase, which was composed of 0.05 mol L−1 Alamine 336 dispersed in petroleum (250 mg L−1 uranium), was exposed to a variety of stripping agents at 25 oC for 30 min. The concentrations of the acidic stripping agents and the neutral and alkaline stripping agents were 4 and 0.5 mol L−1, respectively. The stripping section was carried out by freshly prepared stripping solution at four stages to improve the percentage of the uranium (VI) stripping from the organic phase. The results are shown in (Table 3).
The results showed that for most stripping agents, the major part of uranium (VI) stripping takes place at the first stage. Nonetheless, increasing the number of stages had a positive effect on the stripping caused by HNO3 and H2O. Additionally, the removal percentage increased from the first to the second and the first to the fourth phases. The experiments indicated that 0.5 mol L−1 (NH4)2CO3 exhibited the highest performance among the other stripping agents, resulting in a uranium (VI) extraction percentage of 99.87% during the initial stage. Furthermore, in the process of stripping by 4 mol L−1 sulfuric, nitric, and perchloric acid, red sediment particles were formed on the boundary between two organic and aqueous phases which seemed to be caused as the result of the destruction of Alamine 336.
Effect of temperature on uranium (VI) stripping
Thirteen distinct stripping agents were employed to investigate the impact of temperature on the removal of uranium (VI) from the organic phase. The results of these investigations are summarized in (Table 4). The results indicated that the level of uranium (VI) removal is not significantly affected by an increase in temperature. The enthalpy changes of uranium (VI) stripping reaction were obtained from Eq. (10), which implies that the uranium(VI) stripping reaction was exothermic by Na2CO3 and endothermic by other stripping agents.
Uranium purification (extraction and separation)
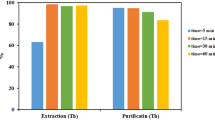
Uranium (VI) extraction from the leach liquor solution was studied using 0.05 mol L− 1 Alamine 336. Table 5 presents the findings. The findings showed that Alamine 336 extracted 97.01% of uranium from the leach solution. Alamine 336 also extracted 15.78% of Fe. There was more than 167 mg L−1 of the Fe in the organic phase. The extraction of other elements was not significant. The major impurities such as Mg, Mn, Al, and Ca were generally extracted at less than 1%. The concentrations of Mg, Mn, Al, and Ca in the organic phase were 28.06, 0, 9.36, and 8.38 mg L−1, respectively. Some interfering elements such as V, P, Zn, and Ni also had low concentrations in the feed solution and were insignificant impurities. Therefore, the main interference in the uranium extraction process with Alamine 336 was caused by iron. To reduce the extraction of iron with Alamine 336, the reduction reaction of Fe(III) to Fe(II) was performed. Based on the reaction (13), ascorbic acid reduces iron (III) to iron (II)29:
For this purpose, 2 g L−1 of ascorbic acid was added to the leach liquor solution as the reduction agent. The percentages of uranium (VI) and iron extractions were equal to 97.01 and 15.78%, respectively, which after the addition of ascorbic acid to the aqueous phase changed to 96.51and 1.18%, respectively. Therefore, the presence of ascorbic acid solved the problem of iron interference to an acceptable extent, and the uranium extraction efficiency changed little. The results show that the presence of a reduction agent has little effect on the extraction of other interferences.
The separation of elements from the loaded organic phase (stripping process) was investigated using 3 agents: 4 mol L−1 HNO3, 0.5 mol L−1 NH4Cl, and 0.5 mol L−1 (NH4)2CO3. The results are presented in (Table 6). To compare the selectivity of the process, the selectivity factor was determined using the following formula30.
where CU and CE are the concentrations of uranium and other elements, respectively. By comparing the concentrations of elements in the leach liquor and stripping solutions, it is observed that the impurities have been significantly reduced. A major part of the purification process occurred in the first stage (extraction), which proved the high selectivity of Alamine 336. In addition, in the stripping stage, relative separation of uranium from other elements occurs.
To indicate uranium purity, the amount of uranium should be reported as a percentage of the total elements. Based on this definition, the purity of uranium in feed solution is less than 3% for the feed solution.While by carrying out the purification process, the purity of uranium in HNO3, NH4Cl, and (NH4)2CO3 solutions has reached 94, 95.7, and 96.7%, respectively.
The promising results of the present study indicate that Alamine 336 has the desirable ability to selectively separate uranium and that uranium can be recovered from it using appropriate agents.
Conclusions
The uranium (VI) solvent extraction from acidic sulfate solutions utilizing Alamine 336 has been investigated. First, using various analyzes, such as XRF, ICP-AES, and titration, the constituent elements of Bandar Abbas Gchin ore leach solution were identified. The UO2SO4 was the predominant form of uranium (VI) in the sulfuric acid medium.
The generation of the preferable form of Alamine 336 in a sulfate medium was studied, and it was concluded that Alamine 336 in interaction with 0.15 mol L−1 H2SO4 was transformed into (R3NH)2SO4.
