Abstract
Accumulating evidence has indicated that exposures to air pollution increase the odds of kidney stones. However, the previous research methods were limited. To address this gap, we employed genome-wide association studies (GWAS) datasets and Mendelian randomization (MR) to verify the causation. Applying publicly accessible summary datasets from UK Biobank, FinnGen consortium and Biobank Japan, a two-sample MR, and further multivariate MR were carried out to calculate the causality between air pollution [particulate matter 2.5 (PM2.5), PM2.5 absorbance, PM2.5–10, PM10, nitrogen dioxide, and nitrogen oxides] and kidney stone risk in three different populations (European, East Asian, and South Asian). The inverse variance weighted (IVW) was utilized for its first-step assessment, supplemented with MR-Egger, weighted median, Cochran’s Q test, MR-Egger intercept and leave-one-out analysis to ensure the robustness. Employing IVW, we discovered in the European population that PM2.5 absorbance was statistically correlated with kidney stone risk (odds ratio (OR) = 1.40; 95% confidence interval (CI), 1.01–1.94; P = 0.04), with no heterogeneity, pleiotropy, or sensitivity observed. Additionally, the MVMR result revealed the directly causative connection between a single PM2.5 absorbance and the increase in kidney stone risk (OR = 1.77, 95%CI: 1.06–2.98, p = 0.03). Our investigation proposed the correlation between PM2.5 absorbance and an increased risk of kidney stones in European populations. The control of air pollution, especially PM2.5, may have crucial implications for the prevention of kidney stones.
Similar content being viewed by others
Introduction
Kidney stones, also known as nephrolithiasis or urolithiasis, affect approximately 10% of the world. Their prevalence is increasing, and their high recurrence rate poses a substantial clinical and economic challenge to healthcare systems1. Previous studies have reported that kidney stone formation is primarily the result of a complex interplay of genetic predisposition, metabolic disorders such as obesity and diabetes mellitus, and lifestyle factors like smoking2,3. Approximately 50% of kidney stone patients may suffer a recurrence of renal colic within 10 years, and roughly over 10% may develop more relapses. In addition, kidney stones have been figured out to be linked to an increased risk of chronic and end-stage renal disease4. Despite the widespread prevalence and high recurrence of kidney stones, their pathophysiology remains poorly studied, which calls for further research for an effective prevention strategy.
Air pollutants, as a long-existing byproduct of industrial activities and human life, present a deteriorating threat to public health, classified as Group I human carcinogens by the International Agency for Research on Cancer (IARC)5. Among these pollutants, particulate matter, especially PM2.5, has been the most extensively researched. Other PM and gases like nitrogen dioxide, sulfur dioxide, and nitrogen oxides also have been frequently studied, while others receive less scientific focus6,7. PM2.5 is one of the most significant health concerns due to its toxic mixed composition and its ability to enter more deeply into the lungs compared to larger particulates. Increasing epidemiological evidence suggests that air pollution exposure is strongly related to systemic inflammation and oxidative stress, contributing to a broad spectrum of health issues, such as respiratory illnesses8, cardiovascular disorders9, damaging kidney function10, and various types of cancer7. Furthermore, air pollution’s influences on metabolism and cardiovascular fitness may promote stone formation by changing the urine composition10,11.
Some observational research indicated the causality of air pollution on renal function. By establishing an air pollution calculation point, it has previously been determined that there are positive correlations between PM, nitrogen oxides, their combined exposure, and the incidence of kidney stones12. A large Taiwanese population research has reported that high levels of PM2.5 and PM10 contributed to a low estimated glomerular filtration rate, signaling impaired renal function13. An additional investigation revealed a positive connection between PM2.5 and the formation of renal stones in Korea14. In a current investigation, a Cox proportional hazards regression model was utilized to measure the correlation between kidney stones and air pollutants, and they identified a positive association between air pollution score, PM2.5, PM10, NO2, NOx,and the risk of kidney stone15.
