Abstract
To investigate the predictive value of breast milk Na+ concentration and Na+/K+ ratio on delayed onset of lactogenesis (DOL) in puerpera with gestational hyperglycemia. Puerpera with gestational hyperglycemia who delivered at The First Affiliated Hospital of Army Medical University in China between October 20, 2023, and April 1, 2024, were enrolled. The concentrations of Na+ and K+ in breast milk samples were examined from study participants on days 1–3 of the postpartum period. The predictive value of Na+ concentration and the Na+/K+ ratio on DOL were assessed using receiver operating characteristic (ROC) curves. Both the Na+ concentration and Na+/K+ ratio in breast milk collected on day 3 could effectively predict DOL, with areas under the curve (AUCs) of the ROC curve and 95%CI values of 94.7% (95%CI = 0.901–0.992) and 94.2% (95%CI = 0.890–0.994), respectively. The optimized cutoffs were Na+ concentration ≥ 26.25 mmol/L (sensitivity = 88.2%, specificity = 92.2.8%) and Na+/K+ ratio ≥ 1.646 (sensitivity = 79.4%, specificity = 98.4%). Both the Na⁺ concentration and Na+/K+ ratio in breast milk were found to be effective in predicting DOL in puerpera with gestational hyperglycemia.
Similar content being viewed by others
Introduction
Breastfeeding is an ideal way to provide infants with the nutrients required for healthy growth and development, and is an important determinant of their short-term and long-term health. The 2022 revision of the “Gestational hyperglycemia diagnosis and treatment guidelines” provides an update on the association between pregnancy, diabetes, and gestational hyperglycemia, including pregestational diabetes mellitus (PGDM) and gestational diabetes mellitus (GDM)1. The presence of gestational hyperglycemia both increases the frequency and prolongs the duration of breastfeeding in the postpartum period, which can regulate the blood glucose and lipid levels of puerpera with GDM and the infant, improve insulin sensitivity, facilitate postpartum weight loss, and reduce the incidence of metabolic diseases in the long term2,3. The guidelines recommend that gravida with GDM without contraindications should begin breastfeeding after delivery and continue breastfeeding for at least 6 months postpartum4. However, breastfeeding in puerpera with gestational hyperglycemia presents some difficulties. Haile et al. found that the rate of breastfeeding by women with GDM at discharge was 62.2%5, while another survey on breastfeeding in women with PGDM showed that only 55% of the women successfully initiated breastfeeding6, and the incidence of DOL after childbirth in women with GDM was 35%7. DOL is the main contributor to reduced lactation volume, leading to the interruption of early breastfeeding, shortening of the feeding time, and reduced breast-feeding rates8,9. It has been found that the Na+ concentration and/or the Na+/K+ ratio in breast milk can predict the onset of lactogenesis with high accuracy and can be used for clinical identification and prevention of DOL10. The decline in postpartum progesterone levels reduces the inhibition of prolactin, which, under the synergistic effect of stimulation by infant suckling, triggers the closure of the paracellular pathway in mammary cells and promotes the formation of tight junctions between mammary epithelial cells. This process maintains high concentrations of lactose and K+ within the mammary alveoli, thereby creating a hypertonic environment in the mammary ducts and alveoli, which facilitates the initiation of lactation and increased milk secretion. Concurrently, Na+ and Cl− are actively transported out of the mammary alveoli, reducing the Na+ concentration11,12. The continuous changes in the Na⁺ concentration and the Na+/K+ ratio reflect the state of the paracellular pathway in mammary cells and serve as effective indicators for predicting delayed onset of lactation. Currently, in China, there have been few clinical studies on Na+ concentrations and Na+/K+ ratios in breast milk for predicting DOL in puerpera with gestational hyperglycemia. Furthermore, no previous studies have continuously monitored dynamic changes in breast milk Na⁺ concentration or the Na+/K+ ratio13. The present study was designed to analyze changes in the Na⁺ concentration and Na+/K+ ratio in the breast milk of postpartum puerpera with gestational hyperglycemia, to investigate the predictive value of these parameters on DOL, and thus provide a basis for the clinical identification of the risk of DOL and determination of the timing of early intervention.
