Abstract
Sleep quality is crucial for recovery in coronary care patients, yet sleep disturbances are prevalent in intensive care settings. This study aimed to validate the Persian version of the Sleep Quality Scale for Coronary Care Patients (SQ-CC-P) in the Iranian population and ensure its psychometric robustness for clinical and research applications. A methodological study was conducted in the Coronary Care Unit (CCU) and heart unit of Imam Khomeini Hospital in Ardabil Province, Iran, from May to September 2024, involving 220 participants diagnosed with acute coronary conditions. The SQ-CC-P, which includes self-assessment and environmental factors, was assessed for content validity, construct validity, and reliability in accordance with COSMIN guidelines. The SQ-CC-P demonstrated strong content validity, with CVI (0.80–0.92), CVR (0.70–1.00), S-CVI/UA (0.85), S-CVI/Ave (0.91), and Adjusted Kappa (0.697) confirming item relevance and necessity. Construct validity was supported by exploratory factor analysis (EFA), which revealed a two-factor structure explaining 62.4% of the variance, and confirmatory factor analysis (CFA), which showed strong factor loadings (0.72–0.82) and excellent model fit. The scale exhibited high internal consistency (Cronbach’s alpha = 0.862, McDonald’s omega = 0.864) and test–retest reliability (ICC = 0.785, 95% CI [0.718, 0.841]). The SQ-CC-P is a valid and reliable tool for assessing sleep quality among coronary care patients, addressing a critical gap in sleep assessment in intensive care settings.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) stands as the leading cause of death globally, with a prevalence of 48.6% among individuals over 20 years of age as of March 2020, totaling 127.9 million cases. The estimated CVD mortality was 19.05 million deaths worldwide in 2020, reflecting a 20% increase since 20101. In 2017, over 17 million people succumbed to cardiovascular disease, nearly double the death rate from cancer, marking a 50% rise compared to 1990 and accounting for 32% of global deaths2. In Iran, the cause of death due to cardiovascular diseases is 6.4 out of every 10,000 population3. Coronary artery disease (CAD) stands as the most prevalent cardiovascular conditio 4. By 2020, an estimated 20.5 million Americans over 20 will have CAD, with a 7.1% prevalence rate among US adult 5.
Due to the potential for severe consequences, CAD patients require hospitalization in specialized care units and continuous monitoring using electrocardiography, blood pressure, heart rate, and blood oxygen saturation6,7. While the intensive care unit offers numerous advantages and careful monitoring, it also presents drawbacks, such as noise and bright lights that can disrupt patient sleep8. Sleep disorders are common in patients hospitalized in the intensive care unit9. These disorders can be attributed to various environmental factors, such as exposure to light and sound and the performance of diagnostic and therapeutic processes10,11. Additionally, the use of mechanical ventilation and the administration of medications during nighttime hours can further contribute to the prevalence of sleep disorders in critically ill patients, which has been estimated to be more than 50% (8)12,13.
Sleep, a state of reduced alertness and activity, is a vital function that is effective in many things and associated with numerous health consequence14. Stressing the importance of adequate sleep for mental and physical health can make the audience feel the significance of their work15. It is related to better cognitive performance, including memory, attention, and decision-making. It can improve the quality of life, perform better executive tasks, regulate better emotions and emotions, and reduce the risk of weight gain6,16. Sleep is essential for efficient energy use, regulation of metabolism and body temperature, immune response, elimination of toxins, and supporting brain development, memory, and learning processes17,18.
Inadequate sleep is associated with adverse health outcomes. Experiencing sleep problems in critical care patients may lead to delirium, irritability, lethargy, confusion, neurocognitive disorders, depression, anxiety, stress, poor impulse control, impaired immune response, Decreased resistance to infections, delay in wound healing, nitrogen imbalance, cardio-respiratory and nervous, long-term hospitalization6,7. Low sleep quality and disturbance can limit sustained attention, while increased sleep latency and daytime sleepiness are associated with severe forgetfulness. On the other hand, daytime sleepiness and efficiency reduce interest in doing work19.
These conditions hurt the patient and may lead to a deterioration of their quality of life in the long run. On the other hand, because the lights may be turned on 24 h a day in intensive care units, these environments are potentially noisy, and coronary care patients are usually alert and awake6,20. Research shows that sleep disorders significantly increase the risk of cardiovascular disease. Irregular sleep patterns can more than double this risk21. Quality sleep is crucial for cardiovascular health, as poor sleep is linked to higher mortality and various health issues, highlighting the need for careful sleep assessment in ICU patients.
Existing sleep quality questionnaires, such as the Pittsburgh Sleep Quality Index (PSQI)22 and the Richards-Campbell Sleep Questionnaire (RCSQ)23, have been widely used in clinical settings. However, these tools often need more specificity to address patients’ unique challenges in intensive care units, particularly in coronary care. For instance, while the PSQI assesses general sleep quality over a month, it may not capture the immediate and dynamic factors affecting sleep in critical care environments. Similarly, the RCSQ primarily evaluates general sleep quality without adequately considering environmental stressors like noise and light, which are common in intensive care settings.