However, considering the inevitable methodological deficiencies of observational studies, previous research results are unable to determine causality due to the potential existence of unmeasured factors, residual confounders, or reverse causation12. Randomized controlled trials (RCTs) are a more suitable approach to resolve the methodological issues but are rarely designed to investigate the correlation between air pollutants and kidney stone risk7. The Mendelian Randomization (MR) method is increasingly applied to analyze the consistency between causal hypotheses and correlations by using genetic variants (single nucleotide polymorphisms; SNPs), which are regarded as instrumental variables (IVs)16. Because the formation of gametes adheres to Mendel’s laws of inheritance, the random distribution of alleles eliminates confounding bias17. Additionally, MR procedures could minimize the potential of confounding variables such as age, gender, lifestyle, etc7. To further explore the causality of air pollution on kidney stone risk, MR analysis was utilized based on large-scale publicly accessible genome-wide association studies (GWAS) data. STROBE-MR was used to examine and improve this study (Supplementary Table 1).
Materials and methods
Design of MR study
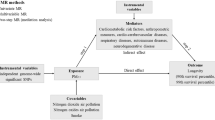
To assess the causality of air pollution on kidney stones, a two-sample MR (TSMR) analysis was designed. SNPs linked to exposures were chosen for subsequent analyses. After adjusting for the confounding effect of various air pollutants, multivariate Mendelian randomization (MVMR) was utilized to eliminate the interference of confounding factors. Three fundamental presumptions guide our MR design: (1) every IV must have a substantial correlation with air pollution; (2) every IV is irrelevant to confounding variables; (3) no IV affects kidney stones independently of air pollution. The flowchart for our MR study is illustrated in Fig. 1.
Sources of GWAS data
All GWAS summary datasets (PM2.5, PM2.5 absorbance, PM2.5–10, PM10, nitrogen dioxide, and nitrogen oxides) were derived from the UK Biobank. It’s a sizable biomedical organization collecting genetic and phenotypic data on approximately 500,000 UK individuals18. The dataset on PM2.5, PM2.5 absorbance, PM2.5–10, and PM10 included 423,796 European participants, and the data on nitrogen dioxide and nitrogen oxides was composed of 456,380 people. In East Asian, the dataset on PM2.5, PM2.5 absorbance, PM2.5–10, and PM10 included 2,505 participants, and data on nitrogen dioxide and nitrogen oxides was composed of 2,625 people. The dataset on PM2.5, PM2.5 absorbance, PM2.5–10, and PM10 included 8,567 South Asian participants, and the data on nitrogen dioxide and nitrogen oxides was composed of 8,746 South Asian participants.
The kidney stone GWAS datasets were sourced from FinnGen, Biobank Japan for East Asia, and UK Biobank for South Asia. FinnGen is a sizable public-private academic initiative that offers fresh insights into disease genetics by integrating newly acquired and existing samples from the Finnish Biobank and digital medical records from the Finnish Health Register (Supplementary Table 2). A total of 412,000 samples have been collected, and 224,737 of those have been assessed so far19. Japan Biobank has operated genome-wide association studies linked to drug reactions and disease susceptibility in the East Asian population, as well as basic infrastructure genetic research for common diseases20. All researches in its project are published on the website (https://biobankjp.org/work/public.html) and accessible to the public. For the population of East Asia, 212,453 participants (206,638 cases and 205,815 controls) were selected (GWAS ID: bbj-a- 155). South Asian participants contained 206 cases and 8,039 controls (GWAS ID: ukb-e- 594_CSA). Since the datasets were retrieved from publicly available GWAS datasets, the respective original studies were ethically approved.
Selection of instrumental variables
A series of quality control procedures were performed to extract SNPs as IVs. Firstly, to satisfy Assumption 1, SNPs that reached the threshold of statistical significance for exposure were selected as IVs21. Due to the limited number of SNPs associated with the 5 × 10−8, 1 × 10−5 was set up to select a sufficient number of SNPs for our study. Secondly, the clumping process (r2< 0.001 and kb > 10,000) was performed to eliminate potential bias caused by the linkage disequilibrium (LD) between IVs22. Thirdly, to minimize the chance of weak-instrument bias, F-statistics were calculated, and SNPs with F-statistics higher than 10 were used for subsequent MR analysis23. The detailed information of all SNPs in the study can be found in Supplementary Table 3.