Subjects and methods
Subjects
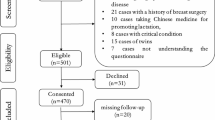
Puerpera with gestational hyperglycemia who delivered at The First Affiliated Hospital of the Army Medical University in China between October 20, 2023, and April 1, 2024, were enrolled. Inclusion criteria: Puerpera that met the diagnosis of gestational hyperglycemia1, encompassing both pregestational diabetes mellitus (PGDM) and gestational diabetes mellitus (GDM); Age ≥ 18 years old; Normal breast and nipple development, and willingness to undergo breastfeeding; No use of hormonal contraceptives or lactation supplements for at least three days after delivery; Basic literacy and normal cognition; Live birth; Voluntarily participation in the study and the provision of written informed consent. Exclusion criteria: Other pregnancy complications or comorbidities, such as thyroid dysfunction, hypertensive disorders of pregnancy, polycystic ovary syndrome, intrahepatic cholestasis of pregnancy, and postpartum hemorrhage; Labor abnormalities, such as amniotic fluid embolism, postpartum shock, and postpartum disseminated intravascular coagulation; Use of insulin therapy during the prenatal or postpartum period. Contraindications to breastfeeding; Puerperal abnormalities, such as puerperal infections and puerperal psychiatric abnormalities; Abandonment of breastfeeding due to various reasons; Neonatal mortality; Dropout from study or incomplete data. The included participants were divided into DOL and non-DOL groups according to the occurrence of postpartum DOL.
Ethics and informed consent
This prospective observational study was approved by the Research and Clinical Trial Ethics Committee of our hospital. A single-center study design was employed to ensure consistency in the research environment, homogeneity of the study population, and the protection of privacy. The ethical approval number was ([A] KY2023148), and all subjects have signed informed consent forms.
Methods
Sample size calculation
This study investigated the predictive value of Na+ concentration and the Na⁺/K⁺ ratio in breast milk on DOL of puerpera with gestational hyperglycemia. The optimal sample size was calculated according to the area under the ROC curve (AUC) for predicting delayed onset of lactogenesis (DOL) with reference to puerpera with GDM14. The DOL rate of puerpera with GDM was 25.2%, and the AUC was 0.757. A two-sided test was conducted using alpha = 0.05 and a power of 80% (β = 0.2). The sample size was calculated to be N = 67 by pass 15. Considering unpredictable postpartum factors and a 20% dropout rate, the sample size was determined to be 98 cases.
Clinical data collection
General information collection
General information on puerpera with gestational hyperglycemia was collected prospectively, recording age, job, education, pre-pregnancy body mass index (BMI), blood glucose level, and obstetric information, including the week of gestation, gestation and parity, mode of delivery, postnatal follow-up, time of lactogenesis onset.
Collection of breast milk samples and measurement of Na+ and K+ concentrations
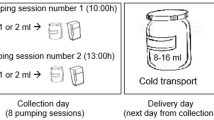
Breast milk sampling: Samples (1–2 mL/time) were collected from puerpera with gestational hyperglycemia on postpartum days 1–3 at 10:00 each day. The milk was extracted manually or with a breast pump, and then injected into cryocontainer with a sterile syringe. The study ID and time and date of collection were recorded. Before sample collection, standardized lactation management was conducted by trained nurses, including guidance on proper breastfeeding positions, latch techniques, and the use of breast pumps. For rooming-in mothers and infants, skin-to-skin contact was initiated within one hour of birth, with breastfeeding performed 8–12 times per 24 h, each lasting 15 min. For mothers and infants who were separated, breast pumping using medical-grade breast pumps was initiated six hours postpartum, performed 8–12 times per 24 h, each lasting 15 min.
Storage of breast milk samples: The samples were stored in the breast milk bank at -20℃ in a medical refrigerator. After collection, the samples were placed in a cold-chain box and sent to the laboratory of the Department of Infectious Diseases of the hospital for testing.
Measurement of Na⁺ and K⁺ concentrations: The indirect ion-selective electrode method was used to measure the concentrations of Na+ and K+ in the breast milk samples. Before testing, frozen samples were thawed rapidly in a 45 °C water bath with shaking, and centrifuged for 4 min at 14 000 rpm and 4 °C. The clarified liquid was aspirated, avoiding the fat, and re-centrifuged under the same conditions. Finally, 300 µL of the milk was placed in an ion-selective electrode sensor (AU5800, Beckman Coulter, Brea, CA, USA) and the internal standard electrolyte solution, a specific reagent for the AU biochemical analysis system, was added. The results were recorded after stabilization. The accuracy of the ion-selective electrode method was confirmed by the results of a controlled experiment investigating changes in the concentrations of Na+ and K+ in breast milk relative to the activation of breast secretion and inflammation15.
