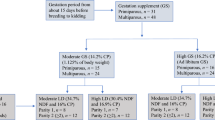
The experiment was designed to consider effects of different amounts of metabolizable energy and protein on mammary gland development, mRNA abundance of genes related to vascular function and angiogenesis of Sistani goats. A total of 32 pregnant Sistani goats were selected on day 100 of gestation and allocated randomly to four treatments: (1) basal diet that meets metabolizable energy (ME) and protein (MP) requirements of goats during late gestation, as recommended by the National Research Council = NRC (2007) (C). (2) A diet with 10% higher levels of ME than recommendations (E). (3) A diet with 10% higher levels of MP than recommendations (P), and (4) a co-addition diet with 10% higher levels of both ME and MP than recommendations (EP). The four diets were fed from 100 days prepartum to 30 days after parturition. Mammary biopsies were obtained 24 h after parturition. Feed intake (g/day), colostrum (kg/day) and milk (kg/month) productions increased when extra E and P were provided together prepartum and early postpartum (P < 0.05). Relative mRNA abundances of vascular endothelial growth factor (VEGF, 4.68-fold), tyrosine kinase tie2 receptor (RTK, 4.61-fold), vascular endothelial growth factor receptor 1 (VEGFR1, 5.15-fold), angiopoietin I (ANGPT1, 4.86-fold), cyclooxygenase I (COX1, 4.5-fold), prostacyclin synthase (PTGIS, 5.66-fold), thromboxane A2 synthase (TBXAS, 98-fold) increased significantly in the mammary gland with the co-addition of E and P. Histological results documented that alveolar area (epithelium + luminal space, µm2), average number of epithelial cells per alveolus, and relative area occupied by overall epithelial cells (per slide) were increased significantly, when co-addition of E and P were fed in late gestation. In summary, increasing the dietary supply of ME and MP peripartum resulted in greater milk production, stimulated mammary gland alveolar development and affected the mRNA abundance of genes associated with both angiogenesis and milk secretion in Sistani goats.
Similar content being viewed by others
Stressors during pregnancy such as heat stress, obesity and increased maternal hepatic metabolism in later stages of gestation, induce an unfavorable intrauterine state that compromises fetal development and survival. Furthermore, postpartum programmatic maternal homeorhetic responses to support maternal lactational drive may be compromised1,2,3. These stressors during pregnancy may put the mother and unborn child at increased risk of metabolic disorders, oxidative stress and gestational inflammation4. Furthermore, low birth weight, delayed growth and development of the offspring are carryover effects that influence, health, wellbeing and productivity1. Moradi et al.2 reported insulin sensitivity and metabolic status of dams around parturition were improved in ewes fed 10% greater metabolizable energy and protein levels, compared with the control group that received 100% E/P according to the National Research Council’s5 recommendations.
The vascular system of the maternal mammary gland undergoes repeated cycles of expansion and regression, accompanied by extensive growth and differentiation of the mammary epithelium during gestation and lactation, as well as epithelial regression after dry-off or prolonged lactation. Germinal angiogenesis and possibly vasculogenesis all influence vascular expansion6. In addition to a variety of local and systemic hormones and growth factors, ability of epithelial cells to promote vascular growth and differentiation are also influenced by changing oxygen and nutrient supply requirements. This leads to the release of angiogenic factors that control vascular architecture and promote endothelial cell growth6.
The process known as angiogenesis, which is associated with mammary gland remodeling in other species, is the division of pre-existing blood vessels into new capillaries7 and is a critical process for normal tissue growth and development8. The mammary microvasculature indirectly contributes significantly to milk synthesis when providing nutrients and oxygen to the mammary endothelium and removing metabolic waste products7,8,9. Many different angiogenic factors and receptors control the vascularity of various tissues8,9. Because of the connection between nutrient exchange and blood flow, angiogenesis is particularly important in vital tissues that transfer nutrients, such as the intestines, placenta, and mammary gland10.