The Sleep Quality Scale for Coronary Care Patients (SQ-CC) was developed to assess sleep quality among critically ill patients in coronary care units. The scale consists of two key dimensions: self-assessment and environmental factors. The self-assessment dimension includes items that allow patients to reflect on their personal sleep experiences, such as sleep duration, disturbances, and overall satisfaction with sleep. This dimension provides a subjective evaluation of sleep quality from the patient’s perspective. The environmental factors dimension evaluates the impact of the clinical setting on sleep quality, focusing on elements such as noise levels, lighting, and the presence of medical interventions. Together, these dimensions offer a comprehensive assessment of sleep quality in coronary care, addressing internal and external factors that may influence patients’ sleep. The development of the SQ-CC was guided by the need for a tool specifically tailored to the unique challenges faced by coronary care patients. While other sleep quality scales exist, the SQ-CC was designed to address the specific sleep-related issues encountered in critical care settings, making it a valuable tool for both clinical practice and research.
Recognizing that adequate sleep is essential for recovery, especially in cardiovascular patients, the SQ-CC facilitates the identification of sleep-related issues and aids healthcare professionals in formulating targeted interventions. By offering a valid and reliable instrument tailored specifically for coronary care patients, the SQ-CC enhances patient outcomes and underscores the importance of individualized care approaches in improving the overall quality of life in intensive care settings. This study aimed to evaluate a psychometrically sound tool that addresses the unique sleep challenges faced by this population, thereby highlighting the necessity for systematic evaluation and intervention to optimize recovery and well-being among coronary care patients. Furthermore, the psychometric properties of the SQ-CC will support its application in Persian-speaking countries, ensuring that it meets the cultural and clinical needs of these populations.
Methods
Design
This study was carried out as a methodological study in the Ardabil province of Iran over four months, from May to September 2024. The psychometric assessment was conducted in strict adherence to the Consensus-based Standards for the Selection of Health Measurement Instruments (COSMIN) guidelines, which are internationally recognized for ensuring a thorough and rigorous evaluation of the measurement tools utilized in the study.
Setting and sample
This study was conducted in the Coronary Care Unit (CCU) and heart unit of Imam Khomeini Hospital in Ardabil. The inclusion criteria for this study comprised participants aged 18 years and older diagnosed with acute myocardial infarction, heart failure, and acute coronary syndrome. All participants were required to provide informed consent and demonstrate proficiency in Persian to ensure comprehension of the study materials. Conversely, individuals were excluded if they exhibited significant cognitive impairments, had severe psychiatric disorders or other primary medical conditions affecting sleep quality, were diagnosed with acute sleep disorders such as insomnia or sleep apnea within the last six months, or were currently taking medications known to significantly impact sleep patterns, unless they had maintained a stable dosage for at least one month before the study.
The sample size determination followed established criteria for confirmatory factor analysis (CFA). Based on recommendations for psychometric studies, a minimum ratio of 5:10 participants per scale item is advised to ensure stable parameter estimates24. For enhanced robustness given the 14-item SQ-CC scale, we adopted a stricter criterion of 15 participants per item (targeting 14 × 15 = 210 participants). To account for potential non-response, this baseline was adjusted upward by 10% using the formula:
A convenience sampling method was employed to recruit 231 eligible participants. Of these, 11 questionnaires (5%) were excluded due to incomplete data, resulting in 220 analyzable responses (95% response rate). This approach aligns with simulation studies showing stable CFA results with ≥ 200 samples and ≥ 10:1 participant-to-item ratios25.
Instrument
The Sleep Quality Scale for Coronary Care Patients (SQ-CC), developed by Pelin and Sert6, consisted of two dimensions designed to evaluate the sleep quality of patients in the coronary care unit: self-assessment (8 items) and environmental factors (6 items), totaling 15 items. Items 2, 3, 8, and 9 were scored inversely. Participants rated each item on a 5-point Likert scale, ranging from 1 (strongly disagree) to 5 (strongly agree). The minimum and maximum possible scores on the scale were 14 and 70, respectively, with higher scores indicating poorer sleep quality. The total score was calculated by summing the scores of the 14 items, as item 1, which gathered information about bedtimes and wake-up times, was not included in the scoring. The overall scale demonstrated a Cronbach’s alpha of 0.816.
Translation procedure
In this research, we received authorization from the tool developer (Dr. Meryem Pelin) to perform a psychometric assessment of the SQ-CC in Persian. The translation process adhered to standard procedures, involving four bilingual translators—two for forward translation and two for back translation—to guarantee linguistic and conceptual precision. The research team reviewed the forward and back-translated versions, addressing discrepancies to produce a coherent final Persian version of the SQ-CC. We conducted preliminary testing with a small group of coronary care patients (n = 10) to further validate the Persian version, collecting feedback on clarity and understanding. Based on this input, adjustments were made to ensure that the instrument was culturally suitable and contextually pertinent for the intended population. This thorough approach reinforced the validity and reliability of the translated tool for use in the Iranian setting (Fig. 1).
