Abstract
Snakebite envenomation remains a significant global health concern, with antivenoms being the primary treatment. However, variations in venom composition can affect antivenom efficacy, leading to differences in immunoreactivity. This study aimed to evaluate and compare the immunological reactivity of venom components in Trimeresurus albolabris and Tropidolaemus wagleri venoms and further investigate the differences in antigenic properties of a key protein between two species that may influence antivenom recognition. The levels of immunological reactivity of monovalent (homospecific) antivenom and hemato polyvalent antivenom to Trimeresurus albolabris and Tropidolaemus wagleri venoms were evaluated using indirect ELISA. The immunoreactive levels of both antivenoms to antigenic proteins in Trimeresurus albolabris venom were comparable. In addition, both antivenoms reacted immunologically with antigens in Tropidolaemus wagleri venom. However, the hemato polyvalent antivenom showed greater reactivity to Tropidolaemus wagleri venom than the monovalent antivenom. The overall reactivity of the antivenoms to Trimeresurus albolabris venom was higher than that to Tropidolaemus wagleri venom. Using two-dimensional (2DE) immunoblotting and liquid chromatography mass-spectrometry-based proteomic technology (LC-MS/MS), immunoreactive and non-reactive proteins in both pit viper venoms were characterized and identified. Trimeresurus albolabris venom comprised a total of 235 spots, while Tropidolaemus wagleri venom contained 72 spots. Immunorecognition between the polyvalent antivenom and specific proteins in both venoms was mostly detected in proteins with a size over 30 kDa. Among the nine protein families identified in both venoms, the most frequently reactive proteins found in Trimeresurus albolabris venom were snake venom metalloproteinases (SVMP) and snake venom serine proteases (SVSP), while in Tropidolaemus wagleri venom, the most frequent were members of the L-amino acid oxidase (LAAO) family. For the non-immunoreactive proteins, we detected the highest identity numbers of phospholipase A2 (PLA2) in Trimeresurus albolabris venom and SVSP in Tropidolaemus wagleri venom. The distinctive characteristics between the non-reactive SVSP in Tropidolaemus wagleri venom and the reactive SVSP in Trimeresurus albolabris venom were investigated. The antigenic properties and predicted B cell epitopes were further analyzed using a computational approach. Structural and physicochemical analyses indicated that Loop 2 (residues 100–110) in the immunoreactive SVSP from Trimeresurus albolabris venom exhibited higher hydrophilicity and surface accessibility compared to the non-immunoreactive SVSP from Tropidolaemus wagleri venom. These findings provide important insights into the differences in antivenom reactivity to specific proteins across different snake venoms and may contribute to future research aimed at optimizing antivenom formulations.
Similar content being viewed by others
Introduction
Snake envenomation is a public health problem in several developing countries across Asia, South America and Africa. Increased human activity in snake habitats due to the expansion of urban and agricultural areas inevitably lead to more frequent snake encounters, and hence more incidence of snakebites1. Among the groups of medically significant snakes, vipers of the genus Trimeresurus (Family Viperidae, Subfamily Crotalinae) have been reported to cause severe snakebites in vast regions across several countries in South Asia, including India2 and Sri Lanka3, and in Southeast Asia, including Myanmar4, Malaysia, Vietnam5 and Thailand6,7. Clinical manifestation due to the hematotoxic properties of Trimeresurus venoms ranges from local tissue inflammation to systemic hemorrhagic symptoms including coagulopathy, hypofibrinogenemia, thrombocytopenia and severe massive bleeding8. In fact, the majority (up to 50–60%) of Trimeresurus venom constituents possess enzymatic function9,10,11. The predominant components are snake venom metalloproteinase (SVMP), phospholipase A2 (PLA2), snake venom serine protease (SVSP) and L-amino acid oxidase (LAAO). Similar compositional proportions of these enzymes have been reported from venomic analyses of Trimeresurus albolabris (white-lipped pit viper), Trimeresurus purpureomaculatus (mangrove pit viper)10, Trimeresurus insularis, Trimeresurus puniceus11, Trimeresurus macrops (large-eyed pit viper) and Trimeresurus hageni (Hagen’s pit viper)9 venoms, which are known to cause hemotoxic effects in their bite victims.
Unlike vipers of the genus Trimeresurus, those of Tropidolaemus (Family Viperidae, Subfamily Crotalinae) are endemic to south India and a more southern range of Southeast Asian countries. Tropidolaemus wagleri (Wagler’s pit viper) is found in southern Thailand, western Malaysia, Indonesia11, the Philippines and Brunei12. Venom of Tropidolaemus wagleri exhibits non-lethal, more neurotoxic characteristics13. Proteomic study showed that more than one-third of its constituents are unique low molecular peptides, namely Waglerins14,15. They bind to nicotinic acetylcholine receptor (nAChR) at the neuromuscular junction and competitively antagonize the action of acetylcholine, causing paralysis. Among the different forms, Waglerin-1 and 3 have been shown to block epsilon subunits of muscle nicotinic acetylcholine receptors, leading to rapid muscle collapse, spasms and dysfunction of myocardium, central nervous and respiratory systems in different mammals including mice and rats16,17. Waglerins have been further developed toward a skincare product which would reduce wrinkles through the disruption of signal transmission of facial muscles18. However, the presence of other viperid enzyme constituents such as PLA2, SVSP, SVMP and LAAO are also detectable in Tropidolaemus wagleri venom, and are capable of causing symptoms in bitten victims14,15.
Viper bite management relies primarily on the administration of antivenom. In Thailand, Queen Saovabha Memorial Institute (QSMI), Thai Red Cross Society supplies antivenom produced from purified F(ab’)2 fragments of horse whole IgG. There are two types of antivenom available for treatment of green pit viper envenomation: monovalent antivenom against Trimeresurus albolabris (white-lipped viper) venom, and hemato polyvalent (trivalent) antivenom against Daboia siamensis (Russell’s viper), Calloselasma rhodostoma (Malayan pit viper) and Trimeresurus albolabris venoms. The immunoreactivity and in vivo neutralizing efficacy of monovalent antivenom has been previously reported against its own Trimeresurus albolabris venom as well as other related Trimeresurus spp., namely Trimeresurus insularis, Trimeresurus purpureomaculatus, Trimeresurus hageni and Trimeresurus puniceus19. However, the profile of specific protein antigens within venoms of these species has never been fully elucidated. This study aimed to investigate the immunoreactive components of Trimeresurus albolabris and Tropidolaemus wagleri venoms by (i) assessing the immunoreactivity levels of monovalent and hemato polyvalent antivenoms using indirect ELISA, (ii) identifying and characterizing immunoreactive and non-reactive venom proteins through two-dimensional electrophoresis (2DE) immunoblotting and liquid chromatography mass-spectrometry-based proteomic technology (LC-MS/MS), and (iii) investigating the antigenic differences between immunoreactive and non-reactive proteins using the approaches in immunoinformatics and protein structure analysis. By characterizing the antigenic and non-antigenic proteins in both venoms, this study provides novel insights into variations in antivenom reactivity. Computational analyses of B cell epitopes and structural features of immunoreactive and non-immunoreactive SVSPs highlight key differences in antigenicity between Trimeresurus albolabris and Tropidolaemus wagleri venom components. These findings may contribute to the refinement of antivenom formulations and the development of improved therapeutic strategies for snakebite management.