The primary objective of this experiment is to determine how different dietary levels of metabolizable energy and proteins in Sistani goats affect the mRNA abundance of genes related to angiogenesis, vascular function factors, and mammary histology.
Materials and methods
The experimental protocols approved by the Ministerial Committee for Animal Experiments were in accordance with the Animal Welfare Protocol of the Iranian Ministry of Agriculture (Experimental Permission no. 1021). Furthermore, the study is reported in accordance with ARRIVE guidelines 2.0. Animals were not euthanized.
The Sistani goat is one of the Iranian goat breeds and is native to the Sistani region in the south part of Iran. This breed produces between 330 and 450 kg of milk in its lactation period of 250 days. The highest daily milk yields per day is 2.8 kg. The average annual fluff is 750 g. Adult weight is approximately 30 kg. The percentage of twins is approximately 30%.
Animals and treatments
Estruses of 100 multiparous Sistani goats (Sistan province, Southeast of IRAN) were synchronized using a Controlled Internal Drug Release Device (CIDR, EAZI-BREEDTM, New Zealand) inserted intravaginally at d 45 after kidding for 7 days. When the CIDR was removed, each goat received 2 ml of PGF2α (Estrumate, 250 µg Cloprostenol/mL, Schering-Plough Animal Health, Germany) and 200 IU of PMSG (Gestavet, HIPRA Co, Spain), and vasectomized bucks were used to detect estrus in all goats. Goats expressed estrus within 48 h following removal of the CIDR and were mated with fertile bucks of the same breed.
A total of 32 healthy pregnant Sistani goats (2.5 ± 1 years old, 25.50 ± 1.60 kg body weight [BW], 2.6 ± 0.50-point body condition score [BCS scale ranging from 1.0 (very thin) to 5.0 (very fat), with 0.5 increments11), and an average of 2.3 ± 0.2 parities were selected on day 100 of gestation. After receiving a 10-day adaptation diet, does were allocated randomly to four dietary treatments (Table 1): (1) adaptation diet that meets metabolizable energy (ME) and protein (MP) requirements of goats during late gestation as recommended by National Research Council = NRC (2007) (C); (2) A diet with 10% higher levels of ME than NRC recommendations (E); (3) A diet with 10% higher levels of MP than NRC recommendations (P); (4) A diet with a 10% higher level of both ME and MP than NRC recommendations (EP). Each of the four groups received their experimental diets from 100 days prepartum to 30 days after kidding. Ration ingredients and compositions (Table l) were described by Shabrandi et al.12. Goats had free access to fresh water and a vitamin-mineral supplement. Goats were milked manually at 0900 h and 1530 h and fed twice a day at 0730 h and 1430 h, with half the daily ration being given at each feeding. Goats were milked for a 30-day period postpartum. The kids were fed 1st milking after kidding and after that transferred to a separate box.
Gene expression
Gene expression was evaluated by quantitative real-time PCR as described by Shabrandi et al.12 and in accordance with MIQE guidelines. Ten mg of mammary tissue was homogenized in 350 mL of RNeasy lysis buffer (Qiagen, Alberts Lund, Denmark) and diluted (1 : 1) with 70% ethanol. The RNA was purified using the RNeasy mini kit (Qiagen) and reverse-transcribed with oligo-dT and Superscript II RNase H reverse transcription kit (Invitrogen, Taastrup, Denmark). The quality assurance control of the RNA was performed through a 2% agarose gel electrophoresis. RNA integrity obtained by observing the staining intensity of the major ribosomal RNA bands. A 28 S:18 S rRNA ratio of 2:1 is generally representative of good quality RNA that was confirmed in present study. Then to digest any contaminating DNA, a total of 1 mg of the RNA was treated with 1 U DNase (Thermo Fisher Scientific, Canada).