Analysis of Mendelian randomization
The inverse variance weighted (IVW) approach was conducted as a principal assessment to figure out the correlation between air pollution and kidney stones (European, East Asian, and South Asian). IVW combined Wald ratios of causality between the exposure and the outcome and provided an accurate assessment of each IV24. To improve accuracy and stability, this study was supplemented with further verification by using MR-Egger and weighted median25. The MR Egger procedure was applied to figure out whether there were any pleiotropic effects on the result. It was also able to provide a consistent evaluation of the causal effect26. The weighted median analysis could estimate the causal effect if more than 50% of SNPs satisfy the criteria of no horizontal pleiotropy27. The independent causal connection between air pollution and kidney stones was examined using MVMR after accounting for the contribution of other air pollutants. A p-value of less than 0.05 in MVMR was regarded to be significant at statistical levels28.
Sensitivity analysis
Cochran’s Q-test was implemented to detect the heterogeneity between various genetic instruments for the IVW approach, with a p-value > 0.05 indicating no possible heterogeneity29. Moreover, the MR-Egger regression intercept served as a crucial predictor of the possible pleiotropy of SNPs, ensuring that IVs have no impact on the result through additional confounding factors irrelevant to pollution exposure26. There was no pleiotropy effect in IVs if the intercept was close to 0 or the p-value > 0.0526. Furthermore, after eliminating one SNP at a time, the leave-one-out analysis was performed to ascertain if a particular SNP drove the link between air pollution and kidney stones30.
The R program (version http://www.rproject.org) package ‘TwoSampleMR’ (version 0.5.11) was used for all analyses in this work.
Results
Instrumental variables extraction
Following strict selection criteria of IVs, ultimately, we screened for SNPs associated with exposures with a GWAS significance and LD clumped. In the European population, we extracted 94 SNPs correlated with PM2.5, 81 SNPs correlated with PM2.5 absorbance, 37 SNPs correlated with PM2.5–10, 55 SNPs correlated with PM10, 117 SNPs correlated with nitrogen dioxide, and 108 SNPs correlated with nitrogen oxides. In the East Asian population, 10, 7, 8, 11, and 8 SNPs for PM2.5 absorbance, PM2.5–10, PM10, nitrogen dioxide, and nitrogen oxides were extracted respectively. In the South Asian population, 28, 14, 14, 19, and 21 SNPs for PM2.5, PM2.5 absorbance, PM2.5–10, nitrogen dioxide, and nitrogen oxides were extracted, respectively. All SNPs’ F-statistics passed the standard threshold of 10, signifying that no weak instrument bias existed.
The causal links between air pollution and kidney stones
Among the European population, we observed a causal connection (odds ratio (OR) = 1.40; 95% confidence interval (CI), 1.01–1.94; P = 0.04) between PM2.5 absorbance and kidney stones in primary IVW assessment. Besides, despite no statistical significance, MR-Egger and the weighted median analysis revealed directionally consistent results with IVW. These results demonstrated that a high level of PM2.5 exposure could strongly increase the risk of kidney stones. The single PM2.5 absorbance exposure was also revealed to have a direct causal impact on the increased risk of kidney stones (OR = 1.77, 95%CI: 1.06–2.98, p = 0.03) through MVMR analysis. Additionally, among the European population, we did not reveal any causal relationships between PM2.5 (p = 0.28), PM2.5–10 (p = 0.67), PM10 (p = 0.44), nitrogen dioxide (p = 0.81), nitrogen oxides (p = 0.78) and the risk of kidney stones. In either East Asian or South Asian populations, the results exhibited no significant causality of air pollution on kidney stone risk (p > 0.05). The MR results are detailed in Fig. 2 and Supplementary Table 4.
Sensitivity analysis
To verify the reliability of the principal MR results, a set of procedures for sensitivity detection were employed. There was no heterogeneity detected in the IVW model regarding Cochran’s Q-test (Q = 92.47; P = 0.16) (Supplementary Table 5). Our analyses yielded no indication of horizontal pleiotropy through MR-Egger intercept (P > 0.05) (Supplementary Table 6). The funnel plot presented a symmetric pattern. The leave-one-out analysis indicated that no possibly specific SNP had an impact on the outcome (Fig. 3).