Psychometric evaluation
Content validity
The SQ-CC-P’s content validity was evaluated using the Content Validity Index (CVI) and the Content Validity Ratio (CVR), with a panel of 10 experts assessing each item for relevance and necessity. The expert panel consisted of five cardiologists (specializing in cardiovascular diseases and critical care), three clinical psychologists (with expertise in sleep disorders and behavioral health), and two nursing specialists (with a focus on critical care nursing and patient sleep management). All experts had at least 10 years of experience in coronary care and sleep-related research. Their diverse expertise ensured a comprehensive evaluation of the scale’s content validity, covering medical and psychological aspects of sleep quality in coronary care patients. A cut-off point of 0.78 or higher was set for the CVI to determine the validity of individual items, based on the recommendations by Polit et al.26for a panel of 10 experts. Items meeting or exceeding this threshold were considered valid. Additionally, the necessity of each item was assessed using the CVR, with a cut-off point of 0.62 or higher indicating essential items, as proposed by Lawshe27. The Scale-Level Content Validity Index for Unanimous Agreement (S-CVI/UA) was calculated with a cut-off point of 0.80 or higher to reflect a high level of consensus among experts, following the guidelines by Lynn28. Similarly, the Scale-Level Content Validity Index Average (S-CVI/Ave) had a cut-off point of 0.90 or higher, indicating strong average agreement across items, as recommended by Polit and Beck29. To further strengthen the validity assessment and account for chance agreement, the Adjusted Kappa statistic was calculated using the formula:
where Pe is the expected agreement due to chance, pi is the proportion of raters who rated the item as relevant or essential, Po is the proportion of items where raters agree (observed agreement among raters), k is the Kappa statistic, and n is the number of raters. The cut-off value for Adjusted Kappa was set at 0.60, as values above this threshold indicate good agreement beyond chance30.
Construct validity
Exploratory factor analysis (EFA)
To assess the construct validity of the SQ-CC-P, an Exploratory Factor Analysis (EFA) was conducted. The EFA was performed using Maximum Likelihood (ML) with Varimax rotation to identify the underlying factor structure of the scale31,32. The Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy and Bartlett’s Test of Sphericity were used to evaluate the suitability of the data for factor analysis32,33. A KMO value > 0.60 and a significant Bartlett’s Test (p < 0.05) were considered acceptable. Factors with eigenvalues > 1.0 were retained, and items with factor loadings ≥ 0.40 were considered to load significantly on a latent factor34. Additionally, communalities were calculated to determine the proportion of variance in each item explained by the extracted factors, with communalities ≥ 0.30 considered acceptable35.
Confirmatory factor analysis (CFA)
The construct validity of the SQ-CC-P was evaluated using Confirmatory Factor Analysis (CFA). A first-order CFA model was utilized to examine the relationships between observed variables and their respective latent constructs. Factor loadings were assessed, with a cut-off point of 0.40 typically used to indicate an acceptable association level between observed variables and their latent constructs. The statistical significance of the factor loadings was determined using T-values, with a threshold of T > 1.96 (p < 0.05) to establish significance36.
Several fit indices were calculated to assess the CFA model’s goodness of fit. The model’s fit was evaluated against established cut-off values for absolute, incremental, and parsimony fit indices. These indices included CMIN/DF < 3, RMSEA < 0.08, GFI > 0.90, CFI > 0.90, TLI > 0.90, IFI > 0.90, NFI > 0.90, AGFI > 0.80, and PNFI > 0.5037.
Convergent and discriminant validity
The convergent validity of the SQ-CC-P was evaluated using Composite Reliability (CR) and Average Variance Extracted (AVE). CR values were determined for self-assessment and environmental factors, ensuring they exceeded the recommended threshold of 0.70. Additionally, AVE values were calculated to confirm that they surpassed the acceptable cutoff of 0.50, indicating that the respective latent constructs account for a significant portion of the variance in the indicators38.
Divergent validity was evaluated using the Heterotrait–Monotrait (HTMT) ratio. This ratio was examined to ensure it remained below the commonly accepted threshold of 0.85, indicating adequate differentiation between the constructs39.
Reliability
The SQ-CC-P’s reliability was assessed through internal consistency and test–retest reliability analysis. Internal consistency was evaluated using Cronbach’s alpha (α) and McDonald’s omega coefficient (ω). These coefficients were calculated for the entire scale and dimensions to ensure they exceeded the recommended threshold of 0.70, signifying that the instrument reliably measures the intended constructs40,41,42,43,44,45. Furthermore, the mean inter-item correlation (ρ), which ranged from 0.15 to 0.5, was computed to further reinforce the scale’s internal consistency46.
Test–retest reliability was evaluated using the Intraclass Correlation Coefficient (ICC), with a threshold greater than 0.75 for the total SQ-CC-P and dimensions. This analysis was conducted by administering the scale to a sample of 30 patients, selected through simple random sampling, at two different time points spaced two weeks apart. This approach aimed to assess the stability of the results over time. Furthermore, the 95% confidence interval (CI) for the ICC was calculated to provide insight into the precision of the reliability estimate47.
Data analysis
We first evaluated the data distribution to identify unusual or missing data points to ensure the data quality. Indifferent respondents and inconsistent responses were identified by examining patterns of straight-lining (i.e., respondents who selected the same response option for a large number of consecutive items). Specifically, respondents with more than 90% of their answers falling within a single response category (e.g., “neutral” or “agree”) were flagged as potentially indifferent and excluded from further analysis. This approach aligns with recommendations by Johnson et al.48for identifying non-engaged respondents.