Real-time PCR was performed using Corbett Rotor GeneTM 3000 (Corbett Life Sciences, Sydney, NSW, Australia) and SYBR Premix Ex Taq kit (Takara Biotechnology Co. Ltd., Tokyo, Japan). A 1.0 µL of cDNA was added to the 12.5 µL of SYBR Premix Ex Taq (2×) mix and 0.5 µL of each specific primer and 10.5 µL of double-distilled water in a total volume of 25-µL. Specific primers used in the present study were published previously by Safayi et al.13 (2010) and Shabrandi et al.12. The roles of the analyzed genes are listed in Table 2. Samples were run in duplicate and Relative quantification of mRNA abundance of genes was calculated based on efficiency and the crossing point deviation of unknown sample vs. a control and expressed in comparison to reference genes (YWHAZ, GAPDH and EMERIN) based on Shabrandi et al.12. Data were normalized to a calibrator sample by using the 2−∆∆Ct method with correction for amplification efficiency14. Because of long time storage, mRNA quality was double checked and classified as good quality it was ok based on absorbance value that was 1.8-2.
Mammary gland biopsy
Biopsy samples were obtained from the mammary gland of each goat 24 h after kidding (right rear udder halves, total 2 samples from each goat) according to the procedure described by Cvek et al.15. Before biopsy, goats were milked to ensure there was no milk left in their mammary glands during sampling. To keep the biopsy needle away from major blood vessels near the dorsal aspect and midline of the udder and ductal area, the insertion point was marked 1.5 to 2 cm away from the udder midline and at the point where the udder protruded the most caudally.
Mammary gland histology
Fixed mammary tissue samples were prepared as described by Norgaard et al.16. Briefly, mammary biopsies were collected from the right rear udder halves immediately after morning milking to minimize milk remaining in the udder. After washing with surgical iodine scrub, the caudal surface of mammary rear udder halves was shaved and disinfected with iodine and 70% ethanol, the biopsy site was anesthetized by injecting about 4 ml of lidocaine hydrochloride (20 mg/ml) subcutaneously in multiple sites surrounding the biopsy site. Biopsies were obtained by using a semi-automatic 14G biopsy gun (TSK Laboratory, Tochigi-Ken, Japan). Each sample contained approximately 15 to 20 mg of tissue. A maximum of three samples was collected at each time. Tissue samples were placed in 4% buffered paraformaldehyde solutions for fixation. Paraffin embedded tissue samples were sectioned at 4 μm. Two to three serial tissue sections were taken at 30 μm intervals into the tissue were mounted on each of two microscope slides. Slides were deparaffinized and stained with Haematoxylum-Eosin (H&E). All slides were studied by light microscopy (OLYMPUS BX40 F4, Olympus, Optical Co., Tokyo, Japan) by the same person. From the stained section, six random images were captured at 20× magnifications on a light microscope with a top-mounted digital microscope camera (SDC-313B, Samsung Engineering Co., Ltd., Seoul, Korea) as described by Brown et al.17.
Image J software was used to examine the images as described by Abramoff et al.18. Each image was subjected to an 88-point digital transparent grid and the number of intersection points displaced across the luminal space, intralobular stroma, or epithelial cells per slide was counted and recorded for each image19. Measurements and tables were created for each image, in particular for the area (µm2) occupied by the lumen space, the epithelial cells or the entire alveolar surface (epithelium + lumen). Values were averaged for each animal in each biopsy period and subjected to statistical analysis. The number of epithelial cells of each identified alveolar profile and the total number of alveoli in each image are counted using the Multipoint Selection Tools function.
Statistical analysis
The Brown-Forsythe and O’Brien tests were used to determine homogeneity of variance. In the F-test, treatment served as the main effect and udder half as a random variable. Analysis of variance was performed using JMP software (SAS Institute, Cary, NC, USA). When comparing the means of multiple comparisons, the DUNCAN test for significant differences was used. Significance was declared at P < 0.05.