Discussion
There is a close association between air pollution and genetic susceptibility. This association is mainly reflected in the fact that air pollution may increase disease risk by affecting gene expression and genetic material stability. Harmful substances in the air may significantly increase the risk of disease in specific populations by interacting with genetic susceptibility. A study has shown that non-smoking women carrying GSTM1 gene deletion have a statistically significant increased risk of developing lung cancer due to exposure to environmental tobacco smoke31. In addition, air pollution may also induce oxidative stress, leading to the production of reactive oxygen species (ROS), which can cause DNA oxidative damage and promote the occurrence of cancer. Another study also reported that the levels of PAH-DNA adduct were significantly increased in individuals exposed to environmental air pollution32, further supporting the association between environmental pollution and genetic susceptibility.
In spite of earlier observational research, our study was the first to calculate the correlated causality of air pollution (including PM2.5, PM2.5 absorbance, PM2.5–10, PM10, nitrogen dioxide, and nitrogen oxides) on kidney stones in three races (European, East Asian, and South Asian). We found a causal relationship between PM2.5 absorbance and the risk of kidney stone at a genetic level in the European population, which was novel compared to the previous study12. However, residual pleiotropy might be the explanation for why the weighted median and MR-Egger test findings were of no statistical significance (P > 0.05). The stability of the result was confirmed by Cochran’s Q test, the plot of the leave-one-out analysis, and the MR-Egger intercept test. There was no evidence of statistical causality for other air pollution on kidney stones.
Earlier research has shown that higher levels of PM2.5 could contribute to oxidative stress and various inflammation indicator responses33. For instance, microalbuminuria, a prior indicator of renal injury34, could be resulted by polycyclic aromatic hydrocarbons (PAHs)35. Another study also reported that the kidney stone risk was significantly related to PAH36. PAH metabolites can generate a substantial quantity of reactive oxygen species (ROS) through metabolic processes and trigger oxidative stress36. Meanwhile, ROS participate in Calcium oxalate (CaOx) crystals, which is the most typical among all kidney stone varieties37. It also can promote renal tubular epithelial cell damage and apoptosis, resulting in crystal adhesion, and ultimately launch a kidney stone cascade38,39. Moreover, ROS can contribute to the development of kidney stones by modulating the expression of stone-related macromolecules, such as Osteopontin (OPN), monocyte chemotactic protein 1 (MCP- 1), and CD44, and by regulating multiple signaling pathways, including NF-κB and mitogen-activated protein kinase (MAPK)40. According to a recent study, PM2.5 exposure-associated oxidative stress can induce the increased renal GRK4 expression, leading to the increased AT1R level. The Ang II/AT1R signaling pathway can mediate CaOx-induced ROS and stone-related protein upregulation41. The expression of IL- 18, IL- 6, and IL- 1β was reported to increase in mice exposed to PM2.542,43, leading to a C-reactive protein(CRP) increase44. A recent finding also revealed a significant association between higher CRP levels and an increased risk of kidney stones45. In clinical practice, CRP measurement in suspected patients has been utilized as a risk indicator for stones to confirm the potential formation of stones44. Moreover, serum creatinine and uric acid content can be impacted by PM2.5, strongly contributing to the occurrence of uric acid stones46,47. The development and deterioration of obesity and diabetes48,49is known risk factors for renal stones1,50, which might be induced by air pollution. Extra research is expected for a complete comprehension of the underlying processes driving the link between air pollution and kidney stones.
Previous observational research has figured out the causality of PM2.5 on kidney stones. Nevertheless, few studies have found the positive causality of PM2.5 absorbance on kidney stones. Our results suggested the relevance of PM2.5 absorbance for kidney stone incidence, but the lack of statistical significance supports the causality of PM2.5 on kidney stone risk. The different associations with kidney stone risk could be reasonably explained by the components of PM2.5. It’s reported that various types of pathogenic pathways would be activated by carbonaceous aerosol that is mainly contained in PM2.5. It’s a complex chemical makeup including both organic and elemental carbon particles51. PM2.5 absorbance is a proxy and indication factor of elemental carbon, which comprises 50% of PM2.5 and can reflecting the carbonaceous components concentration in PM2.5. Studies have shown that PM2.5 absorbance could affect body physiologic function and lead to metabolic syndrome52. Spherical carbonaceous nanoparticle inhalation at elevated levels could trigger an inflammatory reaction53. When PM2.5 was analyzed as a whole factor, no statistical relationship with kidney stone risk was found in our results. However, a positive correlation with kidney stone risk was observed when PM2.5 absorbance was analyzed. It could be interpreted that the carbonaceous compositions in PM2.5 might be independently associated with kidney stone risk, while other components probably diminish or adversely interfere with this causality. It was a more novel and deeper understanding compared to previous studies about PM2.5 and kidney stone risk52.