Definitions and criteria used in determinations
Delayed onset of lactogenesis (DOL)
This was defined as the onset of lactogenesis after 72 h following delivery, or the first time the breasts feel significantly full and engorged compared with the pre-delivery period8,9.
Time of lactogenesis onset
This was calculated as the interval between the first experience of significant breast fullness and swelling and the delivery time. After delivery, the nurse-in-charge assessed feeding and breast milk secretion every 4 h (including nighttime). The time when breast fullness was first experienced was recorded, and the time of lactogenesis onset was calculated. For puerpera discharged before the onset of lactogenesis, the researcher contacted the puerpera by phone each day to determine the time of lactogenesis onset.
Physical measurement
Pre-pregnancy body mass index (BMI) = pre-pregnancy weight (kg) / [height (m)2]. The “Blue Book of Obesity Prevention and Control in China” defines low pre-pregnancy weight as a BMI < 18.5 kg/m2, normal as a BMI of 18.5–23.9 kg/m2, overweight as 24.0–27.9 kg/m2, and obese as BMI ≥ 28.0 kg/m216. In the present study, BMI ≥ 24.0 kg/m2 was considered overweight.
Glycemic control level
The postnatal glycemic control target for GDM or PGDM gravida was fasting plasma glucose (FPG) < 6.1 mmol/L, and 2-h postprandial blood glucose < 7.8 mmol/L17. In the present study, the level of glycemic control meeting this target range was defined as good glycemic control.
Statistical analysis
The data were entered into MS Excel and were organized and analyzed using SPSS 26.0. Measurement data are expressed as mean ± SD and categorical data are expressed as number of cases (%). Independent samples t-tests were used for comparing two groups, while chi-square tests were used for comparing categorical data between two groups. Repeated measures ANOVA was used for the analysis of repeated measurement data, and pairwise comparisons were conducted using simple effects analysis, with Bonferroni corrections, and a significance level α set at 0.016. Associations between variables were assessed using Pearson correlation coefficients, binary logistic regression analysis (Wald forward method) was used for the analysis of influencing factors, receiver operating characteristic (ROC) curves were plotted for the correlation coefficients, and the areas under the curves (AUCs) were compared, with cutoffs calculated using the Youden index, and the test level α was 0.05. P < 0.05 was considered statistically significant.
Results
General information on the study participants
A total of 98 puerpera with gestational hyperglycemia (34 in the DOL group and 64 in the non-DOL group) were enrolled. A total of 294 breast milk specimens were collected. Thirty-four (34.69%) puerpera with gestational hyperglycemia developed postpartum DOL. The percentages of preterm labor and mother-infant separation in the DOL group were higher than those in the non-DOL group, and the proportion of patients with good glycemic control in the DOL group was significantly lower than that in the non-DOL group (P < 0.05). There was no statistically significant difference in terms of age, gestational BMI, educational level, work status, parity, and mode of delivery between the two groups (see Table 1).
Changes Na+ concentrations in the breast milk of puerpera with gestational hyperglycemia
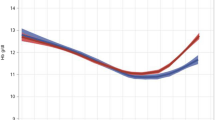
The Na+ concentrations in the breast milk collected on days 1–3 of the postpartum period were observed to be reduced in both the DOL and the non-DOL groups, with those collected on day 3 being 37.00 ± 14.10 and 20.42 ± 3.78 in the two groups, respectively. The decrease in Na+ concentration in the non-DOL group was significantly greater than that in the DOL group. Na+ concentrations in the non-DOL group collected on both days 2 and 3 were significantly lower than those in the DOL group (see Fig. 1; Tables 2 and 3). The Na⁺ concentrations in the breast milk of puerpera with gestational hyperglycemia collected on days 2 and 3 were positively correlated with the onset time of lactogenesis (see Table 4).