Materials and methods
Snakes, venom and antivenom
Trimeresurus albolabris pit vipers were farmed and housed at Snake Farm, QSMI, while Tropidolaemus wagleri were captured in the wild and transferred to Snake Farm, QSMI. After being quarantined, they were given routine care. Fresh snake venom was collected through bites onto a parafilm-covered glass vessel and kept in individual 1.5 ml microcentrifuge tubes. After weighing, the fresh (liquid) venom was immediately frozen at − 20 °C and lyophilized. The lyophilized venom was then pooled from at least three snakes and stored at − 20 °C until use20.
Monovalent antivenom against Trimeresurus albolabris venom (batch no. TA00219, expiry date 09/10/2024) and hemato polyvalent antivenom (against the venom of Calloselasma rhodostoma, Daboia siamensis and Trimeresurus. albolabris) (batch no. HP 00422, expiry date 05/07/2027), both produced by QSMI and available in freeze-dried F(ab’)2 form, isolated from horse immunoglobulins, were used. Following reconstitution with sterile deionized water, 1 ml of monovalent antivenom neutralized 0.7 mg of Trimeresurus albolabris venom, and 1 ml of hematotoxic polyvalent antivenom neutralized 0.7 mg of Trimeresurus albolabris venom, 1.6 mg of Calloselasma rhodostoma venom and 0.6 mg of Daboia siamensis venom, according to the manufacturer’s guideline leaflet.
Determination of protein concentration
Total protein concentration in viper venoms and antivenoms was determined using Quick Start™ Bradford Protein Assay (Bio-Rad, USA) with bovine serum albumin (BSA) as a protein standard according to manufacturer’s instruction.
Indirect enzyme-linked immunosorbent assay (ELISA)
Immunoreactivity of protein antigens in Trimeresurus albolabris and Tropidolaemus wagleri venoms to both monovalent and hemato polyvalent antivenom was assessed by indirect ELISA as previously described21. Briefly, each well of a 96-well Maxisorp Nunc immune plate (Thermo Fisher Scientific, Denmark) was coated with 4 ng of either Trimeresurus albolabris or Tropidolaemus wagleri venom in 0.05 M carbonate/bicarbonate buffer pH 9.6 and kept at 4 °C overnight. Plates were blocked with 2% (w/v) bovine serum albumin (BSA) (Capricorn Scientific GmBH, Ebsdorfergrund, Germany) and incubated for 1.5 h at 37 °C. After being washed, they were incubated again for 1 h at 37 °C with the 2-fold dilution of either monovalent or polyvalent antivenom starting from a master stock of 90 µg/ml antivenom concentration (concentration ranging between 0 and 5.625 µg/ml of protein) in 0.2% BSA-PBS. Normal horse IgG was used as a negative control. Horseradish peroxidase-conjugated goat anti-horse-IgG (Abcam, Cambridge, UK) in PBST (1:1000) was added into each well and further incubated for another hour at 37 °C. Substrate solution (SureBlue TMB microwell peroxidase, Seracare Life Sciences, Milford, MA) was subsequently added to each well, and the plate was kept in the dark for 10 min at room temperature for the reaction to occur. The absorbance at 620 nm was read using a microplate reader (TECAN InfinitePro 200, Switzerland). Average absorbance values ± SEM were plotted against the various concentrations of antivenom. The effective concentration determined at 50% of maximun immunoreativity was referred as the EC50 by non-linear regression analysis using GraphPad Prism software (version 9.0, GraphPad Software Inc, La Jolla, CA).
Venom preparation and two-dimensional polyacrylamide gel electrophoresis (2DE -PAGE)
Venom protein (100 µg) was mixed with IPG sample buffer containing 8 M urea, 2% (w/v) 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), 15 mM dithiothreitol (DTT) and 0.5% IPG sample buffer22. Afterwards, the protein solution was rehydrated overnight into a non-linear immobilized pH gradient (IPG) strip (pH 3–10; Amersham Bioscience). Isoelectric focusing (pI) was done using an Ettan IPGphorII instrument (Amersham Bioscience) with the following settings: 30 V for 14 h, 200 V for 1 h, 500 V for 1 h, 1000 V for 1 h, 3500 V for 1 h and 8000 V for 18 h. The IPG strips were equilibrated with DTT for 15 min and with iodoacetamine for 15 min. After incubation, the strips were placed onto a 12% SDS-PAGE gel. One 2DE gel was stained with silver stain and two other 2DE gels were used for immunoblotting. Both immunoreactive and non-immunoreactive spots in these gels were excised and pooled for mass spectrometric analysis.
Immunoblot analysis
The separated polypeptide spots from 2DE gels were transferred to nitrocellulose membrane for 90 min at 18 V on a Trans-blot semi-dry Transfer CellTM (Biorad) in semi-dry transfer buffer (48 mM Tris and 2.93 g glycine) pH 9.2 containing 20% methanol. The membranes were blocked using 5% (w/v) non-fat milk in PBS for 2 h at room temperature. The membranes were rinsed twice with PBS-T buffer pH 7.4 (8 mM sodium phosphate, 2 mM potassium phosphate, 140 mM NaCl, 2.7 mM KCl and 0.5% v/v Tween) for 30 s each. The blotted membranes were incubated with hemato polyvalent antivenom (1:1000 in 0.2% BSA-PBS). After washing the membrane three times with PBS-T, 50 µL of horseradish peroxidase-conjugated goat anti-horse-IgG (Abcam, Cambridge, UK) in PBS-T (1:2000) was added, and the mixture was incubated for 1 h at ambient temperature under constant agitation. Membranes were washed three times with PBS-T buffer and one time with PBS. Immunogenic spots were visualized by detection of peroxidase activity using Ultra TMB-Blotting Solution (ThermoFisher Scientific, UK).
In-gel digestion
A mixture of 50% acetonitrile (ACN) in 50 mM ammonium bicarbonate was used for de-staining the blue color from gel slides9. Venom proteins were reduced by 4 mM DTT and incubated at 60 °C for 15 min. The reduced proteins were further alkylated by 250 mM iodoacetamine (IAA) (Sigma-Aldrich) and incubated at room temperature for 30 min in darkness. The gel pieces were dehydrated by removing all solution and adding 100% ACN (Thermo Scientific, USA). For tryptic digestion, trypsin (Sigma-Aldrich) in 50 mM ammonium bicarbonate (Sigma-Aldrich) was added to rehydrate the gels, which were then incubated overnight at 37 ˚C. Peptide extraction was performed by adding 100% ACN and incubating for 15 min. The resulting solution was transferred into a new microcentrifuge tube and dried using a centrifugal concentrator (TOMY, Japan). The peptide mixtures were stored at − 20 °C prior to mass spectrometric analysis.