We conducted our analysis by evaluating the data distribution to identify unusual or missing data points. We employed the Mahalanobis D-squared method to detect multivariate outliers, setting a significance level at p < 0.001. This approach effectively identifies outliers in multivariate datasets, as it considers the covariance among variables. To examine the data’s characteristics further, we calculated the Mardia coefficient, which assesses violations of multivariate kurtosis and skewness. A threshold value greater than 8 for the Mardia coefficient indicates potential issues with the normality of the data distribution, suggesting that the data may exhibit significant skewness or kurtosis. Specifically, skewness values greater than 2 and kurtosis values exceeding 7 are often used as cut-off points to indicate deviations from normality, affecting the validity of subsequent analysis49.
Data analysis was conducted using IBM SPSS Statistics for Windows, version 22 (IBM Corp., Armonk, NY, USA) for descriptive data analysis. For CFA and structural equation modeling (SEM), we utilized IBM SPSS AMOS Graphics, version 24. Statistical significance was determined using a two-tailed test, with a p < 0.05 considered significant.
Ethical considerations
The research study was approved by the Research Ethics Committees of Ardabil University of Medical Sciences, approval ID: IR.ARUMS.REC.1403.124. This study adhered rigorously to the ethical principles outlined in the revised Declaration of Helsinki, which serves as an internationally recognized framework for ethical medical research involving human participants. Prior to their involvement in the study, all participants were provided with comprehensive information regarding the purpose and procedures of the research. They signed informed consent forms, which clearly outlined their rights and the nature of their participation. Each participant was assured that their personal information would be handled with the utmost confidentiality, safeguarded against unauthorized access, and used exclusively for the purposes of the study. Additionally, participants were informed of their right to withdraw from the study at any time without any repercussions or impact on their ongoing treatment.
Results
Demographic information
Table 1 presents the demographic characteristics of the participants in this study (N = 220). The mean age of the participants was 56.23 years, with a standard deviation of 10.27 years. Regarding gender distribution, the sample comprised 147 males (65.0%) and 73 females (35.0%). Marital status revealed that most participants were married, accounting for 194 individuals (88.2%), while 26 participants (11.8%) were single. The diagnostic profiles of the participants indicated that myocardial infarction was the most prevalent condition, affecting 102 individuals (46.4%).
Descriptive statistics
The overall mean score for the SQ-CC-P was 45.85, with a standard deviation of 6.96. The self-assessment dimension, consisting of eight items, had a mean score of 26.45 (SD = 4.22). Meanwhile, the environmental factors dimension, comprising six items, had a mean score of 19.40 (SD = 4.61). Findings revealed negative skewness in both dimensions, with values of − 0.412 for self-assessment and − 0.620 for environmental factors. Additionally, the kurtosis values were − 0.987 and − 1.120, respectively. These statistics suggest a tendency toward a positive perception among participants, reflecting a favorable evaluation of the measured constructs.
Furthermore, no floor or ceiling effects (< 15%) were observed in the total instrument and its dimensions. This absence of floor and ceiling effects further supports the reliability of the SQ-CC-P, indicating that the instrument effectively captures the full range of participant responses without clustering at the extremes (Table 2).
Content validity
The CVI for individual items ranged from 0.80 to 0.92, indicating that all items met the threshold of 0.78 for validity. The CVR also supported the necessity of the items, with values ranging from 0.70 to 1.00, confirming that all items were deemed essential by the expert panel. The S-CVI/UA was calculated at 0.85, reflecting a high level of unanimous agreement among experts regarding the relevance of the items. Furthermore, the S-CVI/Ave yielded a score of 0.91, suggesting strong average agreement across the items. The Adjusted Kappa statistic was also calculated to account for chance agreement, yielding a value of 0.697. These findings indicated that the measurement instrument was valid for assessing the intended constructs.
Construct validity
Exploratory factor analysis (EFA)
The KMO measure of sampling adequacy was 0.83, indicating that the data were suitable for factor analysis. Bartlett’s Test of Sphericity was significant (χ2 = 1207.56, p < 0.001), confirming the appropriateness of the data for EFA. The EFA revealed a two-factor solution, consistent with the original scale. The two factors explained 62.4% of the total variance (Table 3).
-
Factor 1 (Self-assessment): This factor included 8 items (items 1–8) and accounted for 42.5% of the variance. All items loaded significantly on this factor, with factor loadings ranging from 0.52 to 0.78.
-
Factor 2 (Environmental factors): This factor included 6 items (items 9–14) and accounted for 19.7% of the variance. All items loaded significantly on this factor, with factor loadings ranging from 0.59 to 0.71.
Confirmatory factor analysis (CFA)
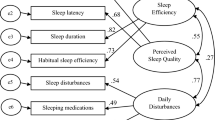
Factor loadings for the first-order CFA model ranged from 0.72 to 0.82, demonstrating strong relationships between observed variables and their respective latent constructs (Fig. 2). The T-values associated with these loadings ranged from 17.11 to 21.95, indicating that all factor loadings were statistically significant (p < 0.001). In addition, we assessed the goodness-of-fit indices of the CFA model with two latent factors. The results indicated that the SQ-CC-P exhibited excellent and acceptable fit values (Table 4).
Convergent and divergent validity
The CR for self-assessment was 0.902, while for environmental factors, it was 0.884, exceeding the recommended threshold of 0.70. Additionally, the AVE values were 0.641 for self-assessment and 0.658 for environmental factors, both surpassing the acceptable cutoff of 0.50. These results suggest that a significant portion of the variance in the indicators can be explained by their respective latent constructs, confirming that the measures effectively capture the intended constructs.