Changes in the Na+/K+ ratio in the breast milk of puerpera with gestational hyperglycemia
The Na+/K+ ratio in the breast milk decreased in both the DOL and non-DOL groups on days 1‒3 of the postpartum period. The Na+/K+ ratios in the DOL and non-DOL groups collected on day 3 were 1.14 ± 0.24 and 2.61 ± 1.59, respectively, indicating that the decrease in the Na⁺/K⁺ ratio in the non-DOL group was higher than that in the DOL group. The Na+/K+ ratios in the non-DOL group collected on both Days 2 and 3 were significantly lower than those in the DOL group (see Fig. 2; Table 5), while the Na+/K+ ratios collected on both days 2 and 3 of the postpartum period were positively correlated with the time of lactogenesis onset (Table 6).
Value of breast milk Na+ concentrations and the Na+/K+ ratios in predicting DOL in puerpera with gestational hyperglycemia
The ROC curves indicated that the AUC for the prediction of DOL by the Na+ concentration in breast milk collected on day 3 of the postpartum period was 0.947 (95%CI: 0.901–0.992) with an optimized cutoff of 26.250, above which there was an increased risk of DOL, with a sensitivity of 0.882, and a specificity of 0.922. The Na+/K+ ratio in breast milk collected on Day 3 of the postpartum period could also accurately predict DOL in puerpera with gestational hyperglycemia, with an AUC of 0.947 (95%CI: 0.890–0.994), and an optimized cutoff of 1.646, above which there was an increased risk of DOL, with a sensitivity of 0.794 and a specificity of 0.984. There was no statistically significant difference in the predictive ability between Na+ concentration and Na+/K+ ratio (p > 0.05) (see Table 7; Fig. 3).
Discussion
Puerpera with gestational hyperglycemia are at high risk for DOL
The results of the present study showed that the incidence of DOL among puerpera with gestational hyperglycemia was 34.69%, which was similar to the incidence of DOL observed among puerpera with GDM reported by Wu, J L et al.7. Consistent with the findings of previous studies18,19, this study confirmed that puerpera with gestational hyperglycemia represent a high-risk population for the development of DOL. The underlying reasons may be associated with a variety of pathophysiological mechanisms, such as abnormal hormonal regulation, impaired mammary gland development and function, and disordered glucose and lipid metabolism, resulting from the maternal hyperglycemic environment in women with gestational hyperglycemia. These factors may contribute to impaired mammary lactation function, thereby increasing the risk of DOL20,21. The results of the present study showed that preterm labor, mother-infant separation, and the level of glycemic control were the factors influencing DOL in puerpera with gestational hyperglycemia.
Regarding preterm labor: Luo et al. developed a DOL risk prediction model for puerpera with GDM and found that preterm labor was a risk factor for DOL in these patients14, consistent with the findings of this study. As breast development in preterm parturients is not yet mature, the biological activity of prolactin is relatively low, resulting in significantly reduced milk production and capacity for secretion. This physiological state results in the opening of epithelial cell connections in the breast and an increase in intercellular gaps, leading to increased Na⁺ leakage and elevating the concentration of Na⁺ in breast milk, resulting in an abnormal Na⁺/K⁺ ratio. The elevation of the Na⁺/K⁺ ratio may further inhibit the initiation of lactation by disrupting the electrolyte balance and osmotic pressure homeostasis in mammary cells, resulting in DOL. Lian et al. showed that mothers with severe postpartum depressive symptoms tended to have DOL. Mothers with anxiety tend to present with lower prolactin and higher cortisol concentrations, which may affect the lactation reflex, leading to DOL22. Puerpera experiences the physical and emotional separation of the mother and infant after preterm labor, and worry about the infant’s condition increase the anxiety level of the mother, increasing the risk of DOL. In addition, the separation of the mother and infant results in missing the most important period in the postnatal period for establishing the breast-feeding connection, and lack of early skin contact and suckling by the newborn lead to delayed onset of breastfeeding, increasing the risk of DOL.
The results of the present study indicated that the percentage of good glycemic control in the DOL group was lower than that in the non-DOL group, suggesting that poor glycemic control aggravated disordered glucose-lipid metabolism. This would affect both lactose synthesis and milk secretion, increasing the risk of DOL. Poor glycemic control may disrupt proteins in the tight junctions, enhancing the passive diffusion of Na+ and elevating the Na+/K+ ratio in breast milk, thereby increasing the risk of DOL. Matias et al. found that the severity of both GDM and blood glucose levels were associated with a risk of DOL and that insulin treatment was an independent risk factor for DOL in women with GDM23. Due to the small sample size in the present study, parturients undergoing insulin therapy were excluded. In the future, a larger sample will be used to perform a detailed investigation into the effects of insulin therapy, along with varying dosage regimens, on breast milk Na+ concentrations and DOL.