Mass spectrometric analysis
Each sample that had undergone tryptic digestion was resuspended in a solution containing 0.1% formic acid and 2% acetonitrile before being added to an UltiMate 3000 nano-LC system (Dionex, Surrey, UK). The LC column used was Acclaim PepMap RSLC 75 μm × 15 cm nanoviper C18 with a 2 μm particle sizes and a 100 Å pore size (Thermo Scientific, Waltham, MA) which connected to a micrOTOF-Q (Bruker Daltonics, Bremen, Germany) for analysis. With a flow rate of 200 nL/min, separation was carried out over a gradient of 58 min. In HPLC grade water, mobile phase A included 2% acetonitrile and 0.1% formic acid, whereas mobile phase B had 0.1% formic acid in acetonitrile. Utilizing Hystar software (Bruker Daltonics, Bremen, Germany), data acquisitions were managed. The mass ranges of the MS and MS/MS spectra were 400–2000 and 50–1500, respectively. DataAnalysisTM software, version 3.4, was used to transform LC-MS/MS data files into the mascot generic file (.mgf) format. The NCBI snake database was searched using Mascot version 2.4.1 (Matrix Science, London, UK). The database was set with the following parameters: missed cleavage site, variable modifications of carbamidomethyl (C) and oxidation (M), 0.8 Da for MS peptide tolerance and 0.8 Da for MS/MS tolerance. The threshold was set at p < 0.05 with 95% significance. Identification was considered positive at least two peptide matched. Three replications were performed for protein identification.
B cell epitope prediction
The potential linear B cell epitopes from SVSP sequences were identified by the prediction algorithm of Bepipred version 3.0 (https://services.healthtech.dtu.dk/services/BepiPred-3.0/)23 The threshold score used was the default of 0.1512. Moreover, various methods for predicting continuous epitope provided by the Immune Epitope Database (IEDB) (http://tools.iedb.org/main/bcell/) were used to assess the physicochemical properties of residues in a protein sequence. The hydrophilicity score was predicted by Parker Hydrophilicity Prediction24. Emini surface accessibility was utilized to compute the surface accessibility scale25. The flexibility of protein structure was predicted by Karplus and Schulz flexibility scale26.
Structure alignment
The 3D protein structures of BATXSVSP15 and serine protease 5 were retrieved from the AlphaFold Structure Database (https://alphafold.ebi.ac.uk/) using their Uniprot IDs, which are A0A1L8D5W3 and T1DGZ7, respectively27. The average per-residue model confidence score (pLDDT) was 89.31 for BATXSVSP1 and 87.30 for serine protease 5, which indicates a high level of model confidence. The structural similarity between two protein structures was computed using the Pairwise Structure Alignment tool from the RCSB Protein Data Bank (PDB) with rigid body alignment (https://www.rcsb.org/alignment)28.
Statistical analysis
Quantitative data are presented as mean ± SEM. Statistical significance between groups was analyzed using standard t-tests or two-way ANOVA followed by the Bonferroni test. Significant p-values are indicated within the figure panels.
Ethics approval
All methodologies conducted in this study were approved by the Mahidol University Institutional Biosafety Committee (MUSC2023-A009). All procedures related to snake care and venom collection were approved by the Ethics and Animal Welfare Committees of the Queen Saovabha Memorial Institute, Thai Red Cross Society (QSMI-ACUC-02-2018), and the Animal Ethics Committee, Faculty of Science, Mahidol University (MUSC66-049-679). This study is performed in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines.
Results
Immunoreactivity levels of Trimeresurus albolabris and Tropidolaemus wagleri venoms to available antivenom by indirect ELISA
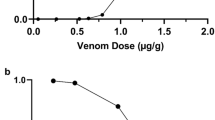
Both readily available monovalent antivenom against Trimeresurus albolabris venom and polyvalent (trivalent) antivenom against hematotoxin were used in this study. Protein concentrations in monovalent antivenom and polyvalent antivenom were 9.8 mg/ml and 20.1 mg/ml, respectively. Trimeresurus albolabris venom exhibited comparable immunoreactivities to both antivenoms in a dose-dependent manner (Fig. 1A). The EC50 of a homospecific monovalent antivenom was similar to that of polyvalent antivenom (Table 1). The overall immunoreactive levels of Tropidolaemus wagleri venom to both antivenoms were lower than those detected in Trimeresurus albolabris venom (Fig. 1B). The reactivity of Tropidolaemus wagleri venom to polyvalent antivenom was greater than that to monovalent antivenom (Table 1). Further experiments on 2DE, immunoblotting and identification of reactive proteins were conducted using this hemato polyvalent antivenom.
Immunoreactivities of homospecific monovalent antivenom and hemato polyvalent antivenom to Trimeresurus albolabris (A) and Tropidolaemus wagleri (B) venoms by indirect ELISA. Data were means ± SEM of triplicates, representative of two independent experiments, * p < 0.05, comparing between monovalent and polyvalent antivenom.
Protein identification from 2DE gel and immunoblotting of Trimeresurus albolabris and Tropidolaemus wagleri venom by LC-MS/MS analysis
The silver stain of 2DE gel revealed a total of 235 spots within Trimeresurus albolabris venom. All spots were numbered, grouped and alphabetically listed from A–P (Fig. 2A). Each group contained one to several spots with similar mass, or similar mass and pI. After being digested, proteins in all spots were identified and categorized by LC-MS/MS into 9 different families: SVMP, PLA2, SVSP, phosphodiesterase, LAAO, C-type lectin, cysteine-rich secretory protein (CRSP), 5’-nucleotidase, and fibrinogenase and other toxins.
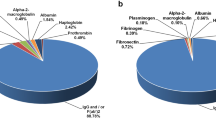
The immunoblot of 2DE showed that 67 spots, located in groups A–D, F–H and O were immunologically reactive with hemato polyvalent antivenom (Fig. 2B). Among all immunoreactive proteins, the most abundant protein types were SVMP (48%), followed by SVSP (30%). Other reactive protein types included coagulation factors and other toxins (8%), 5’-nucleotidase (8%) and PLA2 (6%) (Fig. 2C). A profile of reactive proteins is reported in Table 2 and Supplementary Table 1. Nonetheless, there were 168 protein spots in Trimeresurus albolabris venom that were unrecognized by hemato polyvalent antivenom. By comparing the immunoblot result (Fig. 2B) and the silver staining of 2DE gel (Fig. 2A), we were able to identify the non-immunoreactive proteins. It is also notable that almost all proteins with molecular mass lower than 30 kDa and/or pI higher than 8 (which were located in groups E, I–N and P) did not react with antivenom. PLA2, C-type lectin and SVMP were prominent non-immunoreactive protein types (24%, 20% and 15%, respectively). Other unrecognizable proteins included SVSP (12%), phosphodiesterase (12%), LAAO (7%) and coagulation factors (9%) (Fig. 2D). Detailed identification of the non-immunoreactive categories in Trimeresurus albolabris venom is provided in Table 3 Supplementary Table 2.