In terms of divergent validity, the HTMT ratio value of 0.514 indicated adequate differentiation between the constructs. This value was below the commonly accepted threshold of 0.85, suggesting that while there is some correlation between self-assessment and environmental factors, they remain distinct constructs (Table 5).
Reliability
The reliability analysis of the SQ-CC-P indicated strong internal consistency for the total scale, with a Cronbach’s alpha (α) of 0.862 and a McDonald’s omega coefficient (ω) of 0.864. These values exceeded the recommended threshold of 0.70, confirming that the instrument reliably measures the intended constructs. Additionally, the mean inter-item correlation (ρ) for the total scale was 0.308, further supporting its internal consistency.
Regarding test–retest reliability, the ICC for the total SQ-CC-P was 0.785, with a 95% CI ranging from 0.718 to 0.841. This indicated substantial stability over time, suggesting that the SQ-CC-P produces consistent results when administered at different times (Table 6).
Discussion
The creation and authentication of the SQ-CC-P represents a significant achievement in healthcare, especially in specialized areas like cardiology. This instrument, specifically developed to tackle the distinct sleep difficulties of patients in critical care environments, addresses a crucial need in the field. Considering the significant impact of sleep disruptions on cardiovascular patients’ recovery and overall health outcomes, having a reliable and valid tool like the SQ-CC-P is extremely important. Our research confirms that the SQ-CC-P is vital for assessing sleep quality, providing healthcare professionals with valuable insights to customize interventions and improve patient care.
The SQ-CC-P underwent a rigorous evaluation process to confirm its content validity, ensuring that the diagnostic test accurately identifies the intended condition50. The CVI for each item was meticulously assessed and found to range from 0.80 to 0.92, indicating that every item surpassed the predetermined threshold for validity51. Furthermore, the CVR values were carefully examined and were determined to range from 0.70 to 1.00, affirming that experts considered each item essential for effectively evaluating sleep quality52. The S-CVI/UA of 0.85 and S-CVI/Ave score of 0.91 reflects a substantial level of consensus among experts regarding the relevance of the items53. These results align with the original tool6, emphasizing the critical importance of establishing content validity when developing measurement tools for patient populations. The robust content validity of the SQ-CC underscores its potential for significant utility in clinical settings.
The descriptive statistics reveal an average score of 45.85 ± 6.96 for the SQ-CC-P, indicating that participants perceive their sleep quality positively. The minimal floor and ceiling effects suggest a well-distributed range of scores, reflecting participants’ diverse perceptions regarding sleep quality54. CFA further substantiates these findings, demonstrating robust factor loadings between 0.72 and 0.82. These values signify significant relationships between the observed variables and their respective latent constructs, enhancing the construct validity of the SQ-CC-P55. Additionally, the statistically considerable T-values reinforce the strength of these relationships, providing further evidence of the tool’s reliability56. These findings are consistent with the original tool6, underscoring the reliability of the constructs measured by the SQ-CC-P. These results instill confidence in the SQ-CC’s effectiveness as a tool for evaluating sleep quality, suggesting its valuable application in both research and clinical settings.
The SQ-CC-P has demonstrated strong convergent validity through comprehensive evaluation measures. The CR values of 0.902 for self-assessment and 0.884 for environmental factors surpass the recommended threshold of 0.70, indicating a high level of internal consistency within each construct57,58. Additionally, the AVE values of 0.641 and 0.658 signify that a substantial portion of the variance in the indicators can be attributed to their respective constructs59,60. These results further validate the questionnaire’s ability to precisely measure the fundamental aspects of sleep quality in individuals receiving care for coronary issues. Regarding divergent validity, the Heterotrait–Monotrait (HTMT) ratio value of 0.514 indicates adequate differentiation between constructs, emphasizing the distinct nature of the dimensions assessed by the SQ-CC-P61. These findings are essential for ensuring that the questionnaire covers all aspects of sleep quality for this group of patients. The evidence shows that the SQ-CC-P is a reliable tool for assessing sleep quality in coronary care settings, demonstrating its potential usefulness in clinical practice and research.
The SQ-CC-P has shown strong reliability in assessing sleep quality among coronary care patients. It demonstrates strong internal consistency with a Cronbach’s alpha of 0.862 and a McDonald’s omega coefficient of 0.864, exceeding the recommended threshold of 0.7062,63. These findings indicate that the scale effectively measures the same underlying construct associated with sleep quality. The mean inter-item correlation of 0.308 supports this internal consistency, indicating a moderate correlation among the items64,65. Additionally, the test–retest reliability assessment revealed significant temporal stability, with an ICC of 0.785, indicating consistent results across repeated administrations66,67. These robust findings align with the psychometric evaluations reported in the original tool6, further validating the SQ-CC-P’s effectiveness in assessing sleep quality in coronary care patients. Overall, the evidence supports the SQ-CC-P as a reliable instrument for clinical and research applications.