There are several advantages to this study. We were the first to systematically identify a possible impact of air pollution on kidney stone using MR approaches and finally calculated a potential kidney stone risk factor (PM2.5 absorbance) in European population. Compared to previous studies about PM2.5 and kidney stone risk, we provided a more novel and deeper interpretation. Secondly, all the participants involved were of different ancestries (European, East Asian, and South Asian), which increased the generalizability of our results. Moreover, the two-sample MR method can overcome some limitations, such as confounding variables, causal inversion, and other biases. Finally, the mechanisms of PM2.5 on kidney stones have not been fully studied yet, and our results may provide new insights into prevention strategies in the future.
However, some of the study’s flaws cannot be disregarded. Firstly, it remains to be reconsidered when our findings are applied to other populations and regions. Secondly, we cannot entirely rule out the potential of residual confounding given the undiscovered or unmeasured confounding factors, even if we have taken known confounding factors into account. Thirdly, no information was available on stone composition (such as uric acid, calcium oxalate) in the GWAS datasets and consequently, whether air pollution differentially affected the development of kidney stone subtypes could not be investigated. Finally, theoretical and empirical studies are lacking to further verify related mechanisms involved in the causal effect of PM 2.5 absorbance on kidney stones.
Conclusion
In summary, we assessed the potential causal causality of air pollution on kidney stones in different populations using Mendelian Randomization. This IVW result demonstrated that PM2.5 absorbance was significantly linked with kidney stone risk among European population. Although the mechanism of PM2.5 absorbance leading to kidney stone risk remains to be explored, our findings may provide references between air pollution and human health and may contribute to making more strategies for the clinical prevention of kidney stones.
Data availability
All data could be found in the IEU OpenGWAS project (https://gwas.mrcieu.ac.uk/), Biobank Japan (https://biobankjp.org/work/public.html), and FinnGen R10 (https://r10.risteys.finngen.fi).
Abbreviations
- GAWS:
-
genome-wide association study
- PM:
-
Particulate matter
- MR:
-
Mendelian randomization
- MVMR:
-
multivariate Mendelian randomization
- TSMR:
-
two-sample Mendelian randomization
- SNPs:
-
single nucleotide polymorphisms
- IVs:
-
instrumental variables
- RCTs:
-
Randomized controlled trials
- IVW:
-
inverse variance weighting
- LD:
-
Linkage disequilibrium
- PAHs:
-
polycyclic aromatic hydrocarbons
- ROS:
-
reactive oxygen species
- CaOx:
-
Calcium oxalate
- CRP:
-
C-reactive protein
References
Singh, P. et al. The genetics of kidney stone disease and nephrocalcinosis. Nat. Rev. Nephrol. 18 (4), 224–240 (2022).
Jones, P. et al. Do lifestyle factors including smoking, alcohol, and exercise impact your risk of developing kidney stone disease?? Outcomes of a systematic review. J. Endourol. 35 (1), 1–7 (2021).
Ping, H. et al. New-onset metabolic risk factors and the incidence of kidney stones: a prospective cohort study. BJU Int. 124 (6), 1028–1033 (2019).
Zhe, M. & Hang, Z. Nephrolithiasis as a risk factor of chronic kidney disease: a meta-analysis of cohort studies with 4,770,691 participants. Urolithiasis 45 (5), 441–448 (2017).
Raaschou-Nielsen, O. et al. Air pollution from traffic and cancer incidence: a Danish cohort study. Environ. Health. 10, 67 (2011).
Kim, H. B. et al. Long-term exposure to air pollution and the risk of non-lung cancer: a meta-analysis of observational studies. Perspect. Public. Health. 140 (4), 222–231 (2020).
Xiao, H. et al. Causal association between air pollution and frailty: a Mendelian randomization study. Front. Public. Health. 11, 1288293 (2023).
Liu, S. et al. Long-term air pollution exposure and Pneumonia-related mortality in a large pooled European cohort. Am. J. Respir Crit. Care Med. 205 (12), 1429–1439 (2022).