Two-dimensional (2DE) separations and immunoblot analysis of Trimeresurus albolabris venom. 2DE gels stained with silver stain, spots were numbered, grouped and alphabetically listed from A–P (A). 2DE immunoblot of Trimeresurus albolabris proteins probed with hemato polyvalent antivenom (B). Identification and relative classification of immunoreactive proteins (C) and non-immunoreactive proteins (D) in Trimeresurus albolabris venom .
Compared to Trimeresurus albolabris venom, Tropidolaemus wagleri venom contained fewer protein constituents, with the 2DE gel showing 72 protein spots (Fig. 3A). These were analyzed and grouped into categories A–K. Based on the immunoblotting results, 41 spots were found to react with hemato polyvalent antivenom, predominantly distributed in groups A–F (Fig. 3B). The prominent immunoreactive proteins within Tropidolaemus wagleri venom were LAAO (29%) SVSP (23%) and SVMP (19%). The others included phosphodiesterase (17%), PLA2 (4%), coagulation factors (4%) and 5’-nucleotidase (4%) (Fig. 3C). A full list of protein profiles is shown in Table 4 and Supplementary Table 3. Non-immunoreactive proteins in Tropidolaemus wagleri venom were represented by 31 spots, with the most frequent types being SVSP (41%), followed by PLA2 (27%), SVMP (11%), CRSP (8%), coagulation factors (6%) and C-type lectin (5%) (Fig. 3D). A detailed list of non-immunoreactive toxic proteins is provided in Table 5 and Supplementary Table 4.
2DE separations and immunoblot analysis of Tropidolaemus wagleri venom. 2DE gels stained with silver stain, spots were numbered, grouped and alphabetically listed from A–K (A). 2DE immunoblot of Tropidolaemus wagleri probed with hemato polyvalent antivenom (B). Identification and relative classification of immunoreactive proteins (C) and non-immunoreactive proteins (D) in Tropidolaemus wagleri venom.
Determination of potential SVSP epitope regions
LC-MS/MS results revealed that a high number of SVSPs were immunoreactive in Trimeresurus albolabris venom, while several were categorized as non-reactive in Tropidolaemus wagleri venom. This suggests that serine protease in Trimeresurus albolabris venom may have antigenic regions recognizable by antivenom antibodies, whereas serine protease in Tropidolaemus wagleri venom may not. In order to investigate the antigenic regions in serine protease, the best-matched SVSP from the immunoreactive group in Trimeresurus albolabris venom and the non-immunoreactive group in Tropidolaemus wagleri venom were selected for immunoinformatics analysis (Table 6).
The protein sequences were obtained from the UniProtKB database. The probability of B cell epitope of SVSP sequences from two species was predicted by Bepipred version 3.0. Given that B cell epitopes typically range between 5 and 30 amino acids, the focus was on the longest region with scores above the threshold, differing between the immunoreactive and non-immunoreactive SVSP, as differences in amino acid sequences contribute to antigenic properties.
The predicted results indicated that amino acid residues 80–110 contained many consecutive residues exceeding the threshold score. The scores in this region were generally higher for the immunoreactive SVSP compared to the non-immunoreactive SVSP (Fig. 4). The average score for the full sequence length showed a slight increase for the immunoreactive SVSP, with a more significant difference observed in the average score for amino acid residues 80–110 (Table 7), suggesting that residues 80–110 potentially influence the antigenic property.
The superimposed 3D structure of two SVSP proteins from Trimeresurus albolabris venom and Tropidolaemus wagleri venom revealed that amino acid residues 80–110 contain two loops. The loop region with amino acid residues 80–90 was defined as Loop 1, and the loop with amino acid residues 100–110 was defined as Loop 2 (Fig. 5). Loop regions in proteins are known to play crucial roles in recognition sites, protein-protein interactions and protease inhibitors. The alignment of full-length sequences showed lower RMSD, higher TM-score and higher percent identity compared to the alignment of amino acid residues 80–110, indicating higher similarity in the full-length alignment of the two serine protease proteins. This supports the aforementioned hypothesis that amino acid residues 80–110 differentiate the antigenic properties of SVSP in Trimeresurus albolabris venom from Tropidolaemus wagleri venom (Table 8).
Analysis of antigenic properties in the selective region of Serine protease
The sequence of amino acid residues 80–110 from immunoreactive SVSP in Trimeresurus albolabris venom and non-immunoreactive SVSP in Tropidolaemus wagleri venom was selectively emphasized to assess physicochemical properties related to antigenic ability, including hydrophilicity, surface accessibility and flexibility, using a computational approach.
Parker Hydrophilicity Prediction was used to predict the hydrophilicity of amino acid residues in the selective region. The predicted hydrophilic scores of amino acid residues in the Loop 1 region were similar between immunoreactive and non-immunoreactive proteins. However, the predicted scores in Loop 2 of the immunoreactive were higher than those in the non-immunoreactive SVSP. This prediction indicates that the residues in the Loop 2 region of the immunoreactive SVSP from Trimeresurus albolabris venom have more potential to be exposed to antibodies in antivenom than those in the non-immunoreactive SVSP in Tropidolaemus wagleri venom (Fig. 6A).
Emini Surface Accessibility Prediction was used to evaluate the surface accessibility of amino acid residues, which can indicate their ability to interact with other molecules. The predicted scores of amino acid residues in Loop 1 and Loop 2 of the immunoreactive SVSP were generally higher than those in the non-immunoreactive protein. Specifically, residues 102–107 in the Loop 2 region of the immunoreactive SVSP in Trimeresurus albolabris venom surpassed the threshold, while those in the non-immunoreactive SVSP in Tropidolaemus wagleri venom were close to zero (Fig. 6B). Karplus & Schulz Flexibility Prediction was used to analyze the structural flexibility contributed by amino acid residues in the selective region, as flexibility in protein structure influences the protein’s ability to perform binding interactions. The predicted flexibility scores of amino acid residues in Loop 1 were very similar between the two SVSP proteins from Trimeresurus albolabris and Tropidolaemus wagleri. However, residues 102–107 in Loop 2 of the immunoreactive SVSP in Trimeresurus albolabris venom exceeded the threshold score, while those in Tropidolaemus wagleri venom did not (Fig. 6C).
Altogether, the analysis of physicochemical properties demonstrates the antigenic potential in the Loop 2 region of SVSP in Trimeresurus albolabris venom, especially for residues 102–107. A detailed inspection of the Loop 2 region was conducted to investigate the amino acid properties that account for the differences in antigenicity between SVSP proteins from Trimeresurus albolabris and Tropidolaemus wagleri venoms.