Results
Production performance
Feed intake prepartum was similar among groups (C = 1.9 ± 0.20 kg/d, E = 2.0 ± 0.22 kg/d, P = 1.9 ± 0.28 kg/d, EP = 2.1 ± 0.35 kg/d). Both daily feed intake (g/day) and milk production (kg/day) were significantly greater with the co-addition of E and P (1282 ± 27.5 g/d and 1.6 ± 0.2 kg/d respectively) compared with control groups (883 ± 27.5 g/d and 1.1 ± 0.2 kg/d respectively, P < 0.05). Increasing the E (1.3 ± 0.2 kg/d, 0.4 ± 0.03 kg/d) and P (1.4 ± 0.2 kg/d, 0.3 ± 0.03 kg/d) supplementation levels did not influence milk and colostrum production and was similar with control group (1.3 ± 0.2 kg/d, 0.3 ± 0.03 kg/d, P > 0.05). Shabrandi12 reported that weekly milk yield responses for 4 weeks postpartum were greater for the co-addition of E and P compared to control.
mRNA abundance
The regulation of Vascular Angiogenesis and function of the mammary gland at 24 h after parturition is rather dynamic in association with lactogenesis and timely production of milk. Relative mRNA abundances of genes related to angiogenesis i.e., formation of new and or aged blood vessels and ductular development are described in Fig. 1. Both Vascular Endothelial Growth Factor (VEGF, 4.68-fold) and Tyrosine Kinase Tie2 Receptor (RTK, 4.61-fold) had positive increases of mRNA abundances in response to E and P. When E and P diets were co-fed (EP) mRNA abundances of both VEGF and RTK were greater than stimulated responses to E and P alone and to C (i.e., Stimulatory Additive Effects). Only treatment with EP enhanced mRNA abundance of Vascular Endothelial Growth Factor Receptor 2 (VEGFR2, 5.15-fold) and angiopoietin I (ANGPT1, 4.86-fold). In contrast E, P and EP had similar stimulatory effects on mRNA abundance of VEGFR1 relative to C but they were not synergistically additive.
The mRNA abundance responses of genes related to angiogenesis1. Treatments were as follows: (1) diet providing metabolizable energy (ME) and metabolizable protein (MP) according to NRC recommendations (C), (2) diet with extra 10% ME (E), (3) diet with extra 10% MP (P), and (4) diet 1 with 10% extra of both ME and MP (EP). VEGF = vascular endothelial growth factor; RTK = tyrosine kinase tie2 receptor; VEGFR1 = vascular endothelial growth factor receptor 1; VEGFR2 = vascular endothelial growth factor receptor 2; ANGPT1 = angiopoietin I; ANGPT2 = angiopoietin 2. Values are least squares means ± S.E.
Relative mRNA abundances of genes related to vascular function are depicted in Fig. 2. Cyclooxygenase I (COX1, 4.5-fold); Prostacyclin Synthase (PTGIS, 5.66-fold); Thromboxane A2 synthase (TBXAS, 3.98-fold) increased significantly in responses to E and P and a further additive increase occurred with concurrent treatment of EP in late gestation. . In contrast COX2 gene did not differ among treatments (C, E, P and EP (Fig. 2).
The mRNA abundance responses of genes related to vascular function1. Treatments were as follows: (1) diet providing metabolizable energy (ME) and metabolizable protein (MP) according to NRC recommendations (C), (2) diet with extra 10% ME (E), (3) diet with extra 10% MP (P), and (4) diet 1 with 10% extra of both ME and MP (EP). COX1 = cyclooxygenase I; COX2 = cyclooxygenase II; PTGIS = prostacyclin synthase; TBXAS = thromboxane A2 synthase. Values are least squares means ± S.E.
Histology
The number of mammary gland Epithelial cells was greater in the EP group compared to control group (35.3 ± 8.70 vs. 26.20 ± 4.64), but there was no stimulation due to feeding either E or P alone (Table 3). Likewise, epithelium area (µm) was greater when E and P diets were fed concurrently (EP) compared to Control (P = 0.03), and responses to E and P fed separately were intermediate and not significant. The percent stromal area was reduced by the three metabolizable energy and protein diets (P = 0. 01) in which co-addition of EP markedly reduced percent stroma, E alone approximated the EP reduction, and P diet elicited only a small reduction in stromal area fed in late gestation. Increased feeding of dietary metabolizable energy and protein (E, P and EP) increased percentages of mammary gland epithelium (P = 0.008) and reduced percentages of stromal tissue in late gestation (Table 3).