The validation of the SQ-CC represents a significant step forward in addressing the distinctive sleep challenges encountered by patients in critical care environments. This study underscores the necessity of employing a specialized instrument for assessing sleep quality, particularly given the critical role that sleep disturbances play in the recovery and overall health outcomes of cardiovascular patients. The SQ-CC-P is structured into two subscales: the self-assessment subscale, which captures patients’ subjective perceptions of their sleep quality, and the environmental factors subscale, which examines external conditions affecting sleep. Both subscales exhibit strong psychometric characteristics, including high internal consistency and solid validity, ensuring that the SQ-CC-P accurately reflects the complex dimensions of sleep quality within this demographic. The thorough evaluation of the SQ-CC-P indicates its promising application in both clinical practice and research contexts, equipping healthcare providers with essential insights to tailor interventions and enhance patient care strategies.
It’s important to acknowledge the limitations of this study despite the promising results. While considered adequate, the sample size may only partially capture the diverse population of coronary care patients across various cultural and clinical contexts. The findings may need more generalizability. Additionally, the study’s cross-sectional design restricts our ability to track and assess changes in sleep quality over an extended period. Future research should prioritize conducting longitudinal studies with more extensive and diverse samples to address these limitations. This approach would enhance the generalizability of the results and provide a more comprehensive understanding of sleep quality in coronary care patients across different contexts.
The SQ-CC-P assessment tool has undergone rigorous evaluation, demonstrating robust psychometric properties, including content validity, construct validity, and reliability. It has proven a valuable resource for healthcare professionals working with coronary care patients, enabling practical assessment and addressing sleep quality issues. This tool is crucial in improving patient outcomes within this healthcare setting by addressing a critical gap in evaluating sleep disturbances, especially in coronary care patients. Emphasizing the importance of personalized care approaches, it underscores the need to tailor interventions to the specific needs of patients to enhance their quality of life within intensive care settings. However, it’s important to note that further validation in diverse populations is necessary to strengthen the applicability and utility of the SQ-CC-P in clinical practice, ensuring its adequacy and relevance across a range of patient demographics and healthcare settings.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Tsao, C. W. et al. Heart disease and stroke statistics—2023 update: A report from the American Heart Association. Circulation 147, e93–e621 (2023).
Li, Z. et al. Global, regional, and national death, and disability-adjusted life-years (DALYs) for cardiovascular disease in 2017 and trends and risk analysis from 1990 to 2017 using the global burden of disease study and implications for prevention. Frontiers Public Health https://doi.org/10.3389/fpubh.2021.559751 (2021).
Sheikh, M., Ostadrahimi, P. & Shahraki, E. Evaluation of in-hospital mortality of acute coronary syndrome based on blood glucose at admission. J. Diabetes Nurs. 9, 1408–1418 (2021).
Shahjehan, R. D. & Bhutta, B. S. in StatPearls [Internet] (StatPearls Publishing, 2023).
Kodeboina, M. et al. Challenges and burdens in the coronary artery disease care pathway for patients undergoing percutaneous coronary intervention: A contemporary narrative review. Int J Environ Res Public Health https://doi.org/10.3390/ijerph20095633 (2023).
Pelin, M. & Sert, H. Development of the sleep quality scale for coronary care patients (SQ-CC). Intensive Crit. Care Nurs. 79, 103485 (2023).
Kafantaris, S. & Kadda, O. Advantages and disadvantages of patients’ hospitalization in intensive care units. Health Res. J. 7, 155–159 (2021).
Knauert, M. P. et al. Causes, consequences, and treatments of sleep and circadian disruption in the ICU: An official american thoracic society research statement. Am. J. Respir. Crit. Care Med. 207, e49–e68. https://doi.org/10.1164/rccm.202301-0184ST (2023).
Shih, C.-Y. et al. Dynamic prevalence of sleep disturbance among critically ill patients in intensive care units and after hospitalisation: A systematic review and meta-analysis. Intensive Crit. Care Nurs. 75, 103349. https://doi.org/10.1016/j.iccn.2022.103349 (2023).
Baranwal, N., Yu, P. K. & Siegel, N. S. Sleep physiology, pathophysiology, and sleep hygiene. Prog. Cardiovasc. Dis. 77, 59–69. https://doi.org/10.1016/j.pcad.2023.02.005 (2023).
Nilius, G., Richter, M. & Schroeder, M. Updated perspectives on the management of sleep disorders in the intensive care unit. Nat. Sci Sleep 13, 751–762. https://doi.org/10.2147/NSS.S284846 (2021).
Kakar, E. et al. Sleep assessment in critically ill adults: A systematic review and meta-analysis. J. Crit. Care 71, 154102. https://doi.org/10.1016/j.jcrc.2022.154102 (2022).
Li, Y. et al. Development and validation of a clinical prediction model for sleep disorders in the ICU: A retrospective cohort study. Frontiers Neurosci. https://doi.org/10.3389/fnins.2021.644845 (2021).
Wichlinski, L. J. Adaptive solutions to the problem of vulnerability during sleep. Evol. Psychol. Sci. 8, 442–477 (2022).
Scott, A. J., Webb, T. L., Martyn-St James, M., Rowse, G. & Weich, S. Improving sleep quality leads to better mental health: A meta-analysis of randomised controlled trials. Sleep Med. Rev. 60, 101556. https://doi.org/10.1016/j.smrv.2021.101556 (2021).
Khan, M. A. & Al-Jahdali, H. The consequences of sleep deprivation on cognitive performance. Neurosciences (Riyadh) 28, 91–99. https://doi.org/10.17712/nsj.2023.2.20220108 (2023).