Konduracka, E. & Rostoff, P. Links between chronic exposure to outdoor air pollution and cardiovascular diseases: a review. Environ. Chem. Lett. 20 (5), 2971–2988 (2022).
Lee, W. et al. Associations between long term air pollution exposure and first hospital admission for kidney and total urinary system diseases in the US medicare population: nationwide longitudinal cohort study. BMJ Med. 1 (1), e000009 (2022).
Afsar, B. et al. Air pollution and kidney disease: review of current evidence. Clin. Kidney J. 12 (1), 19–32 (2019).
Gan, X. et al. Positive association between ambient air pollutants and incident kidney stones. Environ. Sci. Pollut Res. Int. 30 (59), 124067–124077 (2023).
Su, W. Y. et al. Association between ambient air pollutant interaction with kidney function in a large Taiwanese population study. Environ. Sci. Pollut Res. Int. 30 (34), 82341–82352 (2023).
Noh, T. I. et al. Association of meteorological factors and ambient air pollution on medical care utilization for urolithiasis: a population-based time-series study. BMC Nephrol. 22 (1), 402 (2021).
Liu, M. et al. Air pollutants, residential greenspace, and the risk of kidney stone disease: a large prospective cohort study from the UK biobank. J. Expo Sci. Environ. Epidemiol., (2024).
Davey Smith, G. & Hemani, G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 23 (R1), R89–98 (2014).
Zheng, J. et al. Recent developments in Mendelian randomization studies. Curr. Epidemiol. Rep. 4 (4), 330–345 (2017).
Bycroft, C. et al. The UK biobank resource with deep phenotyping and genomic data. Nature 562 (7726), 203–209 (2018).
Kurki, M. I. et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 613 (7944), 508–518 (2023).
Kubo, M. & Guest, E. BioBank Japan project: epidemiological study. J. Epidemiol. 27 (3S), S1 (2017).
Burgess, S. et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open. Res. 4, 186 (2019).
Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife, 7. (2018).
Burgess, S., Thompson, S. G. & Collaboration, C. C. G. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 40 (3), 755–764 (2011).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37 (7), 658–665 (2013).
Bowden, J. et al. A framework for the investigation of Pleiotropy in two-sample summary data Mendelian randomization. Stat. Med. 36 (11), 1783–1802 (2017).
Burgess, S. & Thompson, S. G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 32 (5), 377–389 (2017).
Jiang, M. et al. Serum uric acid levels and risk of eight Site-Specific cancers: A Mendelian randomization study. Front. Genet. 12, 608311 (2021).
Hu, X. et al. Causal relationships between air pollutant exposure and bone mineral density and the risk of bone fractures: evidence from a Two-Stage Mendelian randomization analysis. Toxics, 12(1). (2023).
Bowden, J. et al. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int. J. Epidemiol. 48 (3), 728–742 (2019).
Nolte, I. M. Metasubtract: an R-package to analytically produce leave-one-out meta-analysis GWAS summary statistics. Bioinformatics 36 (16), 4521–4522 (2020).
Topinka, J. et al. Influence of GSTM1 and NAT2 genotypes on placental DNA adducts in an environmentally exposed population. Environ. Mol. Mutagen. 30 (2), 184–195 (1997).
Singh, R. et al. The relationship between biomarkers of oxidative DNA damage, polycyclic aromatic hydrocarbon DNA adducts, antioxidant status and genetic susceptibility following exposure to environmental air pollution in humans. Mutat. Res. 620 (1–2), 83–92 (2007).
Gogna, P. et al. Ambient air pollution and inflammatory effects in a Canadian pregnancy cohort. Environ. Epidemiol. 5 (5), e168 (2021).
Glassock, R. J. Is the presence of microalbuminuria a relevant marker of kidney disease? Curr. Hypertens. Rep. 12 (5), 364–368 (2010).
Singh, A. et al. Heat and PAHs emissions in indoor kitchen air and its impact on kidney dysfunctions among kitchen workers in Lucknow, North India. PLoS One. 11 (2), e0148641 (2016).
Sun, S. et al. Polycyclic aromatic hydrocarbons and the risk of kidney stones in US adults: an Exposure-Response analysis of NHANES 2007–2012. Int. J. Gen. Med. 14, 2665–2676 (2021).