Discussion
This experimental model unveiled the effect of feeding different diets on mRNA abundance of factors involved in angiogenesis and vascular remodeling in the mammary gland of dairy goats. There are a limited number of studies in goats that address the regulation of mammary vascular remodeling and function13. These studies sought to determine how different amounts of dietary metabolizable energy and protein affect mRNA abundance of genes related to angiogenesis, vascular function factors, and mammary gland histology in the transitional mammary gland at the onset of early lactation.
Djonov et al.20 suggested that regression of the capillary endothelium during mammary involution could be correlated to or be a consequence of MEC (Mammary Epithelial Cells) involution. In the present study, greater abundances of ANGPT1, VEGF, VEGFR2, and COX1 in mammary tissues of goats fed with increased amounts of both E and P concurrently in late gestation suggest that the mammary glands experienced a higher rate of vascular growth, probably stimulating and/or renewing part of the aging vascular system and new vascularization at parturition from multiparous pregnant goats12. Furthermore, there was a synergism of feeding increased E and P concurrently. The VEGFR1, VEGFR2, ANGPT1, ANGPT2, and RTK are angiogenic factors21 whose gene expression were increased in this study with increased amounts of E and P fed in late gestation. VEGFs are potent proangiogenic factors that bind to three different subtypes of VEGFRs, which are expressed on endothelial and other stromal cells21. VEGF and FGF are believed to be direct-acting positive regulators because they can positively regulate a wide range of endothelial cell functions in vitro, such as: migration, proliferation, extracellular proteolytic activity and tube or duct formation22. For both positive and negative regulators, the endothelial cell is the ultimate target. Thus, regulators of angiogenesis can influence endothelial cells directly or indirectly by stimulating inflammatory and other non-endothelial cells to produce directly acting regulators. Since TGF-β and TNF-α inhibit growth of endothelial cells in vitro, they are directly acting as negative regulators, in contrast to VEGF and FGF, which are direct mitogens of endothelial cells23. Furthermore, VEGF acutely induces microvascular hyperpermeability in vascular smooth muscle cells of blood plasma proteins24,25, Reynolds et al.8 reported that restriction of feed intake to 60% of recommended levels during gestation of ewes results in a 50% reduction in placental abundance of both VEGFR-1 and VEGFR-2 on day 130 of gestation compared with those receiving the maintenance diet. In addition, Redmer et al. reported that capillary size increased and capillary number density decreased in maternal and fetal placental tissue from overfed growing ewes on day 130 of gestation.
Relative mRNA abundances of RTK increased significantly in the mammary gland when both levels of E and P were fed concurrently in the present experiment during late gestation. The process of mammary gland branching morphogenesis is controlled by local paracrine interactions between growing epithelial ducts and the surrounding mesenchymal stroma, as well as by endocrine hormones. Receptor tyrosine kinases (RTKs) are important regulators of branching morphogenesis, although the mediators of the complex interaction in mammary gland development are not yet fully characterized26. The important pathways that are known to be activated by RTKs include mitogen-activated protein kinase (MAPK), Janus kinase (JAK)/ signal transducer and activator of transcription (STAT) and phosphoinositide 3- kinase (PI3K)/Akt27.
This investigation also examined several other genes (COX1, PTGIS, and TBXAS) that encode factors that control vascular tone and function28, which were expressed at a higher level in goats fed greater amounts of E and P concurrently in late gestation. In addition to MEC, capillary endothelial cells also synthesize these regulatory factors. The COX enzyme catalyzes the rate-limiting step in prostanoid biosynthesis29,30. Two isoforms of COX have been identified (COX1 and COX2). COX1 plays an important role in the regulation of normal physiological functions31. COX2 can be induced by growth factors, inflammatory reactions and oncogenes32. Cyclooxygenases are key enzymes involved in synthesis of several vasoactive substances33 in addition, the potent breast vasodilator prostacyclin (PGI2) was found to be specifically regulated by the enzyme PTGIS28. Improved blood perfusion in the EP group could be associated with increased abundance of genes encoding potent vasodilators.