The B cell epitope physicochemical properties analysis in the region of residues 80–110 in immunoreactive SVSP of Trimeresurus albolabris venom and the non-immunoreactive SVSP of Tropidolaemus wagleri venom (A). Parker hydrophilicity prediction (B). Emini surface accessibility prediction (C). Karplus & Schulz flexibility prediction.
As shown in the superimposed structures of the region encompassing amino acid residues 100–110 (Fig. 7), the amino acids in Loop 2 do not align well, resulting in different orientations of this region in the immunoreactive and non-immunoreactive SVSP. The structure of the immunoreactive SVSP from Trimeresurus albolabris venom protrudes from the protein surface due to hydrophilic residues Lys102, Asp104 and Asp105. In contrast, the Loop 2 structure of the non-immunoreactive SVSP from Tropidolaemus wagleri venom curves inward, as residues Thr105, Tyr106 and Thr107 lack hydrophilic side chains. The presence of hydrophilic side chains in the Loop 2 region promotes a protruding structure that potentially enhances exposure to antibodies, facilitating binding interactions.
Discussion
The administration of antivenom has long been used as a mainstay antibody-based therapeutic approach for snake-envenomed victims. Fatality prevention and mitigation of permanent disabilities depends largely on the neutralizing efficacy of antivenom. Currently, the readily available antivenoms for the treatment of green pit viper envenomation in Thailand have been manufactured as purified F(ab’)2 fragments from whole horse IgG by QSMI, Thai Red Cross Society. Both monovalent antivenom as well as the polyvalent antivenom exhibited comparable reactivity against Trimeresurus albolabris and Tropidolaemus wagleri venoms. The binding of the hemato polyvalent antivenom to Trimeresurus albolabris venom exhibited a comparable range of immunoreactivity to the findings previously reported by Tan et al. in 2019. However, our reactivity level was slightly higher, with an EC50 value of 0.202 µg/ml compared to the reported EC50 value of 0.7 µg/ml. It is important to consider that the variation in antibody affinity across different batches and potential dietary differences may contribute to the observed variations in the immunobinding results. The antibodies found in the polyvalent antivenom, which were generated in response to venoms from Calloselasma rhodostoma and Daboia siamensis, showed limited cross-reactivity. This implies that there are variations in the antigenic proteins present in the venoms of those two snake species and in Trimeresurus albolabris and Tropidolaemus wagleri venoms which may be attributed to their distant phylogenetic relationship among these vipers29.
The immuoreactivity level of antivenom to Tropidolaemus wagleri venom was lower than that to Trimeresurus albolabris venom. The immunoreactive spots within the areas of A-C and F&G of Trimeresurus albolabris venom were commonly detected as in those with the area of A-F of Tropidolasemus wagleri venom. Shared antigenic proteins are found to be several members of enzymatic SVMP and SVSP. SVMP are well known for hemorrhage induction through basement membrane component damage and enhanced blood clotting factor cleavage causing coagulopathy30,31. SVSP also plays a critical role as a thrombin-like enzyme in fibrinogen degradation, leading to hypofibrinemia32. The immunoreacivity and neutralizing capacity of hemato polyvalent antivenom against venom of Trimeresurus albolabris and several Southeast Asian species of Trimeresurus has clearly been investigated33,34. In addition, the efficacy of coagulotoxicity neutralization of antivenom which largely counteracts SVMP, SVSP enzymatic functions has been already demonstrated by in vitro35 and in vivo models10. The immunobinding interaction between antivenoms and Tropidolaemus wagleri venom was observed. However, previous evidence presented by Leong et al. in 2014 demonstrated that the polyvalent antivenom lacked neutralizing capacity against this venom36. It is important to note that a high immunoreactive capacity does not necessarily correlate with the ability to neutralize toxins, as exemplified by recent findings in the case of the sharp-nosed pit viper (Deinagkistrodon acutus) reported by Tan et al. 202237. Additionally, it is plausible that Tropidolaemus wagleri venom contains unique low-molecular-weight Waglerin compounds that are not immunologically recognized by the antivenoms used in this particular study. The abundance of Waglerin has been previously investigated ranging from 1%38 to 15–34%14,15 depending on quantification approach and database accessibility39. In this context, we used the public NCBI snake database, where very limited Tropidolaemus proteome numbers were uploaded, hence we were unable to precisely calculate proportion of Waglerin in our work. This contrasts with other previous studies where SwissProt, Serpentes15 and an in-house database14. Different degrees of glycosylation between the venoms of these two species may also contribute to their varying immunoreactivity with antivenom. Studies on the glycoproteomes of Bothrops venoms have revealed significant variability in glycan structures, including sialic acid modifications, which influence venom composition and antibody interactions40. A similar phenomenon has been observed in glycosylated metalloproteases from the venoms of Dispholidus typus and Thelotornis mossambicanus, where glycan patterns affect enzymatic activity and resistance to antivenom neutralization41. Additionally, lectin-bound glycoproteins from different snake families, such as Elapidae and Viperidae, exhibit distinct glycosylation patterns that may impact antibody affinity and recognition42. The role of glycosylation in Trimeresurus and Tropidolaemus venoms requires further investigation.
Our analytical procedures relied on the reducing 2DE gel electrophoresis (12% gel), which could potentially deteriorate the native structure of antigenic proteins, rendering them less immunologically reactive to antivenoms. This could be possible due to this factor that Tropidolaemus wagleri venom toxins reported by Tan et al., 2017b were identified as non-immunoreactive proteins. This also could be the case especially for proteins with low molecular mass (less than 30 kDa), since the immunocharacterization of Trimesurus albolabris venom against hemato polyvalent antivenom by immunoblotting of non-reducing gel exhibited the lowest reactive bands at about 25 kDa43. However, weak immunoreaction of low molecular weight constituent proteins (i.e., PLA2 and C-type lectins) against specific immune horse sera indicating their low immunogenicity has previously been observed in Bothrops jararaca and Bothrops jararacussu venoms44. The enhanced immunostimulatory regime using hepatitis B core antigen virus-like particles could induce immunogenicity of these proteins45.
Our study detected an array of PLA2 and C-type lectins in the non-immunoreactive moiety. Naturally occurring PLA2 in snake venom have low molecular weights, ranging between 13 and 15 kDa46. This corresponds with our results showing that the several PLA2 identities from Trimeresurus albolabris venom were present in groups L, N and P of 2DE immunoblotting. Generally, functional PLA2 hydrolyses the ester bonds in membrane phospholipids at the sn-2 position, releasing an array of free fatty acids including oleic acids and arachinidonic acids47. They are known as biological mediators in various mechanisms including host NF-κB pathway triggering inflammatory responses48. The deteriorating effects of PLA2 on plasma membrane fluidity and permeability inevitably leads to cell death49. Elapid and hydrophid snake venoms contain group I PLA2, while group II PLA2 is commonly found in the venom of viperideans. The latter group includes acidic and basic forms, which are among non-immunorecognized proteins. Therefore, PLA2 potentially contribute to prolonged inflammatory symptoms in snake-envenoming survivors despite the administration of antivenom50.