Khan, S. R., Canales, B. K. & Dominguez-Gutierrez, P. R. Randall’s plaque and calcium oxalate stone formation: role for immunity and inflammation. Nat. Rev. Nephrol. 17 (6), 417–433 (2021).
Kizivat, T. et al. Antioxidant Pre-Treatment reduces the toxic effects of oxalate on renal epithelial cells in a cell culture model of urolithiasis. Int. J. Environ. Res. Public. Health, 14(1). (2017).
Thamilselvan, V., Menon, M. & Thamilselvan, S. Oxalate at physiological urine concentrations induces oxidative injury in renal epithelial cells: effect of alpha-tocopherol and ascorbic acid. BJU Int. 114 (1), 140–150 (2014).
Qin, B. et al. Losartan ameliorates calcium Oxalate-Induced elevation of Stone-Related proteins in renal tubular cells by inhibiting NADPH oxidase and oxidative stress. Oxid. Med. Cell. Longev. 2018, p1271864 (2018).
Hu, C. et al. Paternal long-term PM2.5 exposure causes hypertension via increased renal AT1R expression and function in male offspring. Clin. Sci. (Lond). 135 (22), 2575–2588 (2021).
Wu, X. et al. 2-Undecanone protects against fine Particle-Induced kidney inflammation via inducing mitophagy. J. Agric. Food Chem. 69 (17), 5206–5215 (2021).
Chen, X. et al. Protective effect of lutein on oxidative stress damage caused by acute PM2.5 exposure in rats. Ann. Palliat. Med. 9 (4), 2028–2036 (2020).
Capolongo, G., Ferraro, P. M. & Unwin, R. Inflammation and kidney stones: cause and effect? Curr. Opin. Urol. 33 (2), 129–135 (2023).
Liang, D., Liu, C. & Yang, M. The association between C-reactive protein levels and the risk of kidney stones: a population-based study. BMC Nephrol. 25 (1), 39 (2024).
Trinchieri, A. & Montanari, E. Biochemical and dietary factors of uric acid stone formation. Urolithiasis 46 (2), 167–172 (2018).
Xu, M. X. et al. Activated iRhom2 drives prolonged PM(2.5) exposure-triggered renal injury in Nrf2-defective mice. Nanotoxicology 12 (9), 1045–1067 (2018).
Sun, Q. et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 119 (4), 538–546 (2009).
Furlong, M. A. & Klimentidis, Y. C. Associations of air pollution with obesity and body fat percentage, and modification by polygenic risk score for BMI in the UK biobank. Environ. Res. 185, 109364 (2020).
Wigner, P. et al. The molecular aspect of nephrolithiasis development. Cells, 10(8). (2021).
Martins, V. et al. Origin of inorganic and organic components of PM2.5 in subway stations of Barcelona, Spain. Environ. Pollut. 208 (Pt A), 125–136 (2016).
Liu, C. et al. Causal relationship between particulate matter 2.5 (PM(2.5)), PM(2.5) absorbance, and COVID-19 risk: A two-sample Mendelian randomisation study. J. Glob Health. 13, 06027 (2023).
Andre, E. et al. Inhalation of ultrafine carbon particles triggers biphasic pro-inflammatory response in the mouse lung. Eur. Respir J. 28 (2), 275–285 (2006).
Acknowledgements
As for air pollution GWAS data, we would like to thank Ben Elsworth et al. for their summary in European population, and Pan-UKB team for their summary in East and South Asian population. Also, we would like to thank FinnGen Risteys 10 for calculus of kidney and ureter data in European population, Ishigaki K et al. for urolithiasis data in East Asian population, and Pan-UKB team for urinary calculus data in South Asian population. Finally, we would like to thank the participants and researchers at the UK Biobank.
Funding
There is no funding for this study.
Author information
Authors and Affiliations
Contributions
S.P.L. conceived and designed the study. A.J.M. and Z.N.J drafted the manuscript. Z.Y.L. and X.F.C. contributed to data visualization. J.C.G. contributed to the availability of data and analysis. Y.J.X. and Q.G. made critical revisions to this article. All authors finally approved the edition to be published.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, S., Mu, A., Jing, Z. et al. Cross ethnic Mendelian randomization analysis reveals causal relationship between air pollution and risk of kidney stones. Sci Rep 15, 12132 (2025). https://doi.org/10.1038/s41598-025-97436-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-97436-y