Histologically, alveolar area (epithelium + luminal space, µm2), in contrast to the proportion of area occupied by stromal tissue, was responsive to co-feeding of elevated E and P diets in late gestation, which significantly increased average number of epithelial cells per alveolus and the relative area occupied by total epithelial cells. Reduction of dietary energy level from livestock requirements during gestation reduces development of the mammary gland and milk production after birth in cattle and sheep34. In ewes 98% of mammary growth occurs during late gestation and differentiation and growth stages of alveolar epithelial cells are the highest. The remaining 2% of growth occurred during lactation35. Proliferation of cells in the mammary gland of over fed ewes was greater than feed restricted ewes, which led to an increase in milk production of over fed ewes13. Increased energy and protein levels appear to have a significant impact on the overall pattern of ductular tissue development, as evidenced by the variations in epithelial content in parenchymal tissue. Mammary gland blood flow regulates the substrate supply for milk synthesis36. More specifically, restriction of substrate supply to the mammary gland can alter the function and metabolic activity of mammary gland secretory cells36. Javaheri et al.37 reported both the number of total alveoli in the mammary gland and the proportion of tissue area occupied by epithelial cells increased when dairy cows were fed fish oil37.
Very small capillaries would favor a more efficient nutrient exchange across the capillary–MEC barrier because of smaller diffusion distances, and extraction rates for several nutrients have in fact been found to be higher in early compared with late lactation12. Our findings indicate that in goats with increased amounts of both E and P fed in late gestation, the mammary glands enter the new lactation with a younger population of MEC surrounded by a similarly younger (and possibly less developed) capillary network. As a result, nutrient exchange across the capillary-MEC barrier would occur more efficiently. The mammary capillary network is essential for delivering substrates needed for milk synthesis. Currently, limited data exists regarding the effects of maternal feeding on the development of the mammary capillary network. Meyer et al.38 reported that supranutritional Se would increase activity of the mammary gland. Furthermore nutrient restriction, but not excessive, would decrease activity of the mammary gland. Mammary gland proliferation and other possible cellular activities indicate that the gland is a highly dynamic organ influenced by nutrition during gestation38,39. In the mammary glands of heifer calves, greater energy and protein intake from 2 to 8 weeks of age increased parenchymal mass as well as parenchymal DNA and RNA without increasing parenchymal fat deposition. Histology also was influenced by diet17. At 28 to 120 days of age, heifer calves fed a high-energy diet ad libitum had higher milk and parenchymal volumes compared to control heifers40. The development of goats, as an experimental model for characterizing mammary gland transcriptomics during the processes of mammary gland development and functionality, appears to have potential applicability to peripartum dairy cow’s relative: to dynamics of nutritional, metabolic and potential recrudescence of milk production and reproductive processes.
Conclusion
Improved mammary gland development and function in Sistani goats was achieved with increased maternal feeding in late pregnancy of metabolizable energy (ME) and protein (MP) that enhanced expression of genes associated with angiogenesis and vascular function in mammary tissue at 24 h postpartum and milk production.
Data availability
The data that support the findings of this study are available, on reasonable request, from the corresponding author.
References
Moradi, M. et al. Moderate over-feeding of different sources of metabolizable energy and protein improved gestational insulin resistance markers and maternal metabolic status of sheep around lambing. Theriogenology 161, 332–342 (2021).
Moradi, M., Chashnidel, Y., Teimouri-Yansari, A., Khazari, B. & Mansouryar, M. Moderate overfeeding of different sources of metabolizable energy and protein. II: effects on inflammatory status of sheep in late gestation and growth trajectory of the offspring. Theriogenology 176, 115–121 (2021).