Correlation between Na⁺ concentration and the Na+/K+ ratio in the breast milk of puerpera with gestational hyperglycemia and DOL
The results of this study showed that both the Na+ concentration and Na+/K+ ratio in the breast milk of the two groups decreased on days 1–3 of the postpartum period, which is consistent with previous studies15, The Na+ concentration in breast milk decreased rapidly (from 60 mmol/L on Day 1 to 10 mmol/L on day 5) during the first week. The Na+/K+ ratio was also reduced as the Na+ concentration decreased, implying closure of the tight junctions between breast epithelial cells. High concentrations of lactose and K + are maintained within the alveoli of the mammary glands to sustain a hyperosmolar state within the mammary ducts and alveoli, thereby facilitating milk secretion and the onset of lactogenesis. Na+ and Cl− are transported out of the alveoli as part of an exchange process. During this period, Na+ in breast milk is only actively transported across the cell membrane, resulting in a state of high K⁺ and low Na⁺ within the alveoli12,24. The results of this study showed that the decreases in Na⁺ concentration and Na+/K+ ratio in breast milk in the non-DOL group were more significant than those in the DOL group, and the Na⁺ concentration and Na+/K+ ratio in the non-DOL group collected on day 3 were 20.42 ± 3.78 and 1.14 ± 0.24, respectively, which were significantly lower than those in the DOL group (37.00 ± 14.10 and 2.61 ± 1.59, respectively. The Na+ concentration and Na+/K+ ratio in breast milk thus influence DOL, and relevant studies have also suggested that an increased sodium concentration in breast milk on the seventh day after delivery is a risk factor for interruption of breastfeeding13. The decreases in Na⁺ concentration and the Na+/K+ ratio in breast milk are thus effective indicators for predicting the onset of lactogenesis, whereas persistently high levels of sodium often indicate impaired lactation and lactation failure, which is consistent with the results of Humenick et al.25.Therefore, changes in the Na+ concentration and Na+/K+ ratio in breast milk can be used as early warning signs of DOL, especially in puerpera with gestational hyperglycemia who are at high risk for DOL. In this study, changes in the Na⁺ concentration and Na⁺/K⁺ ratio in the breast milk of postpartum puerpera with gestational hyperglycemia were monitored, finding that in milk collected on days 2 and 3. both parameters were positively correlated with the onset of lactogenesis, with reductions in both the Na⁺ concentration and Na⁺/K⁺ ratio associated with earlier lactogenesis onset. This result provides a quantifiable biomarker for clinical practice to assess the progression of lactation initiation in parturients with gestational hyperglycemia. A significant decrease in the Na+ concentration and Na+/K+ ratio in breast milk can indicate the impending onset of lactation and an increase in milk volume, thereby enhancing the confidence of parturients in breastfeeding. Conversely, timely intervention plans for initiating lactation should be implemented to facilitate the process, which may involve extending the hospital stay or providing lactation support after discharge.