Moraes, K., de Almondes, H., Agudelo, A. M. & Jiménez-Correa, U. Impact of sleep deprivation on emotional regulation and the immune system of healthcare workers as a risk factor for COVID 19: Practical recommendations from a task force of the Latin American association of sleep psychology. Frontiers Psychol https://doi.org/10.3389/fpsyg.2021.564227 (2021).
Vaccaro, A. et al. Sleep loss can cause death through accumulation of reactive oxygen species in the gut. Cell 181, 1307-1328.e1315. https://doi.org/10.1016/j.cell.2020.04.049 (2020).
Liu, X. et al. The mediation role of sleep quality in the relationship between cognitive decline and depression. BMC Geriatr. 22, 178 (2022).
Telias, I. & Wilcox, M. E. Sleep and Circadian Rhythm in Critical Illness. Crit. Care 23, 82. https://doi.org/10.1186/s13054-019-2366-0 (2019).
Zhang, C. & Qin, G. Irregular sleep and cardiometabolic risk: Clinical evidence and mechanisms. Frontiers Cardiovasc. Med. 10, 1059257 (2023).
Buysse, D. J., Reynolds, C. F. 3rd., Monk, T. H., Berman, S. R. & Kupfer, D. J. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213. https://doi.org/10.1016/0165-1781(89)90047-4 (1989).
Richards, K. C., O’Sullivan, P. S. & Phillips, R. L. Measurement of sleep in critically ill patients. J. Nurs. Meas. 8, 131–144 (2000).
Kline, R. B. Principles and practice of structural equation modeling. (Guilford publications, 2023).
Wolf, E. J., Harrington, K. M., Clark, S. L. & Miller, M. W. Sample size requirements for structural equation models: An evaluation of power, bias, and solution propriety. Educ. Psychol. Measur. 73, 913–934 (2013).
Polit, D. F., Beck, C. T. & Owen, S. V. Is the CVI an acceptable indicator of content validity? Appraisal and recommendations. Res. Nurs. Health 30, 459–467 (2007).
Lawshe, C. H. A quantitative approach to content validity. Personnel psychology 28 (1975).
Lynn, M. R. Determination and quantification of content validity. Nurs. Res. 35, 382–386 (1986).
Polit, D. F. & Beck, C. T. The content validity index: are you sure you know what’s being reported? Critique and recommendations. Res. Nurs. Health 29, 489–497 (2006).
Almanasreh, E., Moles, R. & Chen, T. F. Evaluation of methods used for estimating content validity. Res. Social Adm. Pharm. 15, 214–221 (2019).
Pett, M. A., Lackey, N. R. & Sullivan, J. J. Making sense of factor analysis: The use of factor analysis for instrument development in health care research. (sage, 2003).
Field, A. Discovering statistics using IBM SPSS statistics. (Sage publications limited, 2024).
Kaiser, H. F. An index of factorial simplicity. Psychometrika 39, 31–36 (1974).
Tabachnick, B. G. Using multivariate statistics. Alyn and Bacon (2007).
Hair Jr, J. F., Black, W. C., Babin, B. J. & Anderson, R. E. Multivariate data analysis 785–785 (2010).
Marsh, H. W. et al. Exploratory structural equation modeling, integrating CFA and EFA: Application to students’ evaluations of university teaching. Struct. Equ. Modeling 16, 439–476 (2009).
Kline, P. An easy guide to factor analysis (Routledge, 2014). https://doi.org/10.4324/9781315788135.
Fornell, C. & Larcker, D. F. Evaluating structural equation models with unobservable variables and measurement error. J. Mark. Res. 18, 39–50 (1981).
Henseler, J., Ringle, C. M. & Sarstedt, M. A new criterion for assessing discriminant validity in variance-based structural equation modeling. J. Acad. Mark. Sci. 43, 115–135 (2015).
Kalkbrenner, M. T. Alpha, omega, and H internal consistency reliability estimates: Reviewing these options and when to use them. Couns. Outcome Res. Eval. 14, 77–88 (2023).
McNeish, D. Thanks coefficient alpha, we’ll take it from here. Psychol. Methods 23, 412 (2018).
Mayers, A. Introduction to statistics and SPSS in psychology. (2013).
Dunn, T. J., Baguley, T. & Brunsden, V. From alpha to omega: A practical solution to the pervasive problem of internal consistency estimation. Br. J. Psychol. 105, 399–412 (2014).
Streiner, D. L., Norman, G. R. & Cairney, J. Health measurement scales: A practical guide to their development and use (Oxford University Press, 2015).
Spiliotopoulou, G. Reliability reconsidered: Cronbach’s alpha and paediatric assessment in occupational therapy. Aust. Occup. Ther. J. 56, 150–155 (2009).
Clark, L. A. & Watson, D. Constructing validity: Basic issues in objective scale development. (2016).
Koo, T. K. & Li, M. Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 15, 155–163 (2016).
Johnson, T. P., Shavitt, S. & Holbrook, A. L. Survey response styles across cultures. (2011).
Vinzi, V. E., Chin, W. W., Henseler, J. & Wang, H. in Handbook of partial least squares: Concepts, methods and applications 1–20 (Springer, 2009).
Bogduk, N. On understanding the validity of diagnostic tests. Int. Pain Med. 1, 100127. https://doi.org/10.1016/j.inpm.2022.100127 (2022).