Bauman, D. E. & Currie, W. B. Partitioning of nutrients during gestation and lactation: a review of mechanisms involving homeostasis and homeorhesis. J. Dairy. Sci. 63, 1514–1529 (1980).
Duque-Guimarães, D. E. & Ozanne, S. E. Nutritional programming of insulin resistance: causes and consequences. Trends Endocrinol. Metabol. 24, 525–535 (2013).
NRC. Nutrient Requirements of Dairy Cattle 7th Rev ed. (National Academic Science, 2001).
Andres, A. C. & Djonov, V. The mammary gland vasculature revisited. J. Mammary Gland Biol. Neoplasia. 15, 319–328 (2010).
Matsumoto, M., Nishinakagawa, H., Kurohmaru, M., Hayashi, Y. & Otsuka, J. Gestation and lactation affect the microvasculature of the mammary gland in mice. J. Vet. Med. Sci. 54, 937–943 (1992).
Reynolds, L. P. et al. Placental angiogenesis in sheep models of compromised gestation. J. Physiol. 565, 43–58 (2005).
Caton, J. S. et al. Effects of maternal nutrition and stage of gestation on body weight, visceral organ mass, and indices of jejunal cellularity, proliferation, and vascularity in pregnant Ewe lambs. J. Anim. Sci. 87, 222–235 (2009).
Seal, S. J. & Reynold, C. K. Nutritional implications of Gastrointestinal and liver metabolism in ruminants. Nut Res. Rev. 6, 185–208 (1993).
Maurya, V. P. et al. Body Condition Scoring System: A Simple Tool for Optimizing Productivity in Sheep Farms. (Technical Bulletin, Central Sheep and Wool Research Institute, 2008).
Shabrandi, F., Dirandeh, E., Ansari Pirsaraei, Z. & Teimouri-Yansari, A. Increasing metabolisable energy and protein supplementation to stimulate the subsequent milk production during late gestation by increasing proliferation and reducing apoptosis in goat mammary gland prepartum. Anim. Prod. Sci. 59, 1820–1826 (2019).
Safayi, S. et al. Continuous lactation effects mammary remodeling during late gestation and lactation in dairy goats. J. Dairy. Sci. 93, 203–217 (2010).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(– delta delta C(T)) method. Methods 25, 402–408 (2001).
Cvek, K., Dahlborn, K. & Ridderstale, Y. Localization of carbonic anhydrase in the goat mammary gland during Involution and lactogenesis. J. Dairy. Sci. 65, 43–54 (1998).
Norgaard, J. V. et al. Development of mammary glands of fat sheep submitted to restricted feeding during late gestation. Small Rum Res. 76, 155–165 (2008).
Brown, E. G. et al. Effect of increasing energy and protein intake on mammary development in heifer calves. J. Dairy. Sci. 88, 595–603 (2005).
Abramoff, M. D., Magelhaes, P. J. & Ram, S. J. Image processing with ImageJ. Biophotonics Inter. 11, 36–42 (2004).
Chalkey, H. W. Method for the quantitative morphologic analysis of tissues. J. Nation Cancer Inst. 47–53 (1943).
Djonov, V., Andres, A. C. & Ziemiecki, A. Vascular remodelling during the normal and malignant life cycle of the mammary gland. Microsc Res. Tech. 52, 182–189 (2001).
Shibuya, M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti-and proangiogenic therapies. Genes Cancer. 12, 1097–1010 (2011).
Dvorak, H. F., Brown, L. F., Detmar, M. & Dvorak, A. M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 146, 1029–1039 (1995).
Klagsbrun, M. & D’Amore, P. A. Regulators of angiogenesis. Annu. Rev. Physiol. 53, 217–239 (1991).
Senger, D. R. Molecular Framework for Angiogenesis: A Complex Web of Interactions between Extravasated Plasma Proteins and Endothelial Cell Proteins Induced by Angiogenic Cytokines. American J. Pathol. 149(1) (1996).