We were able to detect C-type lectins as well as snaclecs in non-immunological proteins, particularly in groups K and M from immunoblots of 2DE gel. They possess low molecular size, similar to PLA251,52. C-type lectins in snake venom are known to cause platelet aggregation disorders and thrombocytopenia1. Among those, we documented an array of galactose-binding lectins similar to those found in Bothrops spp53. , Protobothrop spp54. and Trimeresurus spp. venoms9. They were previously found to interact with blood coagulation factors, inhibiting platelet aggregation and coagulation55,56. SVSP, SVMP and LAAO were predominately recognized by hemato polyvalent antivenom. However, different SVSP members including thrombin-like enzymes and fibrinogenase were immunologically unrecognizable. Some SVSP were identical to those found in venoms of Bothrops spp. (e.g., Bothrops atrox)57 and Crotalus spp. (e.g., Crotalus horridus)58, which are well known in venom-induced coagulopathy and disturbing homeostasis. Although only weak coagulopathic effect and thrombin-like enzyme activity have been observed in Tropidolaemus wagleri venom13, the possibility of non-reactive SVSP to trigger inflammatory responses is worth consideration59.
As mentioned, SVSP is one of the enzymatic proteins that play an important role in hypofibrinemia. The results of this study showed that several SVSP are the antigenic proteins in Trimeresurus albolabris venom and high numbers of SVSP are identified in the non-reactive proteins of Tropidolaemus wagleri venom. All parameters indicating the antigenic properties in the residues of the Loop 2 structure in immunoreactive SVSP of Trimeresurus albolabris were more immunogenic compared to non-immunoreactive SVSP in Tropidolaemus wagleri venom. The low antigenic ability of SVSP in Tropidolaemus wagleri venom was explained by the hydrophilic side chains of the residues in Loop 2, leading to a buried conformation. Loops in a protein are patternless structures that link two secondary structures and are often located on the protein’s surface, playing important roles such as interacting with other biological objects23. Hence, the Loop 2 region examined in this study could possibly be a significant epitope in SVSP since the differences in this region differentiate the reactivity levels between SVSP proteins in the immunoreactive group of Trimeresurus albolabris and the non-immunoreactive group of Tropidolaemus wagleri. Even though the analysis of immunogenicity and protein structure in this study was solely done by computational approaches, immunoinformatics has been used in a 2023 study by Hiu et al. That study identified potential epitopes in the three-finger loops of cobra venom cytotoxin by immunoinformatics and epitope-omic analyses. Functional tests by site-directed mutagenesis confirmed that those epitopes are located at the functional loop and are responsible for dermonecrosis in snakebite victims60.
Using 2DE immunoblot and advanced proteomic technology, we were able to provide antigenic identification of proteins in Trimeresurus albolabris and Tropidolaemus wagleri venom. Additionally, lists of non-immunologically reactive proteins are now available, which can facilitate the development of next-generation pit viper antivenom. By integrating these lists with the application of immunoinformatics and protein structure characterization, we can aid in the design of specific therapies against non-recognized toxins to alleviate snakebite symptoms.
Data availability
All the mass spectrometry raw data have been deposited in the Science Data Bank repository with accession number 10.57760/sciencedb.18819 (https://www.scidb.cn/en/anonymous/VmJlaUVq).
References
Gutierrez, J. M. et al. Snakebite envenoming. Nat. Rev. Dis. Primers 3, 17063. https://doi.org/10.1038/nrdp.2017.63 (2017).
Vanuopadath, M., Shaji, S. K., Raveendran, D., Nair, B. G. & Nair, S. S. Delineating the venom toxin arsenal of Malabar pit Viper (Trimeresurus malabaricus) from the Western Ghats of India and evaluating its immunological cross-reactivity and in vitro cytotoxicity. Int. J. Biol. Macromol. 148, 1029–1045. https://doi.org/10.1016/j.ijbiomac.2020.01.226 (2020).
Namal Rathnayaka, R., Ranathunga, P. & Kularatne, S. A. M. Epidemiology and clinical features of green pit Viper (Trimeresurus trigonocephalus) envenoming in Sri Lanka. Toxicon 137, 99–105. https://doi.org/10.1016/j.toxicon.2017.07.017 (2017).
Thein, M. M. et al. Characteristics and significance of green snake bites in Myanmar, especially by the pit Vipers trimeresurus Albolabris and trimeresurus erythrurus. Toxicon 203, 66–73. https://doi.org/10.1016/j.toxicon.2021.09.008 (2021).
Blessmann, J. et al. Incidence of snakebites in 3 different geographic regions in Thua Thien Hue Province, central Vietnam: Green pit Vipers and cobras cause the majority of bites. Toxicon 156, 61–65. https://doi.org/10.1016/j.toxicon.2018.11.009 (2018).
Pakmanee, N., Khow, O., Wongtongkam, N., Omori-Satoh, T. & Sitprija, V. Efficacy and cross reactivity of Thai green pit Viper antivenom among venoms of trimeresurus species in Thailand and Japan. J. Nat. Toxins. 7, 173–183 (1998).
Hutton, R. A. et al. Arboreal green pit Vipers (genus Trimeresurus) of South-East Asia: Bites by T. albolabris and T. macrops in Thailand and a review of the literature. Trans. R Soc. Trop. Med. Hyg. 84, 866–874. https://doi.org/10.1016/0035-9203(90)90111-q (1990).
Thumtecho, S. et al. Hematotoxic manifestations and management of green pit Viper bites in Thailand. Ther. Clin. Risk Manag. 16, 695–704. https://doi.org/10.2147/TCRM.S261303 (2020).
Kumkate, S. et al. Venomics and cellular toxicity of Thai pit Vipers (Trimeresurus macrops and T. hageni). Toxins (Basel) 12. https://doi.org/10.3390/toxins12010054 (2020).
Liew, J. L., Tan, N. H. & Tan, C. H. Proteomics and preclinical antivenom neutralization of the Mangrove pit Viper (Trimeresurus purpureomaculatus, Malaysia) and white-lipped pit Viper (Trimeresurus albolabris, Thailand) venoms. Acta Trop. 209, 105528. https://doi.org/10.1016/j.actatropica.2020.105528 (2020).
Anita, S. et al. Venom composition of trimeresurus Albolabris, T. insularis, T. puniceus and T. purpureomaculatus from Indonesia. J. Venom. Anim. Toxins Incl. Trop. Dis. 28, e20210103. https://doi.org/10.1590/1678-9199-jvatitd-2021-0103 (2022).
Vogel, G., David, P., Lutz, M., Van Rooijen, J. & Vidal, N. Revision of the tropidolaemus wagleri-complex (Serpentes: Viperidae: Crotalinae). I. Definition of included taxa and redescription of tropidolaemus wagleri (Boie, 1827). Zootaxa 1644(1–40), 41–40 (2007).