The effect of several parameters on uranium (VI) solvent extraction were studied. Based on the results, the uranium (VI) extraction reaction took place at a relatively fast rate and the reaction was completed in 10 min. The extraction was enhanced by increasing the concentration of sulfuric acid to 0.15 mol L− 1; however, the R3NHHSO4 formation subsequently resulted in a decrease. The extraction was enhanced when the concentration of Alamine 336 and the organic/aqueous phase ratio were increased, while it was reduced when the concentration of uranium (VI) was increased. Log Kex at 0.15 mol L−1 H2SO4 was determined to be 5.92.
Using 0.05 mol L−1 Alamine 336 at 25 oC with an organic/aqueous phase ratio of 1:1, an extraction percentage of almost 99.72% was obtained when the sulfuric acid concentration was 0.15 mol L− 1. The uranium (VI) extraction was an exothermic and spontaneous reaction.
In 4 steps, uranium (VI) stripping from the loaded solvent was conducted by different chemical agents. Uranium (VI) was stripped up to 99.87% at a single stage by making use of 0.5 mol L− 1 (NH4)2CO3. The effect of temperature (20–60 °C) on uranium (VI) stripping was examined. The results demonstrated that the uranium (VI) stripping process was exothermic with Na2CO3 and endothermic with other examined stripping agents.
The uranium (VI) extraction from the leach liquor solution was performed with and without using ascorbic acid as a reduction agent, and the iron(III) removal was scrutinized. Also, the recovery of the loaded organic phase was investigated with different agents. The results showed that the uranium extraction process with Alamine 336 and organic phase stripping has a high selectivity coefficient.
Data availability
Data availability: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Gutorova, S. et al. Disclosing the mechanism of uranium (VI) solvent extraction by polydentate ligands in a polar solvent: the role of ion pairs. J. Mol. Liq. 415, 126382 (2024).
Chen, T. et al. Advanced photocatalysts for uranium extraction: elaborate design and future perspectives. Coord. Chem. Rev. 467, 214615 (2022).
Yuan, J. et al. Bioleaching of uranium from low-grade uranium ore with a high fluorine content by Indigenous microorganisms and their community structure analysis. J. Radioanal. Nucl. Chem., 1–12 (2023).
Wang, Y., Wang, C., Liu, H. & Meng, Y. Extraction of molybdenum and uranium from low-grade molybdenum bearing ore containing uranium through mechanical activation following acid leaching. Miner. Process. Extr. Metall. Rev. 45, 304–313 (2024).
Ahmed, B., Mohammed, A., Kawady, N., Elaasy, I. & Soliman, E. Costly effective bioleaching of valuable metals from low grade ore using aspergillus nidulans. Int. J. Environ. Sci. Technol. 21, 5469–5482 (2024).
Fominykh, A. Y. & Matveev, P. Solvent extraction of uranium (VI) from acidic and alkaline solutions using ionic liquid-based systems. Mosc. Univ. Chem. Bull. 79, 374–386 (2024).
Abdeshahi, A., Sadeghi, M., Nejad, D. G., Zare, M. H. & Outokesh, M. Recovery of uranium from phosphate ore of Iran mine: part II-solvent extraction of uranium from wet-process phosphoric acid. Nucl. Eng. Des. 421, 113093 (2024).
Lee, H. K., Park, W., Chang, S., Jeon, H. & Park, S. Uranium recovery from sulfate-based acidic soil washing effluent using ion-exchange resins. Water Air Soil Pollut. 233, 453 (2022).
Skripchenko, S. Y., Nalivaiko, K. A., Titova, S. M., Rychkov, V. N. & Semenishchev, V. S. Recovery of uranium from conversion production sludge by leaching with nitric acid and subsequent ion-exchange concentration. Hydrometallurgy 224, 106255 (2024).
Sun, H. et al. A high-flux phytic acid functionalized SiO2 blend polysulfone ultrafiltration membrane for extraction of uranium from seawater. J. Environ. Chem. Eng. 12, 113749 (2024).
Zahakifar, F., Keshtkar, A. R. & Talebi, M. Synthesis of sodium alginate (SA)/polyvinyl alcohol (PVA)/polyethylene oxide (PEO)/ZSM-5 zeolite hybrid nanostructure adsorbent by casting method for uranium (VI) adsorption from aqueous solutions. Prog. Nucl. Energy 134, 103642. https://doi.org/10.1016/j.pnucene.2021.103642 (2021).
Zahakifar, F., Keshtkar, A. R. & Talebi, M. Performance evaluation of sodium alginate/polyvinyl alcohol/polyethylene oxide/ZSM5 zeolite hybrid adsorbent for ion uptake from aqueous solutions: a case study of thorium (IV). J. Radioanal. Nucl. Chem. 327, 65–72. https://doi.org/10.1007/s10967-020-07479-w (2021).