Redmer, D. A. et al. Influence of maternal nutrition on placental vascularity during late gestation in adolescent Ewes. Biol. Reprod. 70 (Suppl. 1), 150 (2004).
Sternlicht, M. D., Kouros-Mehr, H., Lu, P. & Werb, Z. Hormonal and local control of mammary branching morphogenesis. Differentiation 74, 365–381 (2006).
Butti, R. et al. Receptor tyrosine kinases (RTKs) in breast cancer: signaling, therapeutic implications and challenges. Mol. Cancer 17, 34.
Nielsen, M. O., Nyborg, S., Jakobsen, K., Fleet, I. R. & Norgaard, J. Mammary uptake and excretion of prostanoids in relation to mammary blood flow and milk yield during gestation-lactation and Somatotropin treatment in dairy goats. Dom Anim. Endocrinol. 27, 345–362 (2004).
Hwang, D., Scollard, D., Byrne, J. & Levine, E. Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J. Natl. Cancer Inst. 90, 455–460 (1998).
Wilson, J. E., Chandrasekharan, N. V., Westover, K. D., Eager, K. B. & Simmons, D. L. Determination of expression of cyclooxygenase-1 and – 2 isozymes in canine tissues and their differential sensitivity to nonsteroidal ARTICLE IN PRESS 184 F.L. Queiroga et al. anti-inflammatory drugs. Am. J. Vet. Res. 65, 810–818 (2004).
Dubois, R. N. et al. Cyclooxygenase in biology and disease. FASEB J. 12, 1063–1073 (1998).
Howe, L. R., Subbaramaiah, K., Brown, A. M. & Dannenberg, A. J. Cyclooxygenase-2: a target for the prevention and treatment of breast cancer. Endocr. Relat. Cancer. 8, 97–114 (2001).
Simmons, D. L., Botting, R. M. & Hla, T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and Inhibition. Pharmacol. Rev. 56, 387–437 (2004).
Anderson, R. R. Mammary gland growth in sheep. J. Anim. Sci. 41, 118–123 (1975).
Blair, H. T. et al. Dam and Granddam feeding during gestation in sheep affects milk supply in offspring and reproductive performance in grand-offspring. J. Anim. Sci. 88, 40–50 (2010).
Davis, S. R., Collier, R. J., McNamara, J. P., Head, H. H. & Sussman, W. Effects of thyroxine and growth hormone treatment of dairy cows on milk yield, cardiac output and mammary blood flow. J. Anim. Sci. 66, 70–79 (1988).
Javaheri Barfourooshi, H. et al. Effect of dietary fish oil on mammary gland development and milk production of Holstein cow. Annal Anim. Sci. 18, 973–990 (2015).
Meyer, A. M. et al. Nutritional plane and selenium supply during gestation impact yield and nutrient composition of colostrum and milk in primiparous Ewes. J. Anim. Sci. 89, 1627–1639 (2011).
Vonnahme, K. A., Lemley, C. O., Caton, J. S. & Meyer, A. M. Impacts of maternal nutrition on vascularity of nutrient transferring tissues during gestation and lactation. Nutrients 7, 3497–3523 (2015).
Petitclerc, D., Dumoulin, P., Ringuet, H., Matte, J. & Girard, C. Plane of nutrition and folic acid supplementation between birth and four months of age on mammary development of dairy heifers. Can. J. Anim. Sci. 79, 227–234 (1999).
Acknowledgements
This research was supported by funds from Sari Agricultural Sciences and Natural Resources University (SANRU) in the form of a research project approved under the number 03-1401-01.
Author information
Authors and Affiliations
Contributions
E.D conceived the study and created the model. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dirandeh, E., Ansari-Pirsaraei, Z., Teimouri, A. et al. Effects of increasing the dietary contents of metabolizable energy and protein during the peripartum period on mammary gland development in Sistani goats. Sci Rep 15, 14722 (2025). https://doi.org/10.1038/s41598-025-96795-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-96795-w