Tan, N. H. & Tan, C. S. The enzymatic activities and lethal toxins of trimeresurus wagleri (speckled pit viper) venom. Toxicon 27, 349–357. https://doi.org/10.1016/0041-0101(89)90182-7 (1989).
Tan, C. H., Tan, K. Y., Yap, M. K. & Tan, N. H. Venomics of tropidolaemus Wagleri, the sexually dimorphic temple pit Viper: Unveiling a deeply conserved atypical toxin arsenal. Sci. Rep. 7, 43237. https://doi.org/10.1038/srep43237 (2017).
Zainal Abidin, S. A. et al. Proteomic characterization and comparison of Malaysian tropidolaemus wagleri and cryptelytrops purpureomaculatus venom using Shotgun-Proteomics. Toxins (Basel) 8. https://doi.org/10.3390/toxins8100299 (2016).
Lin, W. W., Smith, L. A. & Lee, C. Y. A study on the cause of death due to waglerin-I, a toxin from trimeresurus wagleri. Toxicon 33, 111–114. https://doi.org/10.1016/0041-0101(94)00134-t (1995).
McArdle, J. J. et al. Waglerin-1 selectively blocks the epsilon form of the muscle nicotinic acetylcholine receptor. J. Pharmacol. Exp. Ther. 289, 543–550 (1999).
Debono, J. et al. Viper venom Botox: The molecular origin and evolution of the Waglerin peptides used in Anti-Wrinkle skin cream. J. Mol. Evol. 84, 8–11. https://doi.org/10.1007/s00239-016-9764-6 (2017).
Tan, C. H. et al. Cross reactivity and lethality neutralization of venoms of Indonesian trimeresurus complex species by Thai green pit Viper antivenom. Toxicon 140, 32–37. https://doi.org/10.1016/j.toxicon.2017.10.014 (2017).
Chanhome, L., Jintakune, P., Wilde, H. & Cox, M. J. Venomous snake husbandry in Thailand. Wilderness Environ. Med. 12, 17–23. https://doi.org/10.1580/1080-6032(2001)012[0017:vshit]2.0.co;2 (2001).
Sitprija, S. et al. Proteomics and immunocharacterization of Asian mountain pit Viper (Ovophis monticola) venom. PLoS One. 16, e0260496. https://doi.org/10.1371/journal.pone.0260496 (2021).
Danpaiboon, W. et al. Ophiophagus Hannah Venom: proteome, components bound by Naja kaouthia antivenin and neutralization by Naja kaouthia neurotoxin-specific human ScFv. Toxins (Basel) 6, 1526–1558. https://doi.org/10.3390/toxins6051526 (2014).
Clifford, J. N. et al. BepiPred-3.0: Improved B-cell epitope prediction using protein Language models. Protein Sci. 31, e4497. https://doi.org/10.1002/pro.4497 (2022).
Parker, J. M., Guo, D. & Hodges, R. S. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: Correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry 25, 5425–5432. https://doi.org/10.1021/bi00367a013 (1986).
Emini, E. A., Hughes, J. V., Perlow, D. S. & Boger, J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J. Virol. 55, 836–839. https://doi.org/10.1128/JVI.55.3.836-839.1985 (1985).
Kolaskar, A. S. & Tongaonkar, P. C. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 276, 172–174. https://doi.org/10.1016/0014-5793(90)80535-q (1990).
Jumper, J. et al. Highly accurate protein structure prediction with alphafold. Nature 596, 583–589. https://doi.org/10.1038/s41586-021-03819-2 (2021).
Berman, H. M. et al. The protein data bank. Nucleic Acids Res. 28, 235–242. https://doi.org/10.1093/nar/28.1.235 (2000).
Alencar, L. R. V. et al. Diversification in vipers: Phylogenetic relationships, time of divergence and shifts in speciation rates. Mol. Phylogenet Evol. 105, 50–62. https://doi.org/10.1016/j.ympev.2016.07.029 (2016).
Yamashita, K. M., Alves, A. F., Barbaro, K. C. & Santoro, M. L. Bothrops jararaca venom metalloproteinases are essential for coagulopathy and increase plasma tissue factor levels during envenomation. PLoS Negl. Trop. Dis. 8, e2814. https://doi.org/10.1371/journal.pntd.0002814 (2014).
Olaoba, O. T., Dos Santos, K., Selistre-de-Araujo, P., de Ferreira, D. H. & H. S. & Snake venom metalloproteinases (SVMPs): A structure–function update. Toxicon X 7, 100052. https://doi.org/10.1016/j.toxcx.2020.100052 (2020).
Mukherjee, A. K. & Mackessy, S. P. Biochemical and Pharmacological properties of a new thrombin-like Serine protease (Russelobin) from the venom of Russell’s Viper (Daboia russelii russelii) and assessment of its therapeutic potential. Biochim. Biophys. Acta 1830, 3476–3488. https://doi.org/10.1016/j.bbagen.2013.02.007 (2013).
Tan, N. H., Choy, S. K., Chin, K. M. & Ponnudurai, G. Cross-reactivity of monovalent and polyvalent trimeresurus antivenoms with venoms from various species of trimeresurus (lance-headed pit viper) snake. Toxicon 32, 849–853. https://doi.org/10.1016/0041-0101(94)90010-8 (1994).
Tan, C. H. et al. Venomics of trimeresurus (Popeia) nebularis, the Cameron highlands pit Viper from Malaysia: Insights into venom proteome, toxicity and neutralization of antivenom. Toxins (Basel) 11. https://doi.org/10.3390/toxins11020095 (2019).
Debono, J., Bos, M. H. A., Frank, N. & Fry, B. Clinical implications of differential antivenom efficacy in neutralising coagulotoxicity produced by venoms from species within the arboreal Viperid snake genus trimeresurus. Toxicol. Lett. 316, 35–48. https://doi.org/10.1016/j.toxlet.2019.09.003 (2019).
Leong, P. K. et al. Cross neutralization of common Southeast Asian Viperid venoms by a Thai polyvalent snake antivenom (Hemato polyvalent snake Antivenom). Acta Trop. 132, 7–14. https://doi.org/10.1016/j.actatropica.2013.12.015 (2014).
Tan, K. Y., Shamsuddin, N. N. & Tan, C. H. Sharp-nosed pit Viper (Deinagkistrodon acutus) from Taiwan and China: A comparative study on venom toxicity and neutralization by two specific antivenoms across the Strait. Acta Trop. 232, 106495. https://doi.org/10.1016/j.actatropica.2022.106495 (2022).
Weinstein, S. A., Schmidt, J. J., Bernheimer, A. W. & Smith, L. A. Characterization and amino acid sequences of two lethal peptides isolated from venom of Wagler’s pit Viper, trimeresurus wagleri. Toxicon 29, 227–236. https://doi.org/10.1016/0041-0101(91)90107-3 (1991).