Zahakifar, F., Charkhi, A., Torab-Mostaedi, M. & Davarkhah, R. Kinetic study of uranium transport via a bulk liquid membrane containing Alamine 336 as a carrier. J. Radioanal. Nucl. Chem., 1–9 (2018).
Zahakifar, F., Charkhi, A., Torab-Mostaedi, M. & Davarkhah, R. Performance evaluation of Hollow fiber renewal liquid membrane for extraction of uranium (VI) from acidic sulfate solution. Radiochim. Acta (2017).
Zahakifar, F., Charkhi, A., Torab-Mostaedi, M., Davarkhah, R. & Yadollahi, A. Effect of surfactants on the performance of Hollow fiber renewal liquid membrane (HFRLM): a case study of uranium transfer. J. Radioanal. Nucl. Chem. 318, 973–983 (2018).
Cox, M. Solvent Extraction Principles and Practice, Revised and Expanded 466–515 (CRC, 2004).
Kislik, V. S. Solvent Extraction: Classical and Novel Approaches (Elsevier, 2011).
Yu, C., Guoxin, S., Zhenwei, Z., Yufen, H. & Sixiu, S. Extraction of U (VI) with N, N’-dimethyl-N, N’-dioctylsuccinylamide in toluene. J. Radioanal. Nucl. Chem. 272, 199–201 (2007).
Lapka, J. et al. Extraction of uranium (VI) with Diamides of dipicolinic acid from nitric acid solutions. Radiochim. Acta Int. J. Chem. Aspects Nuclear Sci. Technol. 97, 291–296 (2009).
Khanramaki, F. & Torkaman, R. Experimental study on the uranium (VI) extraction rate and droplet mass transfer coefficients from a sulfate leach liquor medium with Alamine 336 in a single drop column. J. Radioanal. Nucl. Chem. 331, 2349–2357 (2022).
YACOUbA, A. R. C., IbRAHIM, S. L., WAgE, A. R. M. & Natatou, I. Optimization of some parameters on the low efficiency of solvent extraction of uranium with Alamine 336. Orient. J. Chem. 37, 213 (2021).
Perdur, N., Nellikalaya, G. B. & Gopalakrishnayya, C. K. Ammonium oxalate as a masking agent for the complexometric determination of manganese (II). Viet. J. Chem. 53, 151–155 (2015).
Charlot, G. Les réactions chimiques en solution aqueuse: et caractérisation des ions (Masson, 1983).
Brubaker, C. H. Jr Textbook errors: XIII. The nature of ionic and molecular species in sulfuric acid. J. Chem. Educ. 34, 325 (1957).
Ramadevi, G., Sreenivas, T., Navale, A. & Padmanabhan, N. Solvent extraction of uranium from lean grade acidic sulfate leach liquor with Alamine 336 reagent. J. Radioanal. Nucl. Chem. 294, 13–18 (2012).
Torkaman, R., Moosavian, M., Torab-Mostaedi, M. & Safdari, J. Solvent extraction of samarium from aqueous nitrate solution by Cyanex301 and D2EHPA. Hydrometallurgy 137, 101–107 (2013).
Milani, S., Zahakifar, F. & Faryadi, M. Reaction stoichiometry and mechanism of tetravalent cerium liquid-liquid extraction in the Ce (IV)-H2SO4-Cyanex 302-kerosene system. Bul. Chem. Commun. 295 https://doi.org/10.34049/bcc.54.4.5431 (2022).
Ali, M., Taha, M., Killa, H., Wanees, E., El-Maadawy, M. & S. A. & Synergistic extraction of uranium from acidic sulfate leach liquor using D2EHPA mixed with TOPO. J. Radioanal. Nucl. Chem. 300, 963–967 (2014).
Elmagirbi, A., Sulistyarti, H. & Atikah, A. Study of ascorbic acid as iron (III) reducing agent for spectrophotometric iron speciation. J. Pure Appl. Chem. Res. 1, 11–17 (2012).
Shadbad, M. R. A., Zaheri, P., Abolghasemi, H. & Zahakifar, F. The performance evaluation of Alamine336 in solvent extraction and polymer inclusion membrane methods for valuable ions extraction: a case study of Te (IV) separation intensification. Chem. Eng. Process. Process Intensif. 109268 https://doi.org/10.1016/j.cep.2023.109268 (2023).
Author information
Authors and Affiliations
Contributions
F.Z.: Experiments, Investigation, Supervision.F. Kh. : Experiments, Writing—Original draft preparation.D. G.: ExperimentsV. G.: ExperimentsA. Y.: Experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zahakifar, F., Khanramaki, F., Nejad, D.G. et al. The solvent extraction and stripping process using Alamine 336 with a case study of uranium. Sci Rep 15, 11749 (2025). https://doi.org/10.1038/s41598-025-96421-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-96421-9