Both Na+ concentration and the Na+/K+ ratio in breast milk are effective in predicting DOL in puerpera with gestational hyperglycemia
The reference ranges of the Na⁺ concentration and Na⁺/K⁺ ratio in breast milk reported in the literature tend to differ26,27. It has been suggested that 23 mmol/L can be used as the high limit of “normal value” for Na+ concentration in breast milk collected on days 1–5 of the postpartum period, while 16 mmol/L and 20 mmol/L can be used as the “optimal value” and “near-optimal value”26. It has also been suggested that Na⁺ concentrations > 18 mmol/L or Na+/ K+ ratios > 0.6 indicate a higher risk of breastfeeding failure27. Meanwhile, it has been found15 that the Na⁺/K⁺ ratio is a better indicator for monitoring, as it has the advantage of having less assay variability and reduces variations in the ratio of water to fat in breast milk due to different detection methods. It has been concluded that a Na+/K+ ratio > 0.8 on day 10 of the postpartum period can predict early lactation failure28. The results of the present study showed that the optimized cutoff of Na+ concentration in the breast milk of puerpera with gestational hyperglycemia collected on day 3 of the postpartum period was 26.250, above which the risk of DOL was increased. Additionally, it was found that the Na+/K+ ratio was also capable of predicting DOL in this population. The Na+/K+ ratio and Na+ concentration in milk collected on days 1–3 reduced together, and the Na+/K+ ratio could also accurately predict DOL, with an optimized cutoff of 1.646, above which the risk of DOL was high. The cutoff values found for Na+ concentration and the Na+/K+ ratio in breast milk in this study were higher than those of previous studies. The reasons for this may be related to the study subjects and the time of milk collection. The samples were collected during days 1–3 of the postpartum period as the critical window of breast activation during this time was essential for the establishment of lactation and the maintenance of an adequate amount of breast milk. The activation of lactogenesis usually occurs on days 2–3 of the postpartum period, when the onset of lactogenesis occurs, the mammary epithelial cell paracellular pathway closes29, and the Na+ concentration in the breast milk starts to decrease. Therefore, Na+ concentration in breast milk collected on d1-3 of the postpartum period is still at a high level. Additionally, monitoring changes in the Na⁺ concentration and Na+/K+ ratio can help to assess early lactation problems, and can be used to adjust the interventional programs in clinical practice. Certainly, continued testing of breast milk Na+ concentrations and Na+/K+ ratios on days 4 or 5 postpartum is also clinically important, as it provides feedback on the effectiveness of the interventional measures. Analyzing the relationship between changes in breastmilk composition and delayed initiation of lactation at different periods postpartum warrants further study. In this study, the AUC of the Na⁺ concentration in the breast milk of puerpera with gestational hyperglycemia was 0.947, with a sensitivity of 0.882 and specificity of 0.922. The AUC of the Na+/K+ ratio was 0.947, with a sensitivity of 0.794 and specificity of 0.984. Both indicators had good efficacy in predicting DOL in puerpera with gestational hyperglycemia, and there was no significant difference between them. Based on the results of this study, it was found that a Na⁺ concentration ≥ 26.25 mmol/L and a Na+/K+ ratio ≥ 1.646 in breast milk on day 3 of the postpartum period indicated a high risk of DOL in puerpera with gestational hyperglycemia. These findings can be applied in clinical nursing practice for the accurate identification of the risk of DOL, thereby enabling the timely implementation of interventional measures. Assessment of problems associated with breastfeeding would enable the use of personalized nursing interventions for inducing lactation, assisting women with hyperglycemia in the successful establishment of breastfeeding.
Conclusions
The findings showed that the optimal cutoff values for the Na+ concentration and Na+/K+ ratio in breast milk on the third postpartum day among puerpera with gestational hyperglycemia are significant predictors of DOL. This finding has important applications in identifying the risk of DOL and determining the timing of early intervention in this patient population. The optimal cutoff values obtained in this study can be used in clinical nursing practice to stratify the risk of DOL in puerpera with gestational hyperglycemia, thereby facilitating the formulation of a stepped and precise lactation support program. During the implementation of this program, the interventional strategies should be adjusted in a timely manner according to dynamic fluctuations in the Na+ concentration and Na+/K+ ratio in the milk, thus reducing the likelihood of DOL, and improving breastfeeding outcomes for high-risk puerpera.
There are a few limitations to our analysis. First, this was an observational study, and the breastfeeding behavior and anxiety of puerpera with gestational hyperglycemia may have affected the Na+ concentration and Na+/K+ ratio. Second, the study was a single-center prospective study with a small sample size, Since breast milk is preferentially used for breastfeeding in the early postpartum period, the number of breast milk samples available for analysis of the Na+ concentration and Na+/K+ ratio was reduced. In subsequent studies, a multicenter design could be adopted to expand the sample size and include participants of different ethnicities to increase diversity, allowing for further research and validation. To improve the feasibility of measuring the characteristics of breast milk in a clinical setting, instruments that can perform real-time analysis at the bedside, such as portable ion-selective probes30 and portable blood gas analyzers with adjustable parameters, would be useful.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Chinese Society Of Obstetrics And Gynecology. Chinese society of perinatal medicine, C. M. A. & commitee of pregnancy with diabetes mellitus, C. M. A. C. Guideline of diagnosis and treatment of hyperglycemia in pregnancy(2022) [Part one]. Chin. J. Obstet. Gynecol. 3–12. https://doi.org/10.3760/cma.j.cn112141-20210917-00528 (2022).