Damm, M., Hempel, B. F., Nalbantsoy, A. & Sussmuth, R. D. Comprehensive snake venomics of the Okinawa Habu pit Viper, protobothrops flavoviridis, by complementary mass spectrometry-guided approaches. Molecules 23. https://doi.org/10.3390/molecules23081893 (2018).
Bras-Costa, C. et al. Profilings of subproteomes of lectin-binding proteins of nine Bothrops venoms reveal variability driven by different glycan types. Biochim. Biophys. Acta Proteins Proteom. 1870, 140795. https://doi.org/10.1016/j.bbapap.2022.140795 (2022).
Debono, J., Dashevsky, D., Nouwens, A. & Fry, B. G. The sweet side of Venom: Glycosylated prothrombin activating metalloproteases from dispholidus typus (boomslang) and Thelotornis mossambicanus (twig snake). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 227, 108625. https://doi.org/10.1016/j.cbpc.2019.108625 (2020).
Nawarak, J., Phutrakul, S. & Chen, S. T. Analysis of lectin-bound glycoproteins in snake venom from the Elapidae and Viperidae families. J. Proteome Res. 3, 383–392. https://doi.org/10.1021/pr034052+ (2004).
Chanhome, L. et al. Biochemical and proteomic analyses of venom from a new pit Viper, protobothrops Kelomohy. J. Venom. Anim. Toxins Incl. Trop. Dis. 28, e20210080. https://doi.org/10.1590/1678-9199-JVATITD-2021-0080 (2022).
Correa-Netto, C. et al. Immunome and venome of Bothrops jararacussu: A proteomic approach to study the molecular immunology of snake toxins. Toxicon 55, 1222–1235. https://doi.org/10.1016/j.toxicon.2009.12.018 (2010).
Menzies, S. K. et al. Virus-like particles displaying conserved toxin epitopes stimulate polyspecific, murine antibody responses capable of snake venom recognition. Sci. Rep. 12, 11328. https://doi.org/10.1038/s41598-022-13376-x (2022).
Hiu, J. J. & Yap, M. K. K. Cytotoxicity of snake venom enzymatic toxins: Phospholipase A2 and l-amino acid oxidase. Biochem. Soc. Trans. 48, 719–731. https://doi.org/10.1042/bst20200110 (2020).
Zambelli, V. O., Picolo, G., Fernandes, C. A. H., Fontes, M. R. M. & Cury, Y. Secreted phospholipases A2 from animal venoms in pain and analgesia. Toxins (Basel) 9. https://doi.org/10.3390/toxins9120406 (2017).
Teixeira, C. F., Landucci, E. C., Antunes, E., Chacur, M. & Cury, Y. Inflammatory effects of snake venom myotoxic phospholipases A2. Toxicon 42, 947–962 (2003). https://doi.org/10.1016/j.toxicon.2003.11.006
Murakami, T. et al. A [Lys49]phospholipase A2 from protobothrops flavoviridis venom induces caspase-independent apoptotic cell death accompanied by rapid plasma-membrane rupture in human leukemia cells. Biosci. Biotechnol. Biochem. 75, 864–870. https://doi.org/10.1271/bbb.100783 (2011).
Bickler, P. E. Amplification of snake venom toxicity by endogenous signaling pathways. Toxins (Basel) 12. https://doi.org/10.3390/toxins12020068 (2020).
Ning, W., Yuanyuan, L., Lipeng, Z., Xiang, L. & Chunhong, H. Targeted identification of C-type lectins in snake venom by 2DE and Western blot. Toxicon 185, 57–63. https://doi.org/10.1016/j.toxicon.2020.06.010 (2020).
Du, X. Y., Sim, D. S., Lee, W. H. & Zhang, Y. Blood cells as targets of snake toxins. Blood Cells Mol. Dis. 36, 414–421. https://doi.org/10.1016/j.bcmd.2006.03.001 (2006).
Sartim, M. A., Pinheiro, M. P., de Pádua, R. A. P., Sampaio, S. V. & Nonato, M. C. Structural and binding studies of a C-type galactose-binding lectin from Bothrops Jararacussu snake venom. Toxicon 126, 59–69. https://doi.org/10.1016/j.toxicon.2016.12.007 (2017).
Aird, S. D. et al. Quantitative high-throughput profiling of snake venom gland transcriptomes and proteomes (Ovophis okinavensis and Protobothrops flavoviridis). BMC Genom. 14, 790. https://doi.org/10.1186/1471-2164-14-790 (2013).
Larréché, S. et al. Bleeding and thrombosis: insights into pathophysiology of Bothrops Venom-related hemostasis disorders. Int. J. Mol. Sci. 22 https://doi.org/10.3390/ijms22179643 (2021).
Cezarette, G. N., Sartim, M. A. & Sampaio, S. V. Inflammation and coagulation crosstalk induced by BJcuL, a galactose-binding lectin isolated from Bothrops Jararacussu snake venom. Int. J. Biol. Macromol. 144, 296–304. https://doi.org/10.1016/j.ijbiomac.2019.12.015 (2020).
Hatakeyama, D. M. et al. Venom complexity of Bothrops atrox (common lancehead) siblings. J. Venom Anim. Toxins Incl. Trop. Dis. 26, e20200018 (2020). https://doi.org/10.1590/1678-9199-JVATITD-2020-0018
Rokyta, D. R., Margres, M. J. & Calvin, K. Post-transcriptional Mechanisms Contribute Little to Phenotypic Variation in Snake Venoms. G3 (Bethesda) 5, 2375–2382 (2015). https://doi.org/10.1534/g3.115.020578
Costa, C. R. C. et al. Edema induced by a crotalus durissus Terrificus venom Serine protease (Cdtsp 2) involves the PAR pathway and PKC and PLC activation. Int. J. Mol. Sci. 19. https://doi.org/10.3390/ijms19082405 (2018).
Hiu, J. J., Fung, J. K. Y., Tan, H. S. & Yap, M. K. K. Unveiling the functional epitopes of Cobra venom cytotoxin by immunoinformatics and epitope-omic analyses. Sci. Rep. 13, 12271. https://doi.org/10.1038/s41598-023-39222-2 (2023).
Acknowledgements
We thank David Anderson for manuscript proofreading and the Central Equipment Unit, Faculty of Tropical Medicine, Mahidol University, Thailand, for use of their proteomics facilities.
Author information
Authors and Affiliations
Contributions
OR, SS, NC, LC , SK : Conceptualization, Methodology; OR, PP, TT: Software; PL, TV, LC, NC: Resources; OR, PP, TT, GS: Investigation; PP, PL, GS, OK, JN: Data curation, Analyzation; OR, PP, LC, SK: Writing- Original draft preparation; SS, NC, SK: Supervision; OR, PP, LC, SK: Writing- Reviewing and Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Reamtong, O., Pearngam, P., Laoungbua, P. et al. Comparative in vitro immunoreactivity and protein analysis of Trimeresurus albolabris and Tropidolaemus wagleri venoms. Sci Rep 15, 12693 (2025). https://doi.org/10.1038/s41598-025-97032-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-97032-0