Dalawi, I., Isa, M. R., Chen, X. W., Azhar, Z. I. & Aimran, N. Development of the Malay Language of understanding, attitude, practice and health literacy questionnaire on COVID-19 (MUAPHQ C-19): content validity & face validity analysis. BMC Public Health 23, 1131. https://doi.org/10.1186/s12889-023-16044-5 (2023).
Invited Sessions at ACPA’s. Annual meeting. Cleft Palate Craniofacial J. 61(1–171), 2024. https://doi.org/10.1177/10556656241234521 (2024).
Tseli, E., Sjöberg, V., Björk, M., Äng, B. O. & Vixner, L. Evaluation of content validity and feasibility of the eVISualisation of physical activity and pain (eVIS) intervention for patients with chronic pain participating in interdisciplinary pain rehabilitation programs. PLoS ONE 18, e0282780. https://doi.org/10.1371/journal.pone.0282780 (2023).
Gulledge, C. M. et al. Floor and ceiling effects, time to completion, and question burden of PROMIS CAT domains among shoulder and knee patients undergoing nonoperative and operative treatment. JB JS Open Access https://doi.org/10.2106/jbjs.Oa.19.00015 (2019).
Pattrawiwat, K., Nilrungratana, P., Deewai, A. & Tuntivivat, S. The measurement model of family strengths for generation alpha in the thai context. Behav. Sci. 14, 921 (2024).
Jaaffar, T. & Samy, N. K. Investigating the complex relationships between leadership, psychological safety, intrinsic motivation, and nurses’ voice behavior in public hospitals using PLS-SEM. Belitung Nurs. J. 9, 165–175. https://doi.org/10.33546/bnj.2556 (2023).
Leavy, P. Research design: Quantitative, qualitative, mixed methods, arts-based, and community-based participatory research approaches. (Guilford Publications, 2022).
Sarstedt, M., Ringle, C. M. & Hair, J. F. in Handbook of market research 587-632 (Springer, 2021)
Sarstedt, M. In the great facilitator: Reflections on the contributions of Joseph F. Hair, Jr. to Marketing and business research 113–119 (Springer, 2019).
Hair, J., Black, W., Babin, B., Anderson, R. & Tatham, R. Multivariate data analysis. Cengage learning. Hampshire, United Kingdom 633 (2019).
Dirgiatmo, Y. in Macroeconomic Risk and Growth in the Southeast Asian Countries: Insight from Indonesia Vol. 33A International Symposia in Economic Theory and Econometrics (eds William A. Barnett & Bruno S. Sergi) 157–170 (Emerald Publishing Limited, 2023).
Hayes, A. F. & Coutts, J. J. Use omega rather than cronbach’s alpha for estimating reliability. but…. Commun. Methods Measur. 14(1), 1–24. https://doi.org/10.1080/19312458.2020.1718629 (2020).
Oluwadiya, K. S., Owoeye, O. K. & Adeoti, A. O. Evaluating the factor structure, reliability and validity of the Copenhagen Burnout Inventory-Student Survey (CBI-SS) among faculty of arts students of Ekiti State University, Ado-Ekiti Nigeria. Sci. Rep. 14, 10476 (2024).
Röschel, A., Wagner, C. & Dür, M. Examination of validity, reliability, and interpretability of a self-reported questionnaire on Occupational Balance in Informal Caregivers (OBI-Care)—A Rasch analysis. PLoS ONE 16, e0261815. https://doi.org/10.1371/journal.pone.0261815 (2021).
Trabelsi, K., Saif, Z., Driller, M. W., Vitiello, M. V. & Jahrami, H. Evaluating the reliability of the athlete sleep behavior questionnaire (ASBQ): a meta-analysis of Cronbach’s alpha and intraclass correlation coefficient. BMC Sports Sci. Med. Rehabil. 16, 1. https://doi.org/10.1186/s13102-023-00787-0 (2024).
Koo, T. & Li, M. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropract. Med. https://doi.org/10.1016/j.jcm.2016.02.012 (2016).
Cordier, R., Speyer, R., Martinez, M. & Parsons, L. Reliability and validity of non-instrumental clinical assessments for adults with oropharyngeal dysphagia: a systematic review. J. Clin. Med. 12, 721 (2023).
Acknowledgements
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors would like to thank all the participants in this study, the Imam Khomeini Hospital affiliated with Ardabil University of Medical Sciences, the Student Research Committee of Ardabil University of Medical Sciences, the Vice Chancellor for Research of Ardabil University of Medical Sciences, and all those who contributed to this study.
Author information
Authors and Affiliations
Contributions
AM: Writing—review & editing, Writing—original draft, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. LV: Writing—review & editing, Writing—original draft, Methodology, Investigation, Formal analysis. RI: Writing—review & editing, Writing—original draft, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. RYS: Writing—review & editing, Writing—original draft, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. RNV: Writing—review & editing, Writing—original draft, Supervision, Resources, Methodology, Data curation, Conceptualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mirzaei, A., Valizadeh, L., Imashi, R. et al. Validation and the psychometric properties of the Persian version of the sleep quality scale for coronary care patients: a methodological survey. Sci Rep 15, 13749 (2025). https://doi.org/10.1038/s41598-025-96728-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-96728-7