Kaul, P. et al. Association between maternal diabetes, being large for gestational age and breast-feeding on being overweight or obese in childhood. Diabetologia 62, 249–258. https://doi.org/10.1007/s00125-018-4758-0 (2019).
Corrado, F. et al. Metabolic effects of breastfeeding in women with previous gestational diabetes diagnosed according to the IADPSG criteria. J. Matern. Fetal Neonatal. Med. 32, 225–228. https://doi.org/10.1080/14767058.2017.1377175 (2019).
Association, A. D. 14. Management of diabetes in pregnancy: Standards of medical care in diabetes-2020. Diabetes Care 43, S183-s192. https://doi.org/10.2337/dc20-S014 (2020).
Haile, Z. T., Oza-Frank, R., Chertok, A., Passen, N. & I. R. & Association between history of gestational diabetes and exclusive breastfeeding at hospital discharge. J. Hum. Lact. 32, Np36–43. https://doi.org/10.1177/0890334415618936 (2016).
Cordero, L., Thung, S., Landon, M. B. & Nankervis, C. A. Breast-feeding initiation in women with pregestational diabetes mellitus. Clin. Pediatr. (Phila) 53, 18–25. https://doi.org/10.1177/0009922813496455 (2014).
Wu, J. L. et al. Gestational diabetes mellitus and risk of delayed onset of lactogenesis: A systematic review and meta-analysis. Breastfeed Med. 16, 385–392. https://doi.org/10.1089/bfm.2020.0356 (2021).
Huang, L. et al. Delayed lactogenesis is associated with suboptimal breastfeeding practices: A prospective cohort study. J. Nutr. 150, 894–900. https://doi.org/10.1093/jn/nxz311 (2020).
Brownell, E., Howard, C. R., Lawrence, R. A. & Dozier, A. M. Delayed onset lactogenesis II predicts the cessation of any or exclusive breastfeeding. J. Pediatr. 161, 608–614. https://doi.org/10.1016/j.jpeds.2012.03.035 (2012).
Geddes, D. T. et al. 25 Years of research in human lactation: from discovery to translation. Nutrients 13 https://doi.org/10.3390/nu13093071 (2021).
Neville, M. C. & Morton, J. Physiology and endocrine changes underlying human lactogenesis II. J. Nutr. 131, 3005s-3008s. https://doi.org/10.1093/jn/131.11.3005S (2001).
McManaman, J. L. & Neville, M. C. Mammary physiology and milk secretion. Adv. Drug Deliv. Rev. 55, 629–641. https://doi.org/10.1016/s0169-409x(03)00033-4 (2003).
Murase, M., Wagner, E. A., Dewey, C. J. C., Nommsen-Rivers, L. A. & K. G. & The relation between breast milk sodium to potassium ratio and maternal report of a milk supply concern. J. Pediatr. 181, 294–297e293. https://doi.org/10.1016/j.jpeds.2016.10.044 (2017).
Luo, F. J., Bao, N. Z. & Li, S. H. Establishment and validation of a predictive model for the risk of delayed lactation initiation in pregnant women with gestational diabetes mellitus. J. Clin. Pathol. Res. 40, 1394–1400. https://doi.org/10.3978/j.issn.2095-6959.2020.06.009 (2020).
Lai, C. T., Gardner, H. & Geddes, D. Comparison of inductively coupled plasma optical emission spectrometry with an ion selective electrode to determine sodium and potassium levels in human milk. Nutrients 10 https://doi.org/10.3390/nu10091218 (2018).
Wang, Y. F. et al. Understanding the China blue paper on obesity prevention and control and policy implications and recommendations for obesity prevention and control in China. Chin. J. Prev. Med. 875–884. https://doi.org/10.3760/cma.j.issn.0253-9624.2019.09.003 (2019).
Berger, H., Gagnon, R., Sermer, M. & Guideline 393-diabetes in pregnancy. J. Obstet. Gynaecol. Can. 41, 1814–1825e1811. https://doi.org/10.1016/j.jogc.2019.03.008 (2019).
Riddle, S. W. & Nommsen-Rivers, L. A. A case control study of diabetes during pregnancy and low milk supply. Breastfeed Med. 11, 80–85. https://doi.org/10.1089/bfm.2015.0120 (2016).
Suwaydi, M. A. et al. Delayed secretory activation and low milk production in women with gestational diabetes: a case series. BMC Pregnancy Childbirth 22, 350. https://doi.org/10.1186/s12884-022-04685-0 (2022).
Nommsen-Rivers, L. A., Dolan, L. M. & Huang, B. Timing of stage II lactogenesis is predicted by antenatal metabolic health in a cohort of primiparas. Breastfeed Med. 7, 43–49. https://doi.org/10.1089/bfm.2011.0007 (2012).
Zhang, Z. et al. Prolactin and maternal metabolism in women with a recent GDM pregnancy and links to future T2D: the SWIFT study. J. Clin. Endocrinol. Metab. 107, 2652–2665. https://doi.org/10.1210/clinem/dgac346 (2022).
Lian, W., Ding, J., Xiong, T., Liuding, J. & Nie, L. Determinants of delayed onset of lactogenesis II among women who delivered via Cesarean section at a tertiary hospital in China: a prospective cohort study. Int. Breastfeed J. 17, 81. https://doi.org/10.1186/s13006-022-00523-3 (2022).
Matias, S. L., Dewey, K. G., Quesenberry, C. P. Jr. & Gunderson, E. P. Maternal prepregnancy obesity and insulin treatment during pregnancy are independently associated with delayed lactogenesis in women with recent gestational diabetes mellitus. Am. J. Clin. Nutr. 99, 115–121. https://doi.org/10.3945/ajcn.113.073049 (2014).
Neville, M. C., Morton, J., Umemura, S. & Lactogenesis. The transition from pregnancy to lactation. Pediatr. Clin. North. Am. 48, 35–52. https://doi.org/10.1016/s0031-3955(05)70284-4 (2001).
Humenick, S. S., Hill, P. D., Thompson, J. & Hart, A. M. Breast-milk sodium as a predictor of breastfeeding patterns. Can. J. Nurs. Res. 30, 67–81 (1998).
Hoban, R. et al. Mother’s own milk biomarkers predict coming to volume in pump-dependent mothers of preterm infants. J. Pediatr. 228, 44–52e43. https://doi.org/10.1016/j.jpeds.2020.09.010 (2021).
Lemay, D. G. et al. RNA sequencing of the human milk fat layer transcriptome reveals distinct gene expression profiles at three stages of lactation. PLoS ONE 8, e67531. https://doi.org/10.1371/journal.pone.0067531 (2013).
Esquerra-Zwiers, A. L. et al. Associations of secretory activation breast milk biomarkers with breastfeeding outcome measures. J. Pediatr. 253, 259–265e252. https://doi.org/10.1016/j.jpeds.2022.09.055 (2023).
Boss, M., Gardner, H. & Hartmann, P. Normal human lactation: closing the gap. F1000Res 7 https://doi.org/10.12688/f1000research.14452.1 (2018).
Esquerra-Zwiers, A., Vroom, A., Geddes, D. & Lai, C. T. Use of a portable point-of-care instrumentation to measure human milk sodium and potassium concentrations. Breastfeed Med. 17, 46–51. https://doi.org/10.1089/bfm.2021.0046 (2022).
Funding
This research was funded by the Chongqing Health Appropriate Technology Promotion Project (China) in 2023, grant number 2022jstg050.
Author information
Authors and Affiliations
Contributions
G.Y.and R.W. conducted most of the experiments. R.W.and K.L. wrote the paper. H.L, X.C, S.L, R.W. and D.W. conceived and supervised all the work. DW coordinated the project and secured funding.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki, and was approved by the Ethics Committee of The First Affiliated Hospital of the Army Medical University ([A] KY2023148) on October 18, 2023.
Consent for publication
All subjects provided informed consent to participate in the study, and written informed consent was obtained from the patients for publication of the paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, R., Liu, K., Chen, X. et al. Breast milk Na+ and Na+/K+ ratio predict delayed onset of lactogenesis in gestational hyperglycemia. Sci Rep 15, 12158 (2025). https://doi.org/10.1038/s41598-025-96519-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